Effects of Climate Change on the Distribution of Prosthechea mariae (Orchidaceae) and within Protected Areas in Mexico
Abstract
:1. Introduction
2. Results
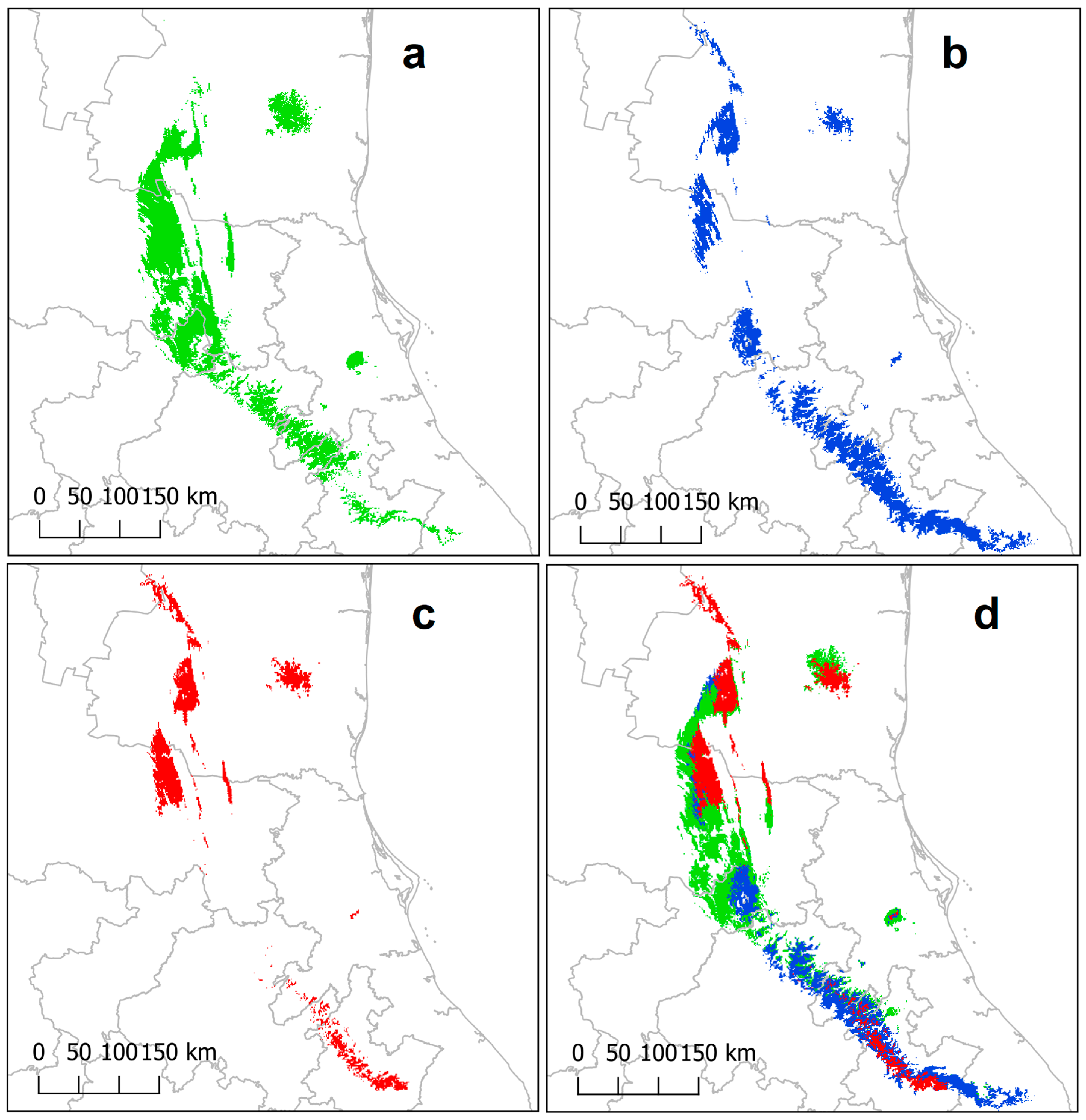
2.1. Potential Distribution and Future Scenarios of P. mariae
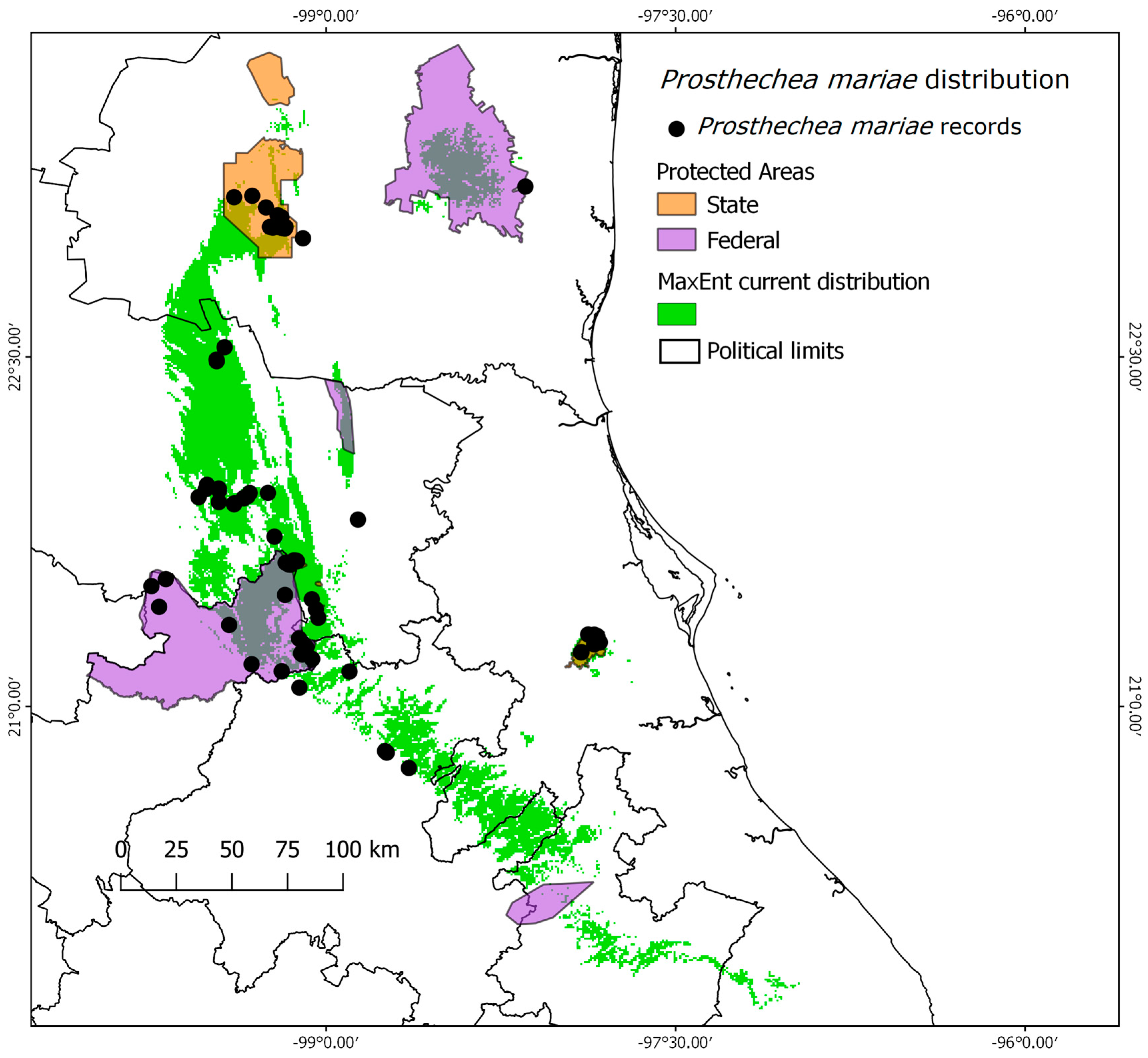
2.2. Species Protection under the Protected Areas Network
3. Discussion
3.1. Potential Distribution and Future Scenarios of P. mariae
3.2. Analysis of the Environmental Protection of Protected Areas
3.3. Ecological Niche Models and Model Evaluation
4. Materials and Methods
4.1. Prosthechea mariae
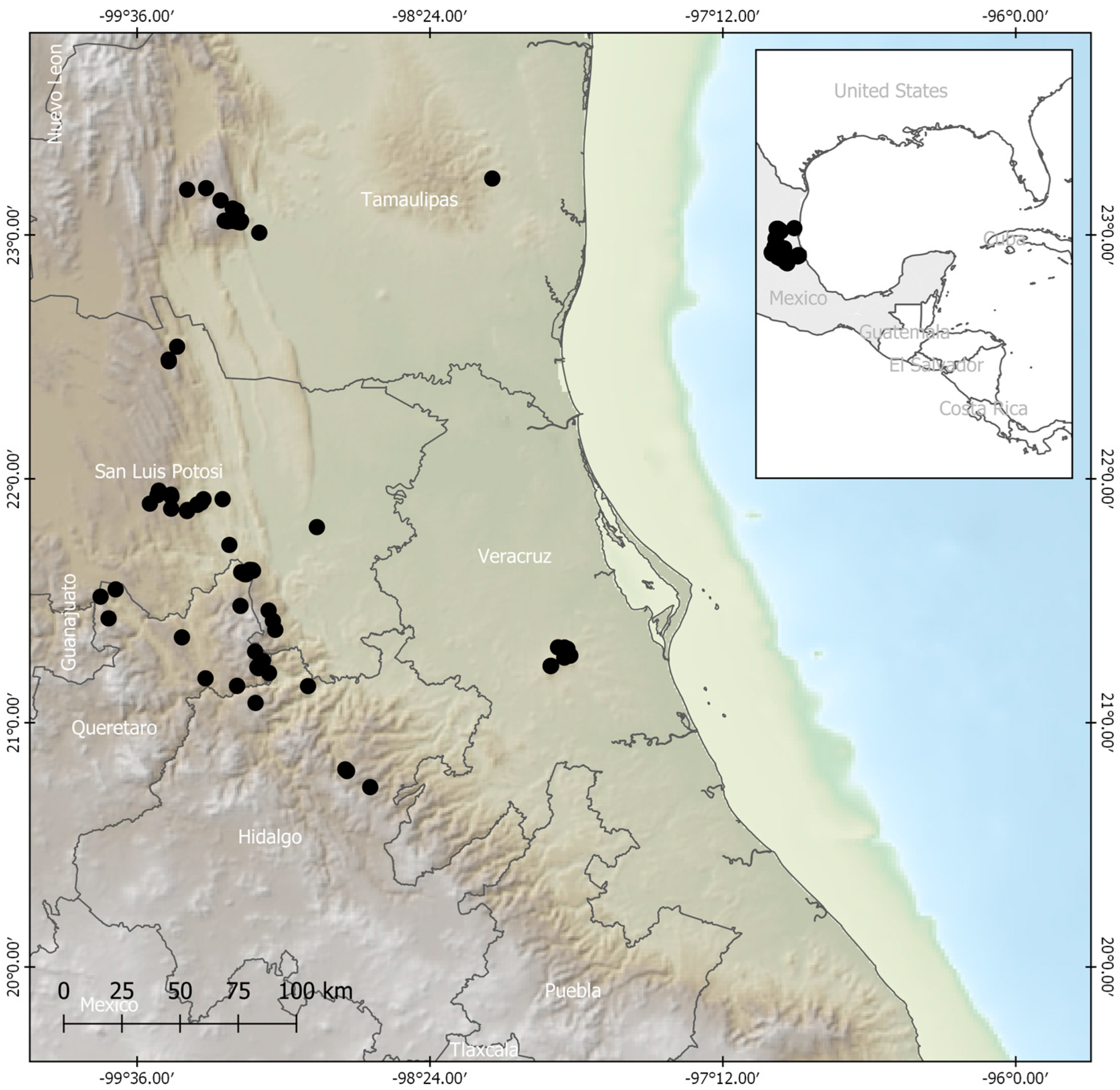
4.2. Presence Data of the Species
4.3. Environmental Variables and Future Climate Scenarios
4.4. Ecological Niche Models and Validation
4.5. Analysis of Distribution Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. The Intergovernmental Panel on Climate Change (IPCC). Available online: https://report.ipcc.ch/ (accessed on 17 July 2023).
- Gómez-Mendoza, L.; Galicia, L. Temperate Forests and Climate Change in Mexico: From Modelling to Adaptation Strategies; Climate change and Variability; SCIYO: Rijeka, Croatia, 2010; pp. 195–210. [Google Scholar]
- Trejo, I.; Martínez-Meyer, E.; Calixto-Pérez, E.; Sánchez-Colón, S.; Vázquez De La Torre, R.; Villers-Ruiz, L. Analysis of the Effects of Climate Change on Plant Communities and Mammals in México. Atmósfera 2011, 24, 1–14. [Google Scholar]
- Jiménez-García, D.; Peterson, A.T. Climate Change Impact on Endangered Cloud Forest Tree Species in Mexico. Rev. Mex. Biodivers. 2019, 90, 1–14. [Google Scholar] [CrossRef]
- Soto, V.H.; Delgado Granados, H. Estimación de La Temperatura Del Aire En La Alta Montaña Mexicana Mediante Un Modelo de Elevación Del Terreno: Caso Del Volcán Nevado de Toluca (México). Ería Rev. Cuatrimest. Geogr. 2020, 40, 167–182. [Google Scholar]
- Gallardo, B.; Aldridge, D.C.; González-Moreno, P.; Pergl, J.; Pizarro, M.; Pyšek, P.; Thuiller, W.; Yesson, C.; Vilà, M. Protected Areas Offer Refuge from Invasive Species Spreading under Climate Change. Glob. Change Biol. 2017, 23, 5331–5343. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Jeschke, J.M.; Leroy, B.; Mace, G.M. Insights from Modeling Studies on How Climate Change Affects Invasive Alien Species Geography. Ecol. Evol. 2018, 8, 5688–5700. [Google Scholar] [CrossRef] [PubMed]
- Lamsal, P.; Kumar, L.; Aryal, A.; Atreya, K. Invasive Alien Plant Species Dynamics in the Himalayan Region under Climate Change. Ambio 2018, 47, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Wilkening, J.; Pearson-Prestera, W.; Mungi, N.A.; Bhattacharyya, S. Endangered Species Management and Climate Change: When Habitat Conservation Becomes a Moving Target. Wildl. Soc. Bull. 2019, 43, 11–20. [Google Scholar] [CrossRef]
- Arar, A.; Nouidjem, Y.; Bounar, R.; Tabet, S.; Kouba, Y. Modeling of the Current and Future Potential Distribution of Atlas Cedar (Cedrus atlantica) Forests Revealed Shifts in the Latitudinal, Longitudinal and Altitudinal Range towards More Humid Conditions. Ecol. Quest. 2020, 31, 49. [Google Scholar] [CrossRef]
- Hufnagel, L.; Garamvölgyi, Á. Impacts of Climate Change on Vegetation Distribution. No. 1: Climate Change Induced Vegetation Shifts in the Palearctic Region. Appl. Ecol. Environ. Res. 2013, 11, 79–122. [Google Scholar]
- Vegas-Vilarrúbia, T.; Nogué, S.; Rull, V. Global Warming, Habitat Shifts and Potential Refugia for Biodiversity Conservation in the Neotropical Guayana Highlands. Biol. Conserv. 2012, 152, 159–168. [Google Scholar] [CrossRef]
- Bridle, J.R.; Vines, T.H. Limits to Evolution at Range Margins: When and Why Does Adaptation Fail? Trends Ecol. Evol. 2007, 22, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xu, X.; Shrestha, N.; Zimmermann, N.E.; Tang, Z.; Wang, Z. Response of Spatial Vegetation Distribution in China to Climate Changes since the Last Glacial Maximum (LGM). PLoS ONE 2017, 12, e0175742. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xue, Y.; MacDonald, G.; Cox, P.; Zhang, Z. Global Vegetation Variability and Its Response to Elevated CO2, Global Warming, and Climate Variability—A Study Using the Offline SSiB4/TRIFFID Model and Satellite Data. Earth Syst. Dyn. 2019, 10, 9–29. [Google Scholar] [CrossRef]
- Singh, J.; Singh, R.P.; Khare, R. Influence of Climate Change on Antarctic Flora. Polar Sci. 2018, 18, 94–101. [Google Scholar] [CrossRef]
- Grabherr, G.; Gottfried, M.; Pauli, H. Climate Change Impacts in Alpine Environments. Geogr. Compass 2010, 4, 1133–1153. [Google Scholar] [CrossRef]
- Nneji, L.M.; Salako, G.; Oladipo, S.O.; Ayoola, A.O.; Onadeko, A.B.; Adedeji, B.E.; Omotoso, O.; Ugwumba, A.A.A.; Adeola, A.C. Species Distribution Modelling Predicts Habitat Suitability and Reduction of Suitable Habitat under Future Climatic Scenario for Sclerophrys perreti: A Critically Endangered Nigerian Endemic Toad. Afr. J. Ecol. 2020, 58, 481–491. [Google Scholar] [CrossRef]
- Dagnino, D.; Guerrina, M.; Minuto, L.; Mariotti, M.G.; Médail, F.; Casazza, G. Climate Change and the Future of Endemic Flora in the South Western Alps: Relationships between Niche Properties and Extinction Risk. Reg. Environ. Chang. 2020, 20, 121. [Google Scholar] [CrossRef]
- CONANP. 100 Años de Conservación en México: Áreas Naturales Protegidas; Semarnat-Conanp: Mexico City, Mexico, 2018. [Google Scholar]
- Williams-Linera, G.; Toledo-Garibaldi, M.; Hernández, C.G. How Heterogeneous Are the Cloud Forest Communities in the Mountains of Central Veracruz, Mexico? Plant Ecol. 2013, 214, 685–701. [Google Scholar] [CrossRef]
- Gómez-Díaz, J.A.; Krömer, T.; Carvajal-Hernández, C.I.; Gerold, G.; Heitkamp, F. Richness and Distribution of Herbaceous Angiosperms along Gradients of Elevation and Forest Disturbance in Central Veracruz, Mexico. Bot. Sci. 2017, 95, 307. [Google Scholar] [CrossRef]
- Gómez-Díaz, J.A.; Brast, K.; Degener, J.; Krömer, T.; Ellis, E.; Heitkamp, F.; Gerold, G. Long-Term Changes in Forest Cover in Central Veracruz, Mexico (1993–2014). Trop. Conserv. Sci. 2018, 11, 1940082918771089. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting Species Distribution: Offering More than Simple Habitat Models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of Consensus Methods in Predictive Species Distribution Modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Tomas, W.M.; Guedes, N.M.R.; Peterson, A.T.; Szabo, J.K.; Júnior, A.S.; Camilo, A.R.; Padovani, C.R.; Garcia, L.C. The Relationship between Scale and Predictor Variables in Species Distribution Models Applied to Conservation. Biodivers. Conserv. 2021, 30, 1971–1990. [Google Scholar] [CrossRef]
- Zurell, D.; Franklin, J.; König, C.; Bouchet, P.J.; Dormann, C.F.; Elith, J.; Fandos, G.; Feng, X.; Guillera-Arroita, G.; Guisan, A.; et al. A Standard Protocol for Reporting Species Distribution Models. Ecography 2020, 43, 1261–1277. [Google Scholar] [CrossRef]
- Beaumont, L.J.; Hughes, L.; Pitman, A.J. Why Is the Choice of Future Climate Scenarios for Species Distribution Modelling Important? Ecol. Lett. 2008, 11, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Booth, T.H. Species Distribution Modelling Tools and Databases to Assist Managing Forests under Climate Change. For. Ecol. Manag. 2018, 430, 196–203. [Google Scholar] [CrossRef]
- Srivastava, V. Species Distribution Models (SDM): Applications, Benefits and Challenges in Invasive Species Management. CAB Rev. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of Species Distributions with Maxent: New Extensions and a Comprehensive Evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making Better MAXENT Models of Species Distributions: Complexity, Overfitting and Evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Gomes, V.H.F.; IJff, S.D.; Raes, N.; Amaral, I.L.; Salomão, R.P.; de Souza Coelho, L.; de Almeida Matos, F.D.; Castilho, C.V.; de Andrade Lima Filho, D.; López, D.C.; et al. Species Distribution Modelling: Contrasting Presence-Only Models with Plot Abundance Data. Sci. Rep. 2018, 8, 1003. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Rodríguez, M.M.; Sajama, M.J.; Gutiérrez-Ortega, J.S.; Ortega-Baes, P.; Estrada-Castillón, A.E. Identification of Endemic Vascular Plant Species Hotspots and the Effectiveness of the Protected Areas for Their Conservation in Sierra Madre Oriental, Mexico. J. Nat. Conserv. 2018, 46, 6–27. [Google Scholar] [CrossRef]
- Antúnez, P.; Suárez-Mota, M.; Valenzuela-Encinas, C.; Ruiz-Aquino, F. The Potential Distribution of Tree Species in Three Periods of Time under a Climate Change Scenario. Forests 2018, 9, 628. [Google Scholar] [CrossRef]
- Vargas-Piedra, G.; Valdez-Cepeda, R.D.; López-Santos, A.; Flores-Hernández, A.; Hernández-Quiroz, N.S.; Martínez-Salvador, M. Current and Future Potential Distribution of the Xerophytic Shrub Candelilla (Euphorbia antisyphilitica) under Two Climate Change Scenarios. Forests 2020, 11, 530. [Google Scholar] [CrossRef]
- Charre-Medellín, J.F.; Mas, J.-F.; Chang-Martínez, L.A. Potential Expansion of Hass Avocado Cultivation under Climate Change Scenarios Threatens Mexican Mountain Ecosystems. Crop Pasture Sci. 2021, 72, 291–301. [Google Scholar] [CrossRef]
- Martínez-Sifuentes, A.R.; Villanueva-Díaz, J.; Crisantos De La Rosa, E.; Stahle, D.W. Current and Future Spatial Modeling of Habitat Suitability of the Mexicanbaldcypress (Taxodium mucronatum Ten.): A Proposal for Conservation in Mexico. Bot. Sci. 2021, 99, 752–770. [Google Scholar] [CrossRef]
- Hernández De La Cruz, M.; Alanís-Méndez, J.L.; Chagoya-Fuentes, J.L.; Enciso-Díaz, O.J. Potential Distribution of Six Endemic Species of Stanhopea (Orchidaceae) Genus in Mexico. Curr. Bot. 2022, 13, 81–88. [Google Scholar] [CrossRef]
- Pulliam, H.R. On the Relationship between Niche and Distribution. Ecol. Lett. 2000, 3, 349–361. [Google Scholar] [CrossRef]
- Soberón, J. Grinnellian and Eltonian Niches and Geographic Distributions of Species. Ecol. Lett. 2007, 10, 1115–1123. [Google Scholar] [CrossRef]
- Kijowska-Oberc, J.; Staszak, A.M.; Kamiński, J.; Ratajczak, E. Adaptation of Forest Trees to Rapidly Changing Climate. Forests 2020, 11, 123. [Google Scholar] [CrossRef]
- Thom, D.; Rammer, W.; Seidl, R. Disturbances Catalyze the Adaptation of Forest Ecosystems to Changing Climate Conditions. Glob. Chang. Biol. 2017, 23, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Holsinger, L.; Parks, S.A.; Parisien, M.-A.; Miller, C.; Batllori, E.; Moritz, M.A. Climate Change Likely to Reshape Vegetation in North America’s Largest Protected Areas. Conserv. Sci. Pract. 2019, 1, e50. [Google Scholar] [CrossRef]
- Anderson, J.T.; Song, B.-H. Plant Adaptation to Climate Change—Where Are We? J. Syst. Evol. 2020, 58, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Hanley, M.E. Plants and Climate Change: Complexities and Surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Soto-Arenas, M.A. Euchile mariae (Ames) Withner; Lámina 584; Soto, E.H.M., Ed.; Asociación Mexicana de Orquideología AC: Mexico City, Mexico, 2002. [Google Scholar]
- Suárez-Quijada, I.; Hernández-Altamirano, M.; Chávez-Ávila, V.M.; Sandoval-Zapotitla, E.; Martinez-Palacios, A. Propagación in vitro y aclimatización de Euchile mariae (Ames) Withner (Orchidaceae). Lankesteriana Int. J. Orchid. 2007, 7, 388–393. [Google Scholar] [CrossRef]
- Suárez-Quijada, I.; Sandoval-Zapotitla, E.; Hernández-Altamirano, M.; Chávez-Ávila, V.M. Determinación Histológica de Regenerantes de Euchile mariae (Ames) Withner, (Orchidaceae), Obtenidos a Partir de Protocormos Cultivados in Vitro. Lankesteriana Int. J. Orchid. 2007, 7, 394–397. [Google Scholar] [CrossRef]
- Ikeda, H.; Senni, K.; Fujii, N.; Setoguchi, H. High Mountains of the Japanese Archipelago as Refugia for Arctic-Alpine Plants: Phylogeography of Loiseleuria procumbens (L.) Desvaux (Ericaceae). Biol. J. Linn. Soc. 2009, 97, 403–412. [Google Scholar] [CrossRef]
- Bocedi, G.; Atkins, K.E.; Liao, J.; Henry, R.C.; Travis, J.M.J.; Hellmann, J.J. Effects of Local Adaptation and Interspecific Competition on Species’ Responses to Climate Change. Ann. N. Y. Acad. Sci. 2013, 1297, 83–97. [Google Scholar] [CrossRef]
- Guevara, L. Altitudinal, Latitudinal and Longitudinal Responses of Cloud Forest Species to Quaternary Glaciations in the Northern Neotropics. Biol. J. Linn. Soc. 2020, 130, 615–625. [Google Scholar] [CrossRef]
- Buchanan, J.; Zuccarello, G.C. Decoupling of Short- and Long-Distance Dispersal Pathways in the Endemic New Zealand Seaweed Carpophyllum maschalocarpum (Phaeophyceae, Fucales). J. Phycol. 2012, 48, 518–529. [Google Scholar] [CrossRef]
- Ghazoul, J. Pollen and Seed Dispersal among Dispersed Plants. Biol. Rev. Camb. Philos. Soc. 2005, 80, 413–443. [Google Scholar] [CrossRef] [PubMed]
- Hetem, R.S.; Fuller, A.; Maloney, S.K.; Mitchell, D. Responses of Large Mammals to Climate Change. Temperature 2014, 1, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Jezkova, T.; Olah-Hemmings, V.; Riddle, B.R. Niche Shifting in Response to Warming Climate after the Last Glacial Maximum: Inference from Genetic Data and Niche Assessments in the Chisel-Toothed Kangaroo Rat (Dipodomys Microps). Glob. Change Biol. 2011, 17, 3486–3502. [Google Scholar] [CrossRef]
- Camacho-Domínguez, E.; Ávila-Díaz, I. Mating System and Female Reproductive Success of the Endemic, Epiphytic Prosthechea Aff. karwinskii (Orchidaceae). Lankesteriana 2011, 11, e18300. [Google Scholar] [CrossRef]
- Ray, H.A.; Stuhl, C.J.; Kane, M.E.; Ellis, J.D.; Daniels, J.C.; Gillett-Kaufman, J.L. Aspects of the Pollination Biology of Encyclia tampensis, the Commercially Exploited Butterfly Orchid, and Prosthechea Cochleata, the Endangered Clamshell Orchid, in South Florida. Fla. Entomol. 2019, 102, 154. [Google Scholar] [CrossRef]
- Ray, H.A.; Kane, M.E.; Gillett-Kaufman, J.L. Effect of Self- and Cross-Pollination Treatments and Microhabitat on Seed Germination of Prosthechea cochleata and Encyclia tampensis (Orchidaceae) in Southern Florida. Southeast. Nat. 2020, 19, 601. [Google Scholar] [CrossRef]
- Geldmann, J.; Barnes, M.; Coad, L.; Craigie, I.D.; Hockings, M.; Burgess, N.D. Effectiveness of Terrestrial Protected Areas in Reducing Habitat Loss and Population Declines. Biol. Conserv. 2013, 161, 230–238. [Google Scholar] [CrossRef]
- Gomes, V.H.F.; Vieira, I.C.G.; Salomão, R.P.; ter Steege, H. Amazonian Tree Species Threatened by Deforestation and Climate Change. Nat. Clim. Chang. 2019, 9, 547–553. [Google Scholar] [CrossRef]
- Guerra, C.A.; Rosa, I.M.D.; Pereira, H.M. Change versus Stability: Are Protected Areas Particularly Pressured by Global Land Cover Change? Landsc. Ecol. 2019, 34, 2779–2790. [Google Scholar] [CrossRef]
- Lehikoinen, P.; Santangeli, A.; Jaatinen, K.; Rajasärkkä, A.; Lehikoinen, A. Protected Areas Act as a Buffer against Detrimental Effects of Climate Change—Evidence from Large-scale, Long-term Abundance Data. Glob. Chang. Biol. 2019, 25, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Adelaide Ferreira, M.; Kenchington, E. Climate Change Is Likely to Severely Limit the Effectiveness of Deep-Sea ABMTs in the North Atlantic. Mar. Policy 2018, 87, 111–122. [Google Scholar] [CrossRef]
- Bruno, J.F.; Bates, A.E.; Cacciapaglia, C.; Pike, E.P.; Amstrup, S.C.; van Hooidonk, R.; Henson, S.A.; Aronson, R.B. Climate Change Threatens the World’s Marine Protected Areas. Nat. Clim. Chang. 2018, 8, 499–503. [Google Scholar] [CrossRef]
- Bruno, J.F.; Côté, I.M.; Toth, L.T. Climate Change, Coral Loss, and the Curious Case of the Parrotfish Paradigm: Why Don’t Marine Protected Areas Improve Reef Resilience? Annu. Rev. Mar. Sci. 2019, 11, 307–334. [Google Scholar] [CrossRef]
- Seiferling, I.S.; Proulx, R.; Peres-Neto, P.R.; Fahrig, L.; Messier, C. Measuring Protected-Area Isolation and Correlations of Isolation with Land-Use Intensity and Protection Status. Conserv. Biol. 2012, 26, 610–618. [Google Scholar] [CrossRef] [PubMed]
- DeFries, R.; Hansen, A.; Newton, A.C.; Hansen, M.C. Increasing Isolation of Protected Areas in Tropical Forests over the Past Twenty Years. Ecol. Appl. 2005, 15, 19–26. [Google Scholar] [CrossRef]
- Oliver, T.; Roy, D.B.; Hill, J.K.; Brereton, T.; Thomas, C.D. Heterogeneous Landscapes Promote Population Stability. Ecol. Lett. 2010, 13, 473–484. [Google Scholar] [CrossRef]
- Curran, L.M.; Trigg, S.N.; McDonald, A.K.; Astiani, D.; Hardiono, Y.M.; Siregar, P.; Caniago, I.; Kasischke, E. Lowland Forest Loss in Protected Areas of Indonesian Borneo. Science 2004, 303, 1000–1003. [Google Scholar] [CrossRef]
- Laurance, W.F.; Carolina Useche, D.; Rendeiro, J.; Kalka, M.; Bradshaw, C.J.A.; Sloan, S.P.; Laurance, S.G.; Campbell, M.; Abernethy, K.; Alvarez, P.; et al. Averting Biodiversity Collapse in Tropical Forest Protected Areas. Nature 2012, 489, 290–294. [Google Scholar] [CrossRef]
- Heino, M.; Kummu, M.; Makkonen, M.; Mulligan, M.; Verburg, P.H.; Jalava, M.; Räsänen, T.A. Forest Loss in Protected Areas and Intact Forest Landscapes: A Global Analysis. PLoS ONE 2015, 10, e0138918. [Google Scholar] [CrossRef]
- Joppa, L.N.; Loarie, S.R.; Pimm, S.L. On Population Growth Near Protected Areas. PLoS ONE 2009, 4, e4279. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.E.; Peterson, A.T.; Barve, N.; Osorio-Olvera, L. Kuenm: An R Package for Detailed Development of Ecological Niche Models Using Maxent. PeerJ 2019, 7, e6281. [Google Scholar] [CrossRef] [PubMed]
- Tourne, D.C.M.; Ballester, M.V.R.; James, P.M.A.; Martorano, L.G.; Guedes, M.C.; Thomas, E. Strategies to Optimize Modeling Habitat Suitability of Bertholletia Excelsa in the Pan-Amazonia. Ecol. Evol. 2019, 9, 12623–12638. [Google Scholar] [CrossRef] [PubMed]
- De Marco, P.; Nóbrega, C.C. Evaluating Collinearity Effects on Species Distribution Models: An Approach Based on Virtual Species Simulation. PLoS ONE 2018, 13, e0202403. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Tarkesh, M.; Jafari, R.; Jetschke, G. Bioclimatic Variables from Precipitation and Temperature Records vs. Remote Sensing-Based Bioclimatic Variables: Which Side Can Perform Better in Species Distribution Modeling? Ecol. Inform. 2020, 57, 101060. [Google Scholar] [CrossRef]
- García-Cruz, J.; Sánches, L.M.; Jimenéz, R.; Solano, R. Orchidaceae, Tribu Epidendraeae. Flora Del Bajío Reg. Adyac. 2003, 119, 19. [Google Scholar]
- Soto-Arenas, M.A.; Hágsater, E.; Jiménez-Machorro, R.S.-C.; Ruíz-Contreras, I. Las Orquídeas de México Catálogo Digital; Herbario AMO, Insituto Chinoin, AC: Miguel Hidalgo, Mexico, 2007. [Google Scholar]
- Téllez, M. de los Á.A. Diagnóstico de la Familia Orchidaceae en México:(Prosthechea Citrina, Prosthechea Vitellina, Stanhopea Tigrina, Encyclia Adenocaula, Laelia Speciosa, Laelia Gouldiana y Rhynchostele Rossii); Chapingo Autonomous University: Texcoco, Mexico, 2011; ISBN 607-12-0206-X. [Google Scholar]
- Soto-Arenas, M.A.; Solano-Gómez, A.R. Ficha Técnica de Euchile Mariae: Proyecto No. W029; Bases de datos SNIB-CONABIO; Herbario de la Asociación Mexicana de Orquideología A.C.: Mexico City, Mexico; Instituto Chinoin A.C.: Mexico City, Mexico, 2007. [Google Scholar]
- Santos Díaz, M.D.S.; Carranza Álvarez, C. Plant Regeneration through Direct Shoot Formation from Leaf Cultures and from Protocorm-like Bodies Derived from Callus of Encyclia mariae (Orchidaceae), a Threatened Mexican Orchid. Vitr. Cell. Dev. Biol.-Plant 2009, 45, 162–170. [Google Scholar] [CrossRef]
- Google. Google Earth Pro, Version 7.3.1; Google: Mountain View, CA, USA, 2019. [Google Scholar]
- GBIF. GBIF (Global Biodiversity Information Facility). Available online: http://gbif.org (accessed on 31 May 2019).
- Peterson, A.T.; Nakazawa, Y. Environmental Data Sets Matter in Ecological Niche Modelling: An Example with Solenopsis Invicta and Solenopsis Richteri. Glob. Ecol. Biogeogr. 2007, 17, 135–144. [Google Scholar] [CrossRef]
- Veloz, S.D. Spatially Autocorrelated Sampling Falsely Inflates Measures of Accuracy for Presence-Only Niche Models. J. Biogeogr. 2009, 36, 2290–2299. [Google Scholar] [CrossRef]
- Monterrubio-Rico, T.C.; Charre-Medellín, J.F.; Pacheco-Figueroa, C.; Arriaga-Weiss, S.; de Dios Valdez-Leal, J.; Cancino-Murillo, R.; Escalona-Segura, G.; Bonilla-Ruz, C.; Rubio-Rocha, Y. Distribución Potencial Histórica y Contemporánea de La Familia Psittacidae En México. Rev. Mex. Biodivers. 2016, 87, 1103–1117. [Google Scholar] [CrossRef]
- Osorio-Olvera, L.; Lira-Noriega, A.; Soberón, J.; Townsend Peterson, A.; Falconi, M.; Contreras-Díaz, R.G.; Martínez-Meyer, E.; Barve, V.; Barve, N. ntbox: An R package with graphical user interface for modeling and evaluating multidimensional ecological niches. Methods Ecol. Evol. 2020, 11, 1199–1206. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very High Resolution Interpolated Climate Surfaces for Global Land Areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Pinedo-Alvarez, C.; Renteria-Villalobos, M.; Aguilar-Soto, V.; Vega-Mares, J.H.; Melgoza-Castillo, A. Distribution Dynamics of Picea chihuahuana Martínez Populations under Different Climate Change Scenarios in Mexico. Glob. Ecol. Conserv. 2019, 17, e00559. [Google Scholar] [CrossRef]
- Hernandez-Guzman, R. Predicting Ambystoma ordinarium Distribution under Differentclimate Scenarios in Central Mexico. Herpetol. J. 2019, 29, 71–81. [Google Scholar] [CrossRef]
- Martínez-Sifuentes, A.; Villanueva-Díaz, J.; Manzanilla-Quiñones, U.; Becerra-López, J.; Hernández-Herrera, J.; Estrada-Ávalos, J.; Velázquez-Pérez, A. Spatial Modeling of the Ecological Niche of Pinus greggii Engelm. (Pinaceae): A Species Conservation Proposal in Mexico under Climatic Change Scenarios. IForest—Biogeosci. For. 2020, 13, 426–434. [Google Scholar] [CrossRef]
- Sierra-Morales, P.; Rojas-Soto, O.; Ríos-Muñoz, C.A.; Ochoa-Ochoa, L.M.; Flores-Rodríguez, P.; Almazán-Núñez, R.C. Climate Change Projections Suggest Severe Decreases in the Geographic Ranges of Bird Species Restricted to Mexican Humid Mountain Forests. Glob. Ecol. Conserv. 2021, 30, e01794. [Google Scholar] [CrossRef]
- Cruz-Jiménez, I.; Delgado-Sánchez, P.; Guerrero-González, M.D.L.L.; Puente-Martínez, R.; Flores, J.; De-Nova, J.A. Predicting Geographic Distribution and Habitat Suitability of Opuntia Streptacantha in Paleoclimatic, Current, and Future Scenarios in Mexico. Ecol. Evol. 2023, 13, e10050. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A Statistical Explanation of MaxEnt for Ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Soberon, J.; Nakamura, M. Niches and Distributional Areas: Concepts, Methods, and Assumptions. Proc. Natl. Acad. Sci. USA 2009, 106, 19644–19650. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Soberon, J.; Peterson, A.T. Interpretation of Models of Fundamental Ecological Niches and Species’ Distributional Areas. Biodivers. Inform. 2005, 2, 1–10. [Google Scholar] [CrossRef]
- Peterson, A.T. Ecological Niche Conservatism: A Time-Structured Review of Evidence. J. Biogeogr. 2011, 38, 817–827. [Google Scholar] [CrossRef]
- Barve, N.; Barve, V.; Jiménez-Valverde, A.; Lira-Noriega, A.; Maher, S.P.; Peterson, A.T.; Soberón, J.; Villalobos, F. The Crucial Role of the Accessible Area in Ecological Niche Modeling and Species Distribution Modeling. Ecol. Model. 2011, 222, 1810–1819. [Google Scholar] [CrossRef]
- Feng, X.; Park, D.S.; Walker, C.; Peterson, A.T.; Merow, C.; Papeş, M. A Checklist for Maximizing Reproducibility of Ecological Niche Models. Nat. Ecol. Evol. 2019, 3, 1382–1395. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the Black Box: An Open-source Release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papeş, M.; Soberón, J. Rethinking Receiver Operating Characteristic Analysis Applications in Ecological Niche Modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- SEMARNAT-CONANP. Áreas Naturales Protegidas Estatales, Municipales, Ejidales, Comunitarias y Privadas de México 2020; Catálogo de Metadatos Geográficos; SEMARNAT-CONANP: Mexico City, Mexico, 2020. [Google Scholar]

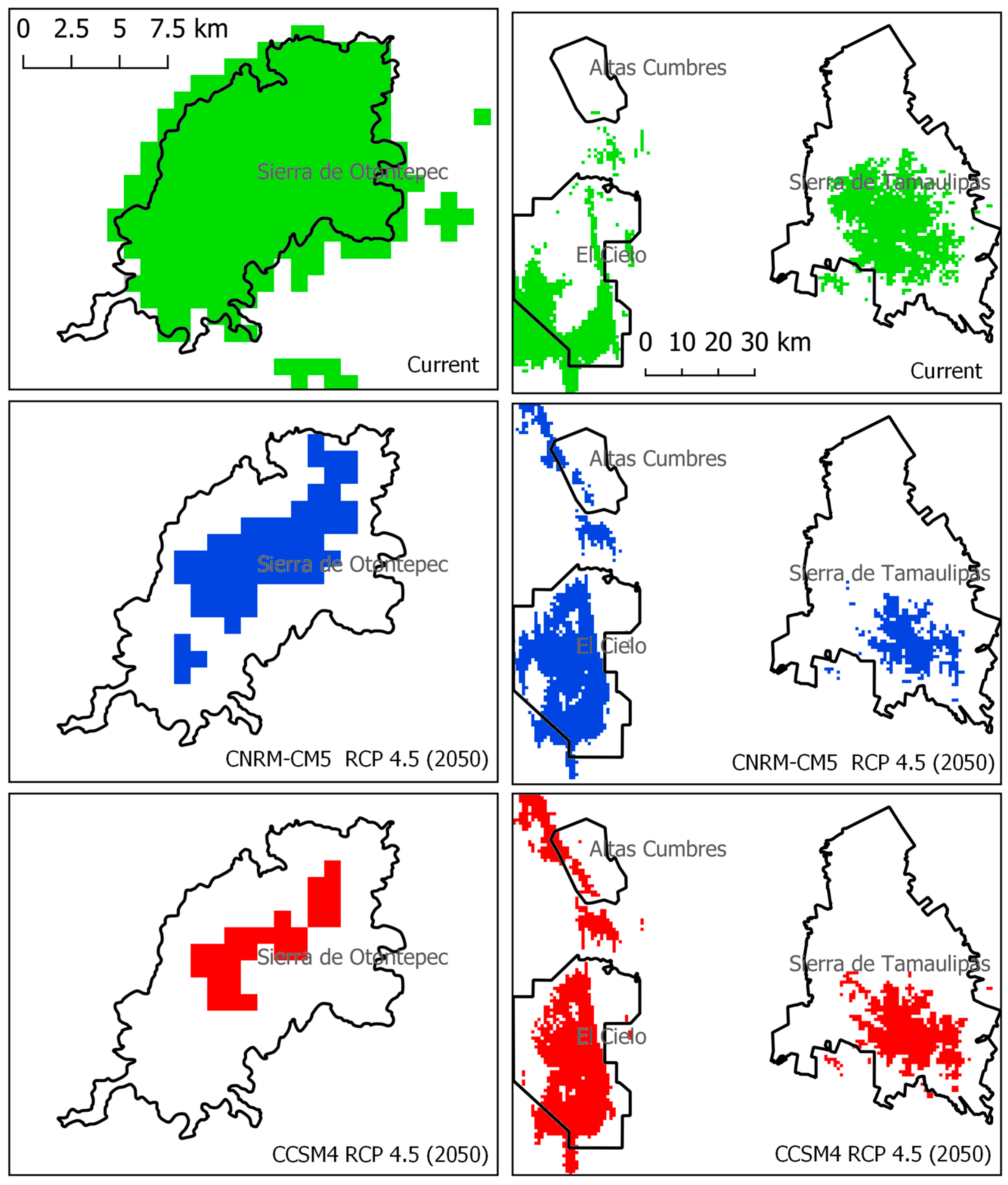
| Protection Type | Name | PAs Decreed Area km2 | Current Surface Area km2 (%) | Projected Surface Area 2050 CNRM-CM5 km2 (%) | Projected Surface Area 2050 CCSM4 km2 (%) |
|---|---|---|---|---|---|
| Federal | Sierra Gorda | 3835.67 | 1142.45 (29.78) | 400.21 (10.43) | - |
| Federal | Sierra de Tamaulipas | 3088.88 | 827.03 (26.77) | 324.63 (10.51) | 487.26 (15.77) |
| State | El Cielo | 1375.01 | 451.79 (32.86) | 720.49 (52.40) | 677.13 (49.25) |
| Federal | Cuenca Hidrográfica del Rio Necaxa | 421.53 | 10.99 (2.61) | 184.69 (43.81) | 98.31 (23.32) |
| State | Altas Cumbres | 315.94 | 2.35 (0.75) | 35.84 (11.34) | 54.40 (17.22) |
| Federal | Sierra del Abra Tanchipa | 214.84 | 134.61 (62.65) | 3.13 (1.46) | 72.71 (33.84) |
| State | Sierra de Otontepec | 151.59 | 143.41 (94.61) | 46.29 (30.53) | 21.55 (14.21) |
| State | La Hoya de las Huahuas | 4.07 | 3.32 (81.61) | - | - |
| State | El Sótano de Las Golondrinas | 2.82 | 2.82 (100) | 2.18 (77.30) | 0.08 (2.82) |
| State | Las Cuevas del Viento y la Fertilidad | 0.08 | 0.08 (100) | - | - |
| Total | 9410.44 | 2718.85 (28.89) | 1717.45 (18.25) | 1411.43 (15) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanís-Méndez, J.L.; Soto, V.; Limón-Salvador, F. Effects of Climate Change on the Distribution of Prosthechea mariae (Orchidaceae) and within Protected Areas in Mexico. Plants 2024, 13, 839. https://doi.org/10.3390/plants13060839
Alanís-Méndez JL, Soto V, Limón-Salvador F. Effects of Climate Change on the Distribution of Prosthechea mariae (Orchidaceae) and within Protected Areas in Mexico. Plants. 2024; 13(6):839. https://doi.org/10.3390/plants13060839
Chicago/Turabian StyleAlanís-Méndez, José Luis, Víctor Soto, and Francisco Limón-Salvador. 2024. "Effects of Climate Change on the Distribution of Prosthechea mariae (Orchidaceae) and within Protected Areas in Mexico" Plants 13, no. 6: 839. https://doi.org/10.3390/plants13060839
APA StyleAlanís-Méndez, J. L., Soto, V., & Limón-Salvador, F. (2024). Effects of Climate Change on the Distribution of Prosthechea mariae (Orchidaceae) and within Protected Areas in Mexico. Plants, 13(6), 839. https://doi.org/10.3390/plants13060839






