Antioxidative Response and Phenolic Content of Young Industrial Hemp Leaves at Different Light and Mycorrhiza
Abstract
:1. Introduction
2. Results
2.1. Morphological Parameters of Young Hemp Plants
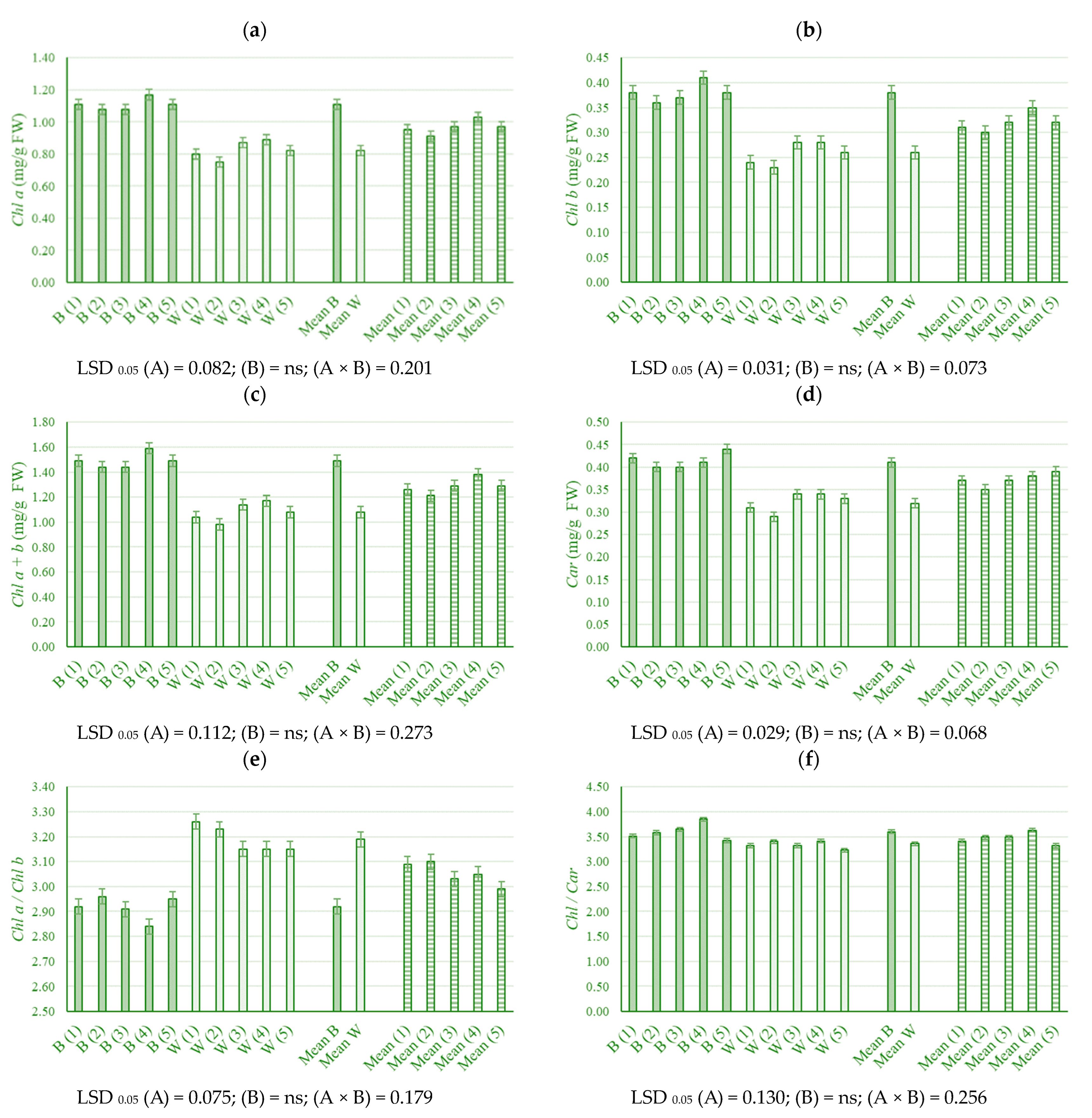
2.2. Pigment Content in Young Leaves
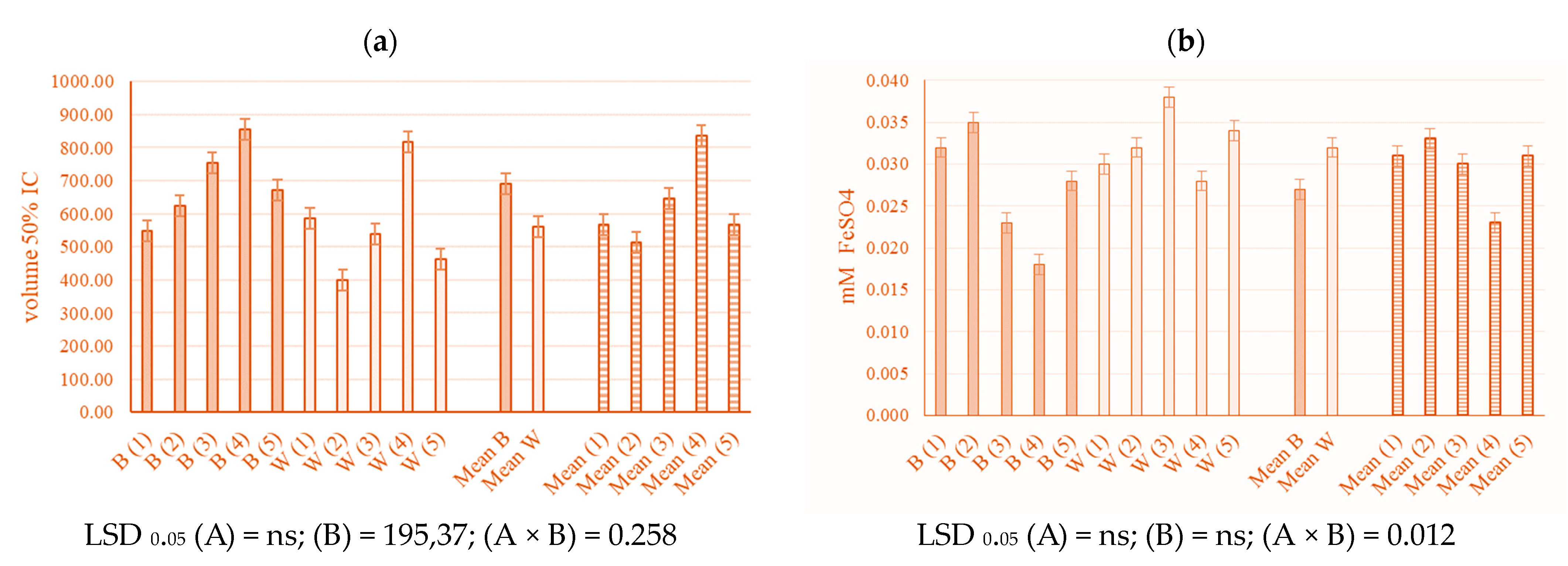
2.3. Antioxidant Activity
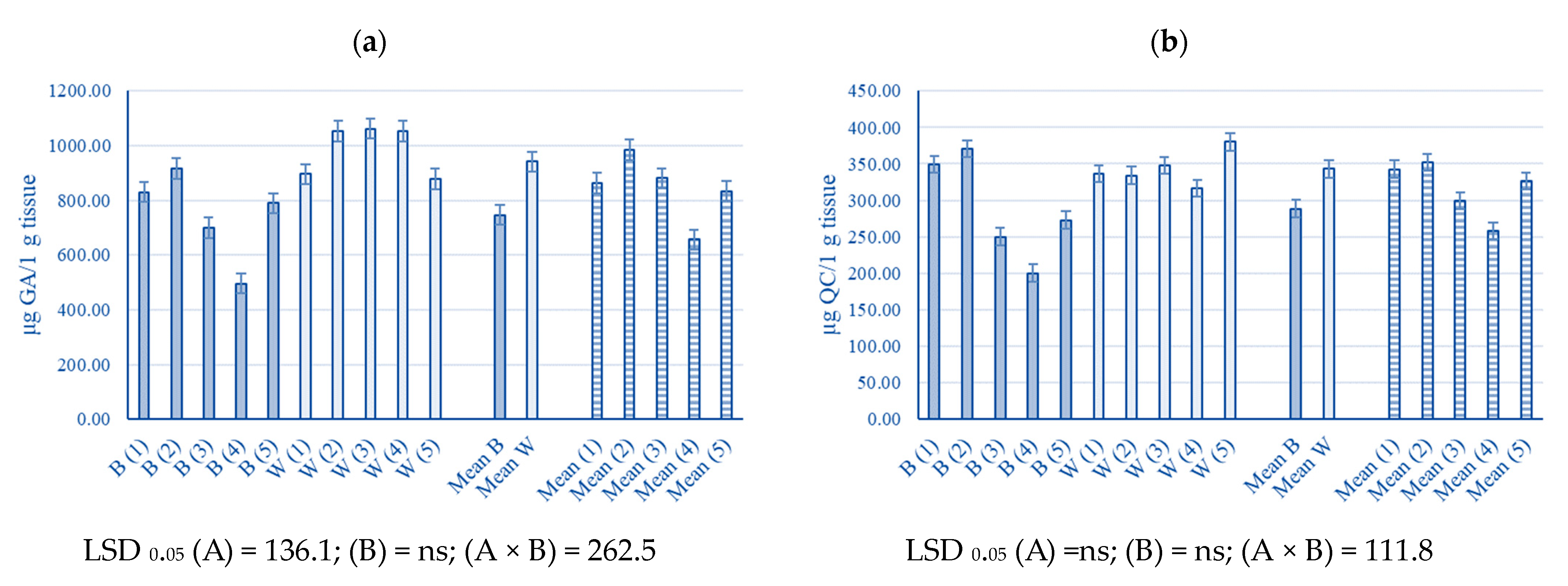
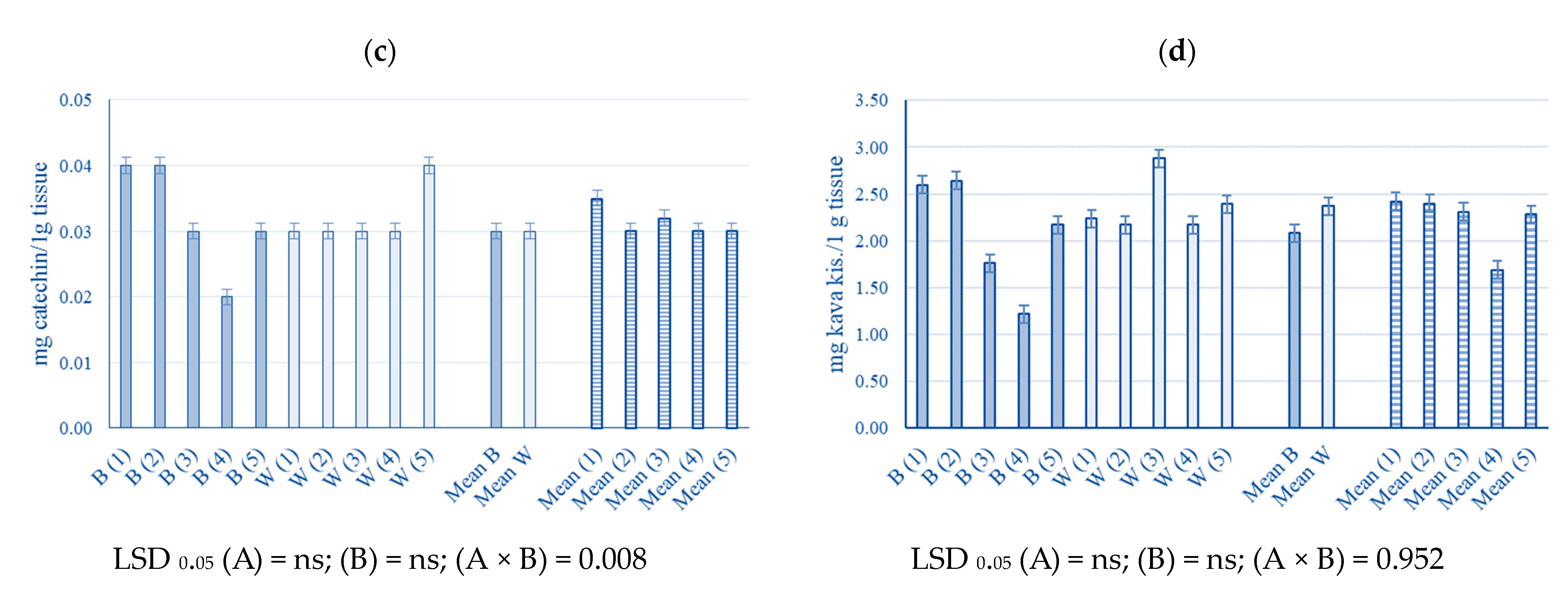
2.4. The Phenolic Content of Young Industrial Hemp Leaves
3. Discussion
3.1. Morphological Parameters of Young Hemp Plants
3.2. Pigment Content in Young Leaves
3.3. Antioxidant Activity
3.4. The Phenolic Content of Young Industrial Hemp Leaves
4. Materials and Methods
4.1. Plant Material, Growth Conditions and Mycorrhiza Application
4.2. Sample Preparation
4.3. Determination of Chlorophyll and Carotenoid Content
4.4. Determination of Antioxidant Activity with DPPH Method
4.5. Determination of Antioxidant Capacity with FRAP Method
4.6. Determination of Total Phenols and Flavonoids
4.7. Determination of Total Flavanols and Phenolic Acids
4.8. Data Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rupasinghe, H.P.V.; Davis, A.; Kumar, S.K.; Murray, B.; Zheljazkov, V.D. Industrial Hemp (Cannabis sativa subsp. sativa) as an Emerging Source for Value-Added Functional Food Ingredients and Nutraceuticals. Molecules 2020, 25, 4078. [Google Scholar] [CrossRef]
- Lančaričová, A.; Kuzmiaková, B.; Porvaz, P.; Havrlentova, M.; Nemeček, P.; Kraic, J. Nutričná kvalita semena konopy siatej (Cannabis sativa L.) pestovanej v rôznom prostredí. JCEA 2021, 22, 748–761. [Google Scholar] [CrossRef]
- Varga, I.; Varga, D.; Antunović, M. The potential of Cannabis sp. in pain medicine: A perspective. Food Health Dis. Sci.-Prof. J. Nutr. Diet. 2021, 10, 104–111. [Google Scholar]
- Official Gazette 39/2019: Zakon o Izmjenama i Dopunama Zakona o Suzbijanju Zlouporabe Droga. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_04_39_799.html (accessed on 23 July 2023). (In Croatian).
- Aloo, S.O.; Mwiti, G.; Ngugi, L.W.; Oh, D.H. Uncovering the secrets of industrial hemp in food and nutrition: The trends, challenges, and new-age perspectives. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–20. [Google Scholar] [CrossRef]
- Varga, I.; Kraus, I.; Iljkić, D.; Jonjić, A.; Antunović, M. Tradicija proizvodnje industrijske konoplje u Hrvatskoj. Sjemenarstvo 2022, 33, 25–40. [Google Scholar] [CrossRef]
- Visković, J.; Zheljazkov, V.D.; Sikora, V.; Noller, J.; Latković, D.; Ocamb, C.M.; Koren, A. Industrial Hemp (Cannabis sativa L.) Agronomy and Utilization: A Review. Agronomy 2023, 13, 931. [Google Scholar] [CrossRef]
- Mougin, G. Hemp: Industrial Production and Uses; Bouloc, P., Allegret, S., Arnaud, L., Eds.; CABI: Wallingford, UK, 2013. [Google Scholar] [CrossRef]
- Kraszkiewicz, A.; Kachel, M.; Parafiniuk, S.; Zając, G.; Niedziółka, I.; Sprawka, M. Assessment of the Possibility of Using Hemp Biomass (Cannabis sativa L.) for Energy Purposes: A Case Study. Appl. Sci. 2019, 9, 4437. [Google Scholar] [CrossRef]
- Galić Subašić, D.; Jurišić, M.; Rebekić, A.; Josipović, M.; Radočaj, D.; Rapčan, I. Odnos komponenata prinosa i prinosa zrna soje (Glycine max L. Merr.) u uvjetima navodnjavanja. Poljoprivreda 2022, 28, 32–38. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Radočaj, D.; Samac, D.; Jurišić, M. Influence of Thermal Pretreatment on Lignin Destabilization in Harvest Residues: An Ensemble Machine Learning Approach. AgriEngineering 2024, 6, 171–184. [Google Scholar] [CrossRef]
- Krüger, M.; van Eeden, T.; Beswa, D. Cannabis sativa Cannabinoids as Functional Ingredients in Snack Foods—Historical and Developmental Aspects. Plants 2022, 11, 3330. [Google Scholar] [CrossRef]
- Buranji, I.; Varga, I.; Lisjak, M.; Iljkić, D.; Antunović, M. Morphological characteristic of fiber flax seedlings regard to different pH water solution and temperature. JCEA 2019, 20, 1135–1142. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and Microgreens—Novel Food Sources for Healthy Diets. Plants 2022, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Pannico, A.; Kyriacou, M.C.C.; El-Nakhel, C.; Graziani, G.; Carillo, P.; Corrado, G.; Ritieni, A.; Rouphael, Y.; De Pascale, S. Hemp microgreens as an innovative functional food: Variation in the organic acids, amino acids, polyphenols, and cannabinoids composition of six hemp cultivars. Food Res. Int. 2022, 161, 111863. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Iljkić, D.; Tkalec Kojić, M.; Dobreva, T.; Markulj Kulundžić, A.; Antunović, M. Germination of Industrial Hemp (Cannabis sativa L.) at Different Level of Sodium Chloride and Temperatures. Agric. Conspec. Sci. 2022, 87, 11–15. [Google Scholar]
- Žalac, H.; Herman, G.; Lisjak, M.; Teklić, T.; Ivezić, V. Intercropping in Walnut Orchards–Assessing the Toxicity of Walnut Leaf Litter on Barley and Maize Germination and Seedlings Growth. Poljoprivreda 2022, 28, 46–52. [Google Scholar] [CrossRef]
- Kristić, M.; Grubišić, S.; Rebekić, A.; Rupčić, J.; Teklić, T.; Lisjak, M. The influence of variety and cutting on the wheatgrass (Triticum aestivum L.) functional properties. Poljoprivreda 2022, 28, 35–43. [Google Scholar] [CrossRef]
- Knezevic, F.; Nikolai, A.; Marchart, R.; Sosa, S.; Tubaro, A.; Novak, J. Residues of herbal hemp leaf teas—How much of the cannabinoids remain? Food Control 2021, 127, 108146. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef]
- Radočaj, D.; Vinković, T.; Jurišić, M.; Gašparović, M. The Relationship of Environmental Factors and the Cropland Suitability Levels for Soybean Cultivation Determined by Machine Learning. Poljoprivreda 2022, 28, 53–59. [Google Scholar] [CrossRef]
- Stošić, M.; Popović, B.; Ranogajec, L. Soil Tillage Systems in the Function of Ecological Sustainability. Poljoprivreda 2022, 28, 27–34. [Google Scholar] [CrossRef]
- Litskas, V.D. Environmental Impact Assessment for Animal Waste, Organic and Synthetic Fertilizers. Nitrogen 2023, 4, 16–25. [Google Scholar] [CrossRef]
- Liu, T.; Sheng, M.; Wang, C.Y.; Chen, H.; Li, Z.; Tang, M. Impact of arbuscular mycorrhizal fungi on the growth, water status, and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica 2015, 53, 250–258. [Google Scholar] [CrossRef]
- Kristek, S.; Lenart, L.; Jović, J.; Marček, T.; Zmaić, K.; Rešić, I.; Rašić, S. The influence of beneficial microorganisms on yield and quality of soybean grains under conditions of reduced nitrogen fertilization. Poljoprivreda 2017, 23, 25–30. [Google Scholar] [CrossRef]
- Ma, J.; Janoušková, M.; Ye, L.; Bai, L.Q.; Dong, R.R.; Yu, X.C.; Zou, Z.R.; Li, Y.S.S.; He, C.X.X. Role of arbuscular mycorrhiza in alleviating the effect of cold on the photosynthesis of cucumber seedlings. Photosynthetica 2019, 57, 86–95. [Google Scholar] [CrossRef]
- Adavi, Z.; Tadayoun, M.R. Effect of mycorrhiza application on plant growth and yield in potato production under field conditions. Iran. J. Plant Physiol. 2014, 4, 1087–1093. [Google Scholar]
- Baričević, M.; Vrandečić, K.; Zorica, M.; Kos, T. Zeoliti i njihova primjena u zaštiti bilja. Poljoprivreda 2023, 29, 33–42. [Google Scholar] [CrossRef]
- Kristek, S.; Brkić, S.; Jović, J.; Stanković, A.; Ćupurdija, B.; Brica, M.; Karalić, K. The application of nitrogen-fixing bacteria in order to reduce the mineral nitrogen fertilizers in sugar beet. Poljoprivreda 2020, 26, 65–71. [Google Scholar] [CrossRef]
- Kristek, S.; Jović, J.; Martinović, M.; Jantoš, J.; Popović, B.; Lončarić, Z. The Application of Biopreparations as an Alternative to Chemical Fungicides in the Protection of Wheat. Poljoprivreda 2023, 29, 24–32. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Natsiopoulos, D.; Tziolias, A.; Lagogiannis, I.; Mantzoukas, S.; Eliopoulos, P.A. Growth-Promoting and Protective Effect of Trichoderma atrobrunneum and T. simmonsii on Tomato against Soil-Borne Fungal Pathogens. Crops 2022, 2, 202–217. [Google Scholar] [CrossRef]
- Karadzhova, N.; Georgieva, O. The role of Trichoderma and Gliocladium fungi in the soil biocenosis of greenhouse cucumbers. JCEA 2023, 24, 447–454. [Google Scholar] [CrossRef]
- Zielonka, D.; Sas-Paszt, L.; Derkowska, E.; Lisek, A.; Russel, S. Occurrence of arbuscular mycorrhizal fungi in hemp (Cannabis sativa) plants and soil fertilized with sewage sludge and phosphogypsum. J. Nat. Fibers 2021, 18, 250–260. [Google Scholar] [CrossRef]
- Babaei, M.; Ajdanian, L.; Lajayer, B.A. Morphological and phytochemical changes of Cannabis sativa L. affected by light spectra. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 119–133. [Google Scholar]
- Li, M.; Roman, M.; Yuan, J.; Rehman, M.; Liu, L. Varying light intensity can alter metabolic profile and cannabispiradienone content of industrial hemp. Ind. Crops Prod. 2023, 202, 117031. [Google Scholar] [CrossRef]
- Cheng, X.; Wang, R.; Liu, X.; Zhou, L.; Dong, M.; Rehman, M.; Fahad, S.; Liu, L.; Deng, G. Effects of Light Spectra on Morphology, Gaseous Exchange, and Antioxidant Capacity of Industrial Hemp. Front. Plant Sci. 2022, 13, 937436. [Google Scholar] [CrossRef] [PubMed]
- Vitale, L.; Vitale, E.; Guercia, G.; Turano, M.; Arena, C. Effects of different light quality and biofertilizers on structural and physiological traits of spinach plants. Photosynthetica 2020, 58, 932–943. [Google Scholar] [CrossRef]
- Hayes, M.; Ferruzzi, M.G. Update on the bioavailability and chemopreventative mechanisms of dietary chlorophyll derivatives. Nutr. Res. 2020, 81, 19–37. [Google Scholar] [CrossRef]
- Schoefs, B. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Mishra, V.K.; Bacheti, R.K.; Husen, A. Medicinal uses of chlorophyll: A critical overview. In Chlorophyll: Structure, Function and Medicinal Uses; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 177–196. [Google Scholar]
- Ferruzzi, M.G.; Blakeslee, J. Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr. Res. 2007, 27, 1–12. [Google Scholar] [CrossRef]
- Derrien, M.; Aghabararnejad, M.; Gosselin, A.; Desjardins, Y.; Angers, P.; Boumghar, Y. Optimization of supercritical carbon dioxide extraction of lutein and chlorophyll from spinach by-products using response surface methodology. LWT-Food Sci. Technol. 2018, 93, 79–87. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Markulj Kulundžić, A.; Viljevac Vuletić, M.; Matoša Kočar, M.; Antunović Dunić, J.; Varga, I.; Zdunić, Z.; Sudarić, A.; Cesar, V.; Lepeduš, H. Effect of Elevated Temperature and Excess Light on Photosynthetic Efficiency, Pigments, and Proteins in the Field-Grown Sunflower during Afternoon. Horticulturae 2022, 8, 392. [Google Scholar] [CrossRef]
- Markulj Kulundžić, A.; Josipović, A.; Matoša Kočar, M.; Viljevac Vuletić, M.; Antunović Dunić, J.; Varga, I.; Cesar, V.; Sudarić, A.; Lepeduš, H. Physiological Insights on Soybean Response to Drought. Agric. Water Manag. 2022, 268, 107620. [Google Scholar] [CrossRef]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in human health. IJCEA 2014, 5, 393–396. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of distribution, extraction methods, and health benefits of bound phenolics in food plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef]
- Magagnini, G.; Grassi, G.; Kotiranta, S. The effect of light spectrum on the morphology and cannabinoid content of Cannabis sativa L. Med. Cannabis Cannabinoids 2018, 1, 19–27. [Google Scholar] [CrossRef]
- Klir, Ž.; Novoselec, J.; Antunović, Z. An overview on the use of hemp (Cannabis sativa L.) in animal nutrition. Poljoprivreda 2019, 25, 52–61. [Google Scholar] [CrossRef]
- Muniz, C.R.; Freire, F.C.O.; Viana, F.M.P.; Cardoso, J.E.; Sousa, C.A.F.; Guedes, M.I.F.; van der Schoor, R.; Jalink, H. Monitoring cashew seedlings during interactions with the fungus Lasiodiplodia theobromae using chlorophyll fluorescence imaging. Photosynthetica 2014, 52, 529–537. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Magic Blue Light: A Versatile Mediator of Plant Elongation. Plants 2024, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Reichel, P.; Munz, S.; Hartung, J.; Kotiranta, S.; Graeff-Hönninger, S. Impacts of Different Light Spectra on CBD, CBDA and Terpene Concentrations in Relation to the Flower Positions of Different Cannabis sativa L. Strains. Plants 2022, 11, 2695. [Google Scholar] [CrossRef] [PubMed]
- Tkalec Kojić, M.; Kujundžić, S.; Parađiković, N.; Bošnjak, D.; Vinković, T.; Ravnjak, B.; Stošić, M.; Zeljković, S.; Kujundžić, T. Establishment of indigenous garlic varieties in vitro under influence of growth regulator and light. JCEA 2023, 24, 491–497. [Google Scholar] [CrossRef]
- Glowacka, B. The effect of blue light on the height and habit of the tomato (Lycopersicon esculentum Mill.). Folia Hortic. 2004, 16, 3–10. [Google Scholar]
- Javanmardi, J.; Emami, S. Response of tomato and pepper transplants to light spectra provided by light emitting diodes. Int. J. Veg. Sci. 2013, 19, 138–149. [Google Scholar] [CrossRef]
- Kakabouki, I.; Tataridas, A.; Mavroeidis, A.; Kousta, A.; Karydogianni, S.; Zisi, C.; Kouneli, V.; Konstantinou, A.; Folina, A.; Konstantas, A.; et al. Effect of Colonization of Trichoderma harzianum on Growth Development and CBD Content of Hemp (Cannabis sativa L.). Microorganisms 2021, 9, 518. [Google Scholar] [CrossRef]
- Balthazar, C.; Joly, D.L.; Filion, M. Exploiting Beneficial Pseudomonas spp. for Cannabis Production. Front. Microbiol. 2022, 12, 833172. [Google Scholar] [CrossRef]
- Seemakram, W.; Paluka, J.; Suebrasri, T.; Lapjit, C.; Kanokmedhakul, S.; Kuyper, T.W.; Ekprasert, J.; Boonlue, S. Enhancement of growth and Cannabinoids content of hemp (Cannabis sativa) using arbuscular mycorrhizal fungi. Front. Plant Sci. 2022, 13, 845794. [Google Scholar] [CrossRef]
- Freire Cruz, A. Effect of light-emitting diodes on arbuscular mycorrhizal fungi associated with bahiagrass (Paspalum notatum Flügge) and millet [Pennisetum glaucum (L.) R. Br]. Bioagro 2016, 28, 163–170. [Google Scholar]
- Nishio, J.N. Why are higher plants green? Evolution of the higher plant photosynthetic pigment complement. Plant Cell Environ. 2000, 23, 539–548. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. PCP 2009, 50, 684–697. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Snowden, M.C.; Cope, K.R.; Bugbee, B. Sensitivity of Seven Diverse Species to Blue and Green Light: Interactions with Photon Flux. Control. Environ. 2016, 11, e0163121. Available online: https://digitalcommons.usu.edu/cpl_env/10 (accessed on 12 February 2024). [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. EEB 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Akoyunoglou, G.; Anni, H. Blue light effect on chloroplast development in higher plants. In Blue Light Effects in Biological Systems; Springer: Berlin/Heidelberg, Germany, 1984; pp. 397–406. [Google Scholar]
- Cope, K.; Snowden, M.C.; Bugbee, B. Photobiological interactions of blue light and photosynthetic photon flux: Effects of monochromatic and broad-spectrum light sources. Photochem. Photobiol. 2014, 90, 574–584. [Google Scholar] [CrossRef] [PubMed]
- van Grondelle, R.; Boeker, E. Limits on Natural Photosynthesis. J. Phys. Chem. B. 2017, 121, 7229–7234. [Google Scholar] [CrossRef] [PubMed]
- Salehi, A.; Tasdighi, H.; Gholamhoseini, M. Evaluation of proline, chlorophyll, soluble sugar content and uptake of nutrients in the German chamomile (Matricaria chamomilla L.) under drought stress and organic fertilizer treatments. Asian Pac. J. Trop Biomed. 2016, 6, 886–891. [Google Scholar] [CrossRef]
- Kook, H.S.; Park, S.H.; Jang, Y.J.; Lee, G.W.; Kim, J.S.; Kim, H.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Blue LED (light-emitting diodes)-mediated growth promotion and controlof Botrytis disease in lettuce. Acta Agric. Scand. Sect. B Soil Plant Sci. 2013, 63, 271–277. [Google Scholar]
- Vaštakaitė, V.; Viršilė, A.; Brazaitytė, A.; Samuolienė, G.; Jankauskienė, J.; Sirtautas, R.; Novičkovas, A.; Dabašinskas, L.; Sakalauskienė, S.; Miliauskienė, J.; et al. The effect of blue light dosage on growth and antioxidant properties of microgreens. Sodinink. Daržinink 2015, 34, 25–35. [Google Scholar]
- He, R.; Wei, J.; Zhang, J.; Tan, X.; Li, Y.; Gao, M.; Liu, H. Supplemental Blue Light Frequencies Improve Ripening and Nutritional Qualities of Tomato Fruits. Front. Plant Sci. 2022, 13, 888976. [Google Scholar] [CrossRef]
- Lee, S.; Park, C.H.; Kim, J.K.; Ahn, K.; Kwon, H.; Kim, J.K.; Park, S.U.; Yeo, H.J. LED Lights Influenced Phytochemical Contents and Biological Activities in Kale (Brassica oleracea L. var. acephala) Microgreens. Antioxidants 2023, 12, 1686. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz-Krzemień, A.; Sip, S.; Szulc, P.; Cielecka-Piontek, J. Determining antioxidant activity of Cannabis leaves extracts from different varieties—Unveiling ‘nature’s treasure trove. Antioxidants 2023, 12, 1390. [Google Scholar] [CrossRef]
- Genzel, F.; Dicke, M.D.; Junker-Frohn, L.V.; Neuwohner, A.; Thiele, B.; Putz, A.; Usadel, B.; Wormit, A.; Wiese-Klinkenberg, A. Impact of moderate cold and salt stress on the accumulation of antioxidant flavonoids in the leaves of two Capsicum cultivars. J. Agric. Food Chem. 2021, 69, 6431–6443. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of exogenous Ca2+ on phenolic accumulation and physiological changes in germinated wheat (Triticum aestivum L.) under UV-B radiation. Food Chem. 2019, 288, 368–376. [Google Scholar] [CrossRef]
- Toscano, S.; Cavallaro, V.; Ferrante, A.; Romano, D.; Patané, C. Effects of Different Light Spectra on Final Biomass Production and Nutritional Quality of Two Microgreens. Plants 2021, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, P.; Lozowicka, B. Biochemical compounds and stress markers in lettuce upon exposure to pathogenic Botrytis cinerea and fungicides inhibiting oxidative phosphorylation. Planta 2022, 255, 61. [Google Scholar] [CrossRef] [PubMed]
- Iwaniuk, P.; Łuniewski, S.; Kaczy´nski, P.; Łozowicka, B. The Influence of Humic Acids and Nitrophenols on Metabolic Compounds and Pesticide Behavior in Wheat under Biotic Stress. Agronomy 2023, 13, 1378. [Google Scholar] [CrossRef]
- Wallis, C.M.; Galarneau, E.R.A. Phenolic compound induction in plant-microbe and plant-insect interactions: A meta-analysis. Front. Plant Sci. 2020, 11, 580753. [Google Scholar] [CrossRef]
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–461. [Google Scholar] [CrossRef]
- Wettstein, D. Chlorophyll–letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–487. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Quality and nutritional value of strawberry fruit under long term salt stress. Food Chem. 2008, 107, 1413–1420. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagent. AJEV 1965, 16, 144–158. [Google Scholar] [CrossRef]
| Light (A) | Biopreparation (B) | |||||
|---|---|---|---|---|---|---|
| Control | Seed | Seed and Substrate | Average | |||
| (1) | (2) | (3) | (4) | (5) | ||
| Root length (cm) | ||||||
| Blue light | 5.2 | 4.5 | 4.5 | 4.5 | 4.2 | 4.6 |
| White light | 6.5 | 6.0 | 4.9 | 6.5 | 7.3 | 6.2 |
| Average | 5.9 | 5.2 | 4.7 | 5.5 | 5.7 | 5.4 |
| LSD (A)0.05 = 0.32; LSD (B)0.05 = 0.53; LSD (A × B)0.05 = 0.69 | ||||||
| Stem length (cm) | ||||||
| Blue light | 15.0 | 14.8 | 13.6 | 9.9 | 12.3 | 13.0 |
| White light | 17.5 | 17.8 | 19.6 | 17.8 | 18.9 | 18.3 |
| Average | 16.3 | 16.3 | 16.4 | 13.8 | 15.6 | 15.7 |
| LSD (A)0.05 = 0.74; LSD (B)0.05 = 1.29; LSD (A × B)0.05 = 1.59 | ||||||
| Plant length (cm) | ||||||
| Blue light | 20.2 | 19.4 | 17.7 | 14.3 | 16.4 | 17.6 |
| White light | 24.0 | 23.8 | 24.6 | 24.0 | 26.1 | 24.6 |
| Average | 22.1 | 21.6 | 21.1 | 19.3 | 21.3 | 21.1 |
| LSD (A)0.05 = 0.83; LSD (B)0.05 = 1.51; LSD (A × B)0.05 = 1.80 | ||||||
| Light (A) | Biopreparation (B) | |||||
|---|---|---|---|---|---|---|
| Control | Seed | Seed and Substrate | Average | |||
| (1) | (2) | (3) | (4) | (5) | ||
| Fresh biomass root (g plant−1) | ||||||
| Blue light | 0.10 | 0.09 | 0.05 | 0.07 | 0.07 | 0.08 |
| White light | 0.07 | 0.11 | 0.04 | 0.04 | 0.11 | 0.08 |
| Average | 0.08 | 0.10 | 0.05 | 0.06 | 0.05 | 0.08 |
| LSD (A)0.05 = ns; LSD (B)0.05 = 0.03; LSD (A × B)0.05 = 0.04 | ||||||
| Fresh biomass stem (g plant−1) | ||||||
| Blue light | 0.44 | 0.41 | 0.32 | 0.25 | 0.29 | 0.34 |
| White light | 0.42 | 0.44 | 0.45 | 0.40 | 0.42 | 0.43 |
| Average | 0.43 | 0.42 | 0.38 | 0.42 | 0.38 | 0.38 |
| LSD (A)0.05 = 0.02; LSD (B)0.05 = 0.04; LSD (A × B)0.05 = 0.05 | ||||||
| Fresh biomass leaves (g plant−1) | ||||||
| Blue light | 0.24 | 0.16 | 0.21 | 0.13 | 0.13 | 0.18 |
| White light | 0.17 | 0.20 | 0.18 | 0.17 | 0.16 | 0.18 |
| Average | 0.21 | 0.19 | 0.20 | 0.15 | 0.14 | 0.18 |
| LSD (A)0.05 = ns; LSD (B)0.05 = 0.01; LSD (A × B)0.05 = 0.03 | ||||||
| Fresh biomass plant (g plant−1) | ||||||
| Blue light | 0.78 | 0.72 | 0.53 | 0.49 | 0.49 | 0.59 |
| White light | 0.67 | 0.73 | 0.69 | 0.60 | 0.70 | 0.68 |
| Average | 0.72 | 0.72 | 0.61 | 0.53 | 0.60 | 0.64 |
| LSD (A)0.05 = 0.04; LSD (B)0.05 = 0.04; LSD (A × B)0.05 = 0.09 | ||||||
| Biopreparation Treatment | Added Quantity |
|---|---|
| (1) Control | 0 |
| (2) Vesicular arbuscular mycorrhiza fungi (VAM) on biolith | seed: 10 g kg−1 |
| (3) Trichoderma spp. on biolith | |
| (4) VAM on biolith + VAM and Azotobacter chroococum in liquid media | seed: 10 g kg−1 substrate: 50 g kg−1 and 30 mL kg−1 |
| (5) Trichoderma spp. on biolith + Trichoderma spp. in liquid media |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, I.; Kristić, M.; Lisjak, M.; Tkalec Kojić, M.; Iljkić, D.; Jović, J.; Kristek, S.; Markulj Kulundžić, A.; Antunović, M. Antioxidative Response and Phenolic Content of Young Industrial Hemp Leaves at Different Light and Mycorrhiza. Plants 2024, 13, 840. https://doi.org/10.3390/plants13060840
Varga I, Kristić M, Lisjak M, Tkalec Kojić M, Iljkić D, Jović J, Kristek S, Markulj Kulundžić A, Antunović M. Antioxidative Response and Phenolic Content of Young Industrial Hemp Leaves at Different Light and Mycorrhiza. Plants. 2024; 13(6):840. https://doi.org/10.3390/plants13060840
Chicago/Turabian StyleVarga, Ivana, Marija Kristić, Miroslav Lisjak, Monika Tkalec Kojić, Dario Iljkić, Jurica Jović, Suzana Kristek, Antonela Markulj Kulundžić, and Manda Antunović. 2024. "Antioxidative Response and Phenolic Content of Young Industrial Hemp Leaves at Different Light and Mycorrhiza" Plants 13, no. 6: 840. https://doi.org/10.3390/plants13060840
APA StyleVarga, I., Kristić, M., Lisjak, M., Tkalec Kojić, M., Iljkić, D., Jović, J., Kristek, S., Markulj Kulundžić, A., & Antunović, M. (2024). Antioxidative Response and Phenolic Content of Young Industrial Hemp Leaves at Different Light and Mycorrhiza. Plants, 13(6), 840. https://doi.org/10.3390/plants13060840








