Comparative Evaluation of Transient Protein Expression Efficiency in Tissues across Soybean Varieties Using the Tsukuba System
Abstract
:1. Introduction
2. Results
2.1. Microscopic Analysis of Green Fluorescent Protein Expression
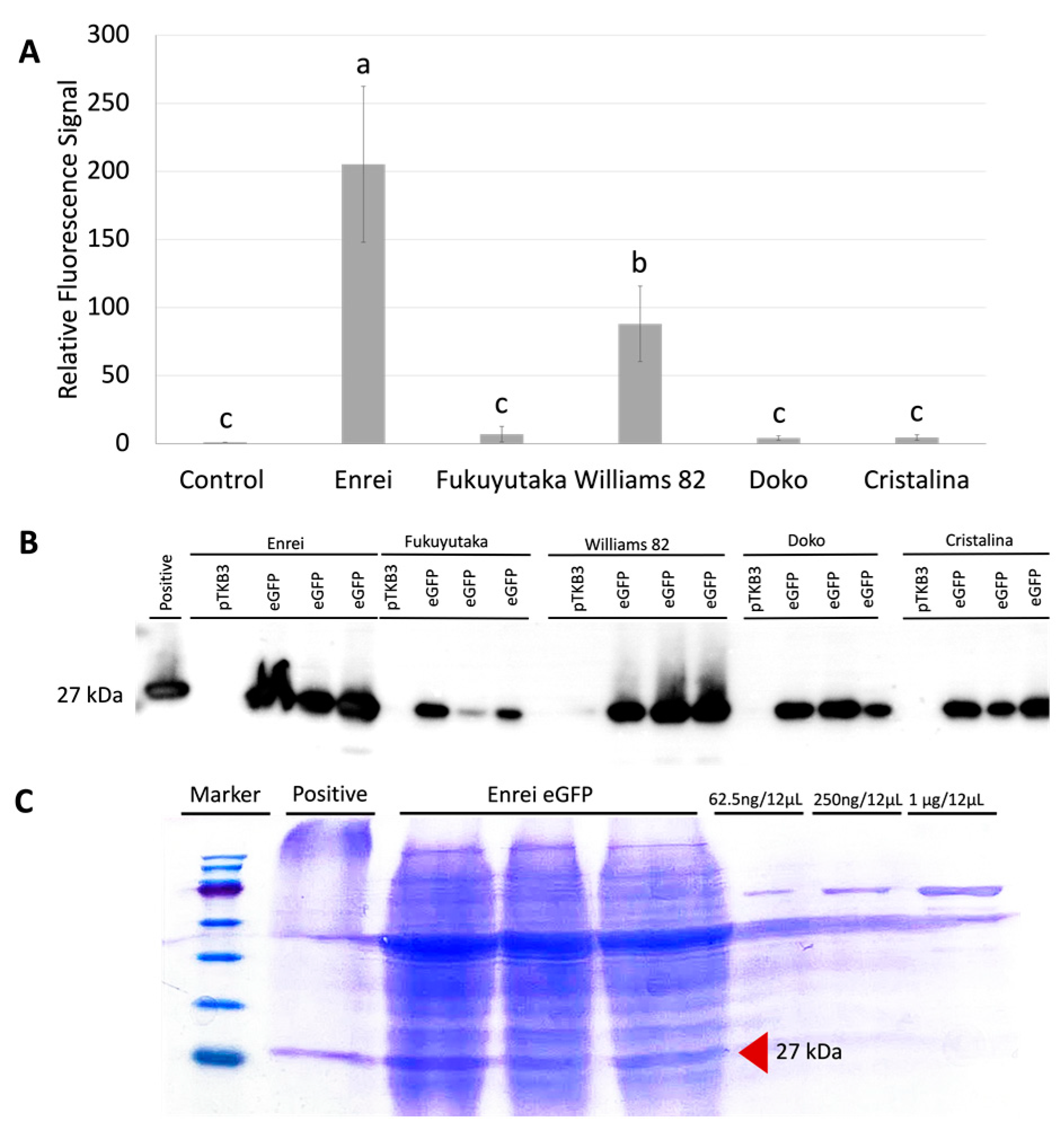
2.2. Comparative GFP Expression Levels across Soybean Varieties
2.3. Protein Quantification
3. Discussion
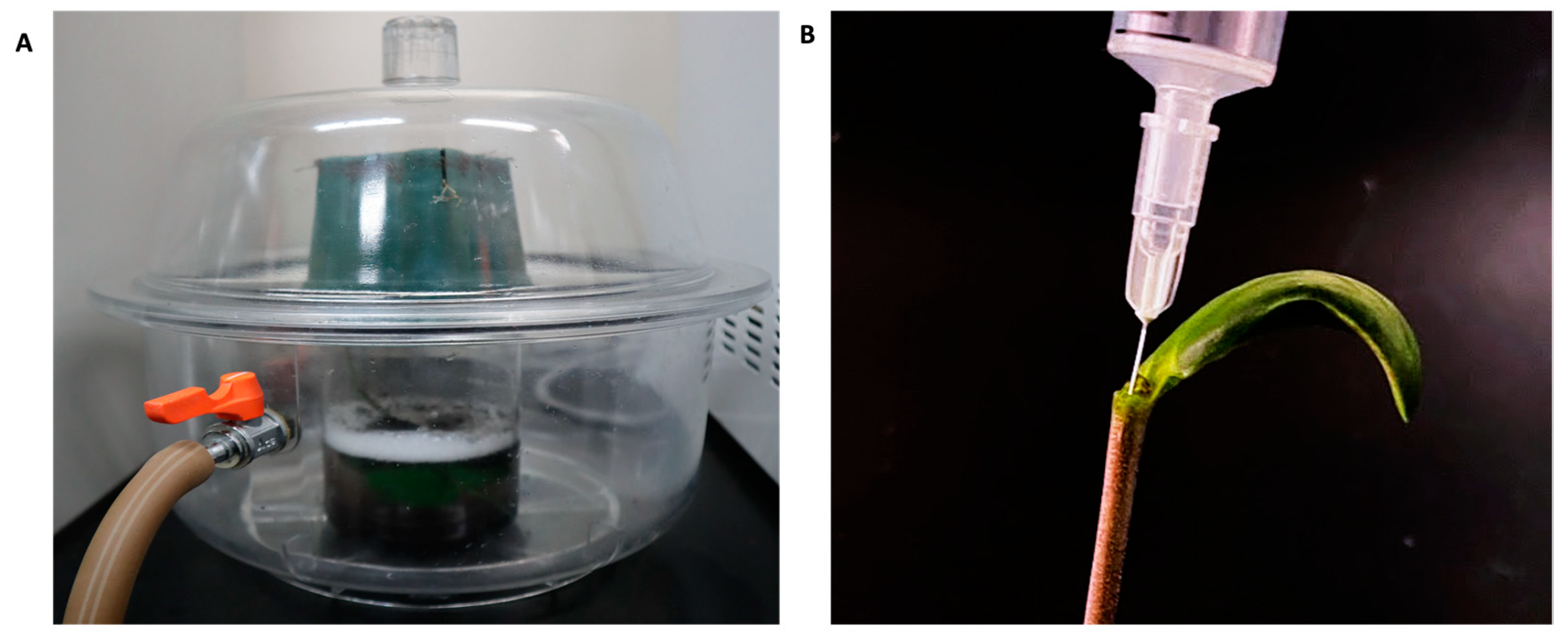
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Agrobacterium Preparation
4.3. Infiltration Procedure and GFP Expression Verification in Agro-Infiltrated Plants
4.4. Agrobacterium-Mediated Seed Transformation
4.5. Protein Expression Evaluation
4.6. Protein Expression Quantification
4.7. Image Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, G.; Chen, P.; Zhang, J.; Florez-Palacios, L.; Zeng, A.; Wang, X.; Bowen, R.A.; Miller, A.; Berry, H. Genetic Analysis of Sugar Composition and Its Relationship with Protein, Oil, and Fiber in Soybean. Crop Sci. 2018, 58, 2413–2421. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B.; Schlueter, J.; Ma, J.; Mitros, T.; Nelson, W.; Hyten, D.L.; Song, Q.; Thelen, J.J.; Cheng, J.; et al. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Cannon, S.B. The model legume genomes. Methods Mol. Biol. 2013, 1069, 1–14. [Google Scholar] [CrossRef]
- Hudson, L.C.; Garg, R.; Bost, K.L.; Piller, K.J. Soybean Seeds: A Practical Host for the Production of Functional Subunit Vaccines. BioMed Res. Int. 2014, 2014, 340804. [Google Scholar] [CrossRef] [PubMed]
- Vianna, G.R.; Cunha, N.B.; Murad, A.M.; Rech, E.L. Soybeans as bioreactors for biopharmaceuticals and industrial proteins. Genet. Mol. Res. 2011, 10, 1733–1752. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hoshikawa, K.; Ezura, K.; Okazawa, R.; Fujita, S.; Takaoka, M.; Mason, H.S.; Ezura, H.; Miura, K. Improvement of the transient expression system for production of recombinant proteins in plants. Sci. Rep. 2018, 8, 4755. [Google Scholar] [CrossRef]
- Nosaki, S.; Hoshikawa, K.; Ezura, H.; Miura, K. Transient protein expression systems in plants and their applications. Plant Biotechnol. 2021, 38, 297–304. [Google Scholar] [CrossRef]
- Yuan, S.; Kawasaki, S.; Abdellatif IM, Y.; Nishida, K.; Kondo, A.; Ariizumi, T.; Ezura, H.; Miura, K. Efficient base editing in tomato using a highly expressed transient system. Plant Cell Rep. 2021, 40, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Kapila, J.; De Rycke, R.; Van Montagu, M.; Angenon, G. An Agrobacterium-mediated transient gene expression system for intact leaves. Plant Sci. 1997, 122, 101–108. [Google Scholar] [CrossRef]
- Suzaki, T.; Tsuda, M.; Ezura, H.; Day, B.; Miura, K. Agroinfiltration-based efficient transient protein expression in leguminous plants. Plant Biotechnol. 2019, 36, 119–123. [Google Scholar] [CrossRef]
- Picard, K.; Lee, R.; Hellens, R.; Macknight, R. Transient gene expression in Medicago truncatula leaves via Agroinfiltration. Methods Mol. Biol. 2013, 1069, 215–226. [Google Scholar] [CrossRef]
- Juranić, M.; Nagahatenna, D.S.K.; Salinas-Gamboa, R.; Hand, M.L.; Sánchez-León, N.; Leong, W.H.; How, T.; Bazanova, N.; Spriggs, A.; Vielle-Calzada, J.-P.; et al. A detached leaf assay for testing transient gene expression and gene editing in cowpea (Vigna unguiculata [L.] Walp.). Plant Methods 2020, 16, 88. [Google Scholar] [CrossRef]
- Yin, X.; Luo, X.; Yang, F.; Wang, Y.; Song, L. An effective transient expression system for gene function identification in Lotus japonicus. Plant Cell Tissue Organ Cult. 2024, 156, 57. [Google Scholar] [CrossRef]
- Guy, E.; Boulain, H.; Aigu, Y.; Le Pennec, C.; Chawki, K.; Morlière, S.; Schädel, K.; Kunert, G.; Simon, J.-C.; Sugio, A. Optimization of Agroinfiltration in Pisum sativum Provides a New Tool for Studying the Salivary Protein Functions in the Pea Aphid Complex. Front. Plant Sci. 2016, 7, 1171. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, L.; Golkar, P.; Mirakhorli, N.; Jalali SA, H.; Mohammadinezhad, R. Transient expression of the full-length glycoprotein from infectious hematopoietic necrosis virus in bean (Phaseolus vulgaris) leaves via agroinfiltration. Biotechnol. Appl. Biochem. 2021, 68, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Yang, Q.; Yang, T.; Wu, Y.; Wang, G.; Yang, F.; Wang, R.; Lin, X.; Li, G. Development of Agrobacterium-mediated transient expression system in Caragana intermedia and characterization of CiDREB1C in stress response. BMC Plant Biol. 2019, 19, 249. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Li, C.; Li, H.; Lyu, X.; Zhao, T.; Liu, J.; Zuo, Z.; Liu, B. A transient expression system in soybean mesophyll protoplasts reveals the formation of cytoplasmic GmCRY1 photobody-like structures. Sci. China Life Sci. 2019, 62, 1070–1077. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; Bao, Y.; Wang, D.; Wang, W.; Chen, R.; Li, Y.; Xu, G.; Feng, X.; Liang, X.; et al. Functional Diversification Analysis of Soybean Malectin/Malectin-Like Domain-Containing Receptor-Like Kinases in Immunity by Transient Expression Assays. Front. Plant Sci. 2022, 13, 938876. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Takagi, K.; Ishimoto, M. Recent advances in soybean transformation and their application to molecular breeding and genomic analysis. Breed. Sci. 2012, 61, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Bao, A.; Zhang, C.; Huang, Y.; Chen, H.; Zhou, X.; Cao, D. Genome editing technology and application in soybean improvement. Oil Crop Sci. 2020, 5, 31–40. [Google Scholar] [CrossRef]
- Shamloul, M.; Trusa, J.; Mett, V.; Yusibov, V. Optimization and Utilization of Agrobacterium-mediated Transient Protein Production in Nicotiana. J. Vis. Exp. 2014, 86, e51204. [Google Scholar] [CrossRef]
- Shimomura, M.; Kanamori, H.; Komatsu, S.; Namiki, N.; Mukai, Y.; Kurita, K.; Kamatsuki, K.; Ikawa, H.; Yano, R.; Ishimoto, M.; et al. The Glycine max cv. Enrei Genome for Improvement of Japanese Soybean Cultivars. Int. J. Genom. 2015, 2015, 358127. [Google Scholar] [CrossRef]
- Sugano, S.; Hirose, A.; Kanazashi, Y.; Adachi, K.; Hibara, M.; Itoh, T.; Mikami, M.; Endo, M.; Hirose, S.; Maruyama, N.; et al. Simultaneous induction of mutant alleles of two allergenic genes in soybean by using site-directed mutagenesis. BMC Plant Biol. 2020, 20, 513. [Google Scholar] [CrossRef]
- Yamagishi, N.; Yamagishi, N.; Yoshikawa, N. Virus-induced gene silencing in soybean seeds and the emergence stage of soybean plants with Apple latent spherical virus vectors. Plant Mol. Biol. 2009, 71, 15–24. [Google Scholar] [CrossRef] [PubMed]
- King, J.L.; Finer, J.J.; McHale, L.K. Development and optimization of agroinfiltration for soybean. Plant Cell Rep. 2015, 34, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, C.G.; de Silva, A.C., Jr.; Pereira, M.R.R.; Gasparino, E.C.; Martins, D. Morphological modifications in soybean in response to soil water management. Plant Growth Regul. 2017, 83, 105–117. [Google Scholar] [CrossRef]
- Hussain, S.; Iqbal, N.; Pang, T.; Khan, M.N.; Liu, W.L.; Yang, W.Y. Weak stem under shade reveals the lignin reduction behavior. J. Integr. Agric. 2019, 18, 496–505. [Google Scholar] [CrossRef]
- Paradiso, R.; Arena, C.; De Micco, V.; Giordano, M.; Aronne, G.; De Pascale, S. Changes in leaf anatomical traits enhanced photosynthetic activity of soybean grown in hydroponics with plant growth-promoting microorganisms. Front. Plant Sci. 2017, 8, 674. [Google Scholar] [CrossRef]
- Leuzinger, K.; Dent, M.; Hurtado, J.; Stahnke, J.; Lai, H.; Zhou, X.; Chen, Q. Efficient Agroinfiltration of Plants for High-level Transient Expression of Recombinant Proteins. J. Vis. Exp. 2013, 77, 50521. [Google Scholar] [CrossRef]
- Fan, J.; Wang, Y.; Huang, S.; Xing, S.; Wei, Z. Production of active human FGF21 using tobacco mosaic virus-based transient expression system. Biotechnol. Lett. 2022, 39, 37–44. [Google Scholar] [CrossRef]
- Pervitasari, A.N.; Nugroho AB, D.; Jung, W.H.; Kim, D.H.; Kim, J. An efficient Agrobacterium tumefaciens-mediated transformation of apical meristem in radish (Raphanus sativus L.) using a needle perforation. Plant Cell Tissue Organ Cult. 2022, 148, 305–318. [Google Scholar] [CrossRef]
- Amal, T.C.; Karthika, P.; Dhandapani, G.; Selvakumar, S.; Vasanth, K. A simple and efficient Agrobacterium-mediated in planta transformation protocol for horse gram (Macrotyloma uniflorum Lam. Verdc.). J. Genet. Eng. Biotechnol. 2020, 18, 9. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Dong, J.; Ying, J.; Wang, Y.; Xu, L.; Ma, Y.; Wang, L.; Li, J.; Liu, L. Functional analysis of RsWUSb with Agrobacterium-mediated in planta transformation in radish (Raphanus sativus L.). Sci. Hortic. 2024, 323, 112504. [Google Scholar] [CrossRef]
- Benzle, K.A.; Finer, K.R.; Marty, D.; McHale, L.K.; Goodner, B.W.; Taylor, C.G.; Finer, J.J. Isolation and characterization of novel Agrobacterium strains for soybean and sunflower transformation. Plant Cell Tissue Organ Cult. 2015, 121, 71–81. [Google Scholar] [CrossRef]
- Paz, M.M.; Martinez, J.C.; Kalvig, A.B.; Fonger, T.M.; Wang, K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006, 25, 206–213. [Google Scholar] [CrossRef]
- Miura, K.; Shiba, H.; Ohta, M.; Kang, S.W.; Sato, A.; Yuasa, T.; Inoue, M.I.; Kamada, H.; Ezura, H. SlICE1 encoding a MYC-type transcription factor controls cold tolerance in tomato, Solanum lycopersicum. Plant Biotechnol. 2012, 29, 253–260. [Google Scholar] [CrossRef]
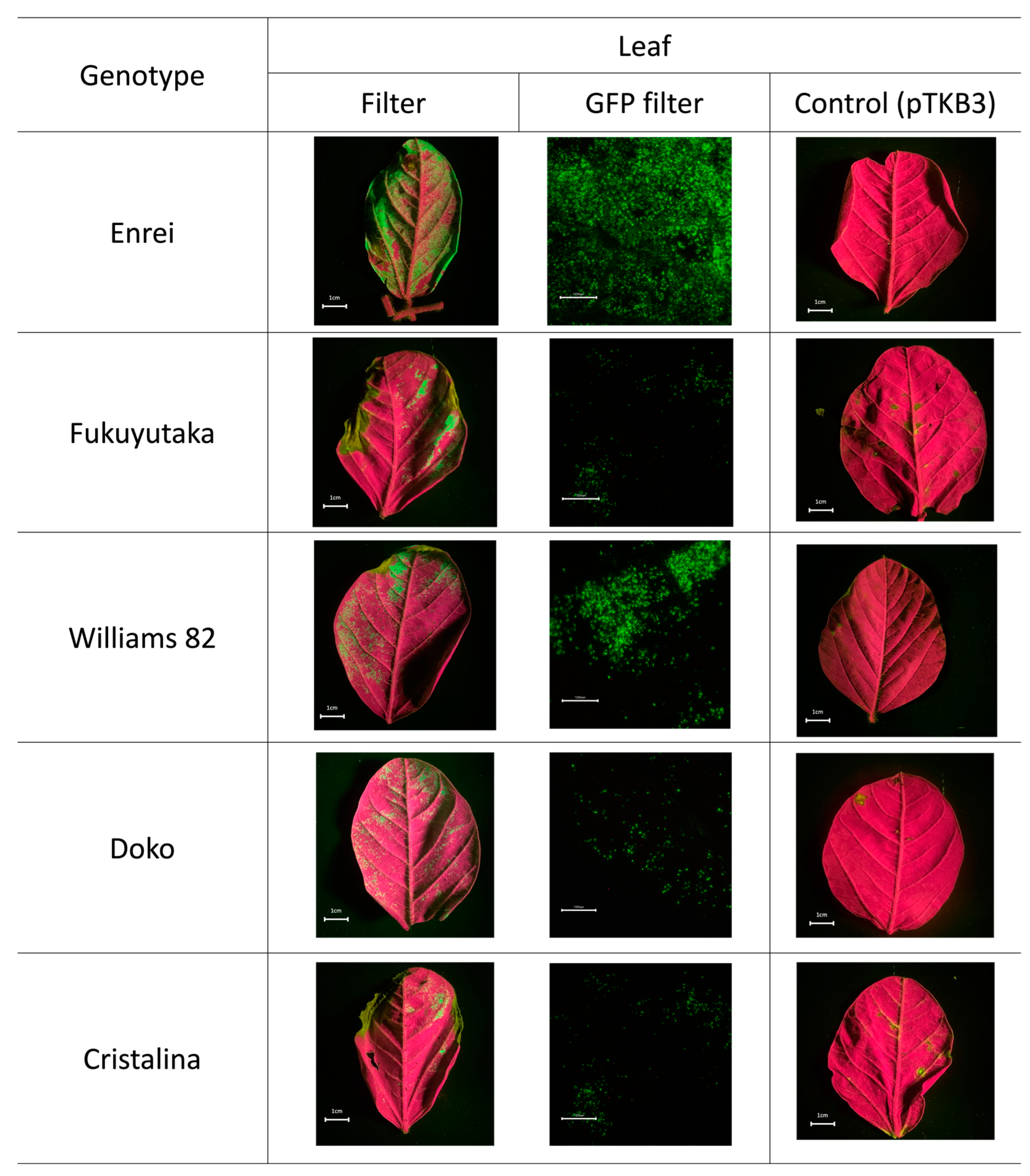
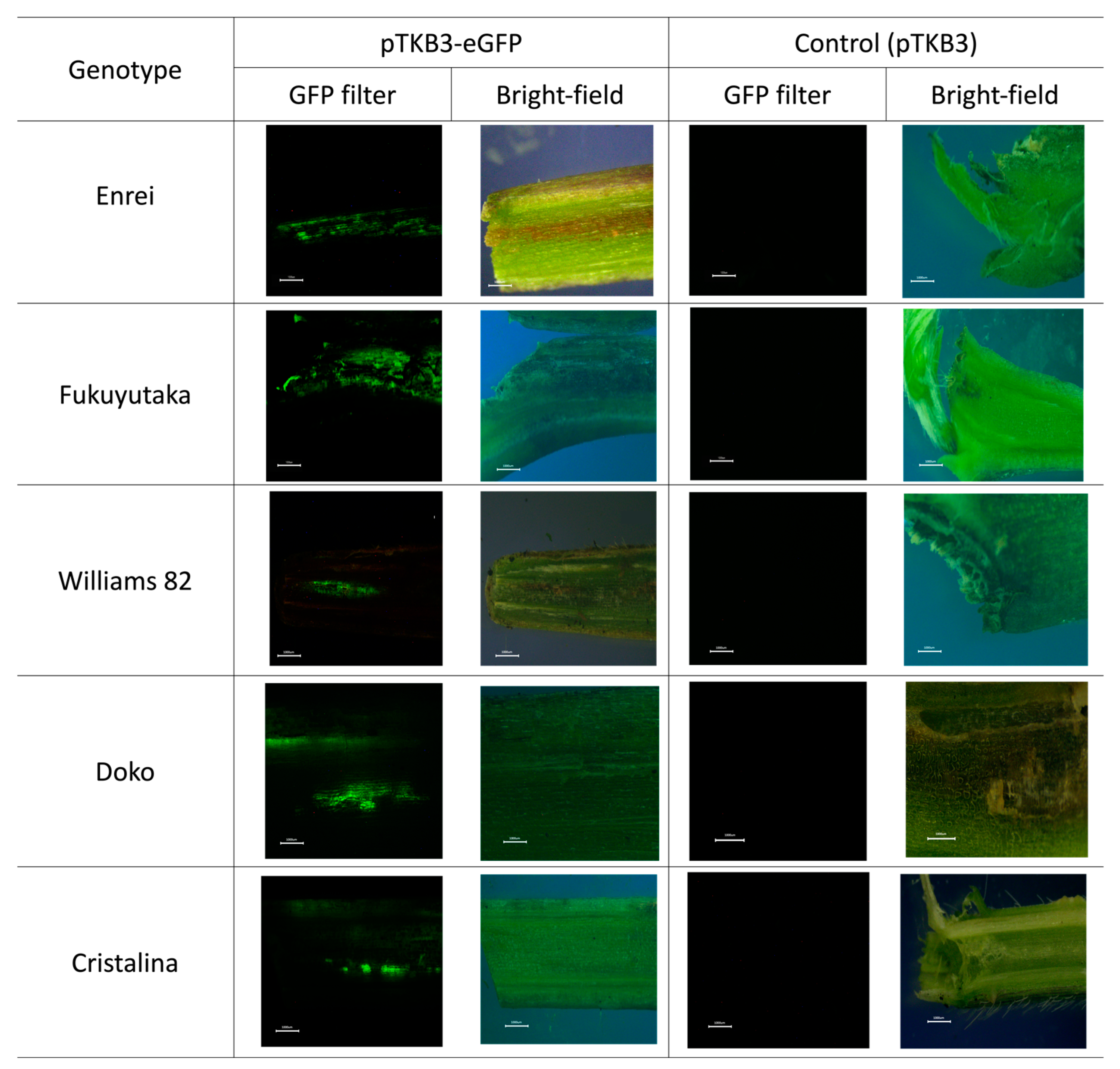
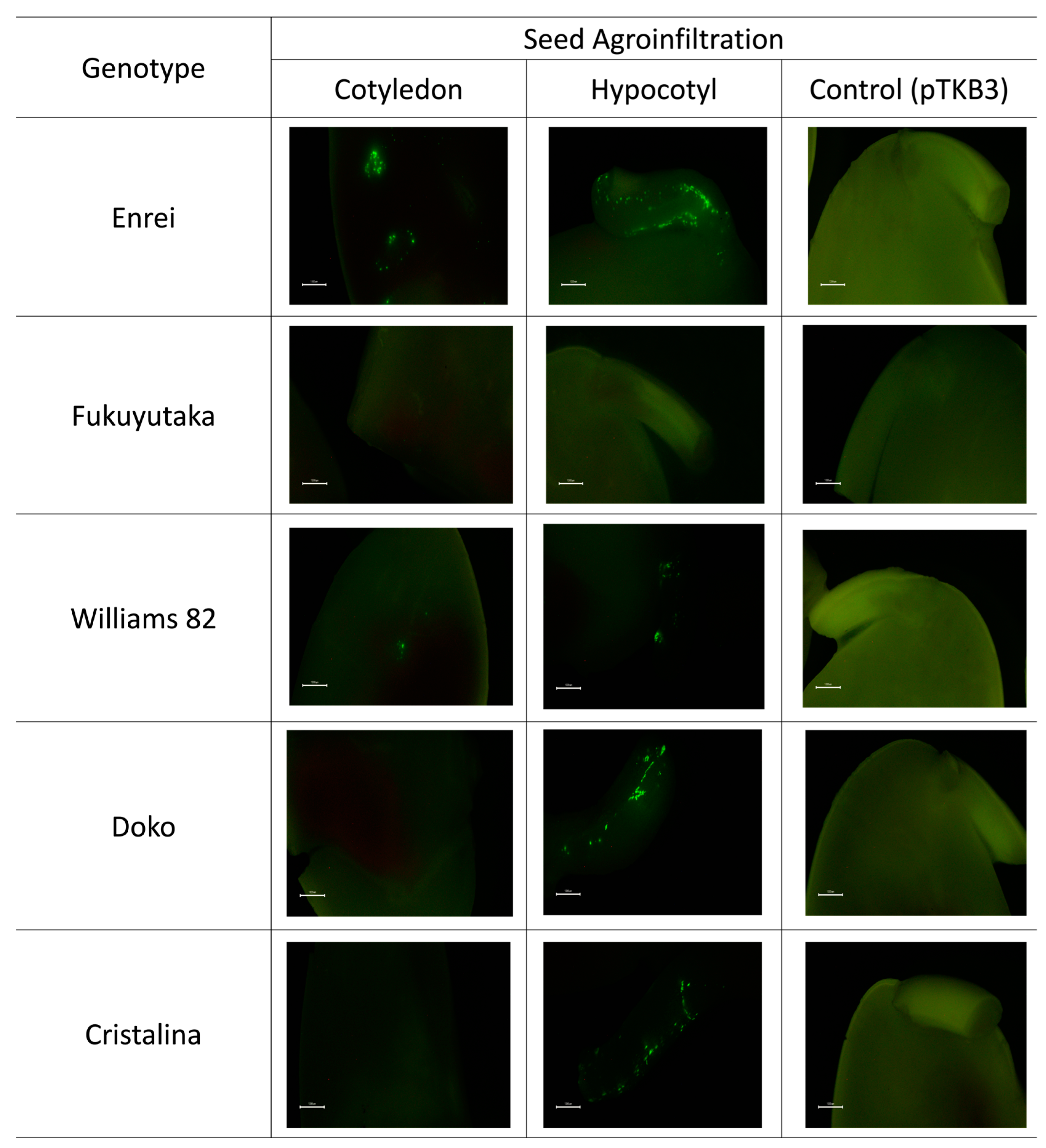
| Tissue | ||||
|---|---|---|---|---|
| Variety | Leaf | Seedling | Cotyledon | Hypocotyl |
| Glycine max cv. Enrei | ++ | ++ | + | + |
| Glycine max cv. Fukuyutaka | + | + | − | − |
| Glycine max cv. Williams 82 | + | + | + | + |
| Glycine max cv. Doko | + | + | − | + |
| Glycine max cv. Cristalina | + | + | − | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuhrmann-Aoyagi, M.B.; Igarashi, S.; Miura, K. Comparative Evaluation of Transient Protein Expression Efficiency in Tissues across Soybean Varieties Using the Tsukuba System. Plants 2024, 13, 858. https://doi.org/10.3390/plants13060858
Fuhrmann-Aoyagi MB, Igarashi S, Miura K. Comparative Evaluation of Transient Protein Expression Efficiency in Tissues across Soybean Varieties Using the Tsukuba System. Plants. 2024; 13(6):858. https://doi.org/10.3390/plants13060858
Chicago/Turabian StyleFuhrmann-Aoyagi, Martina Bianca, Saki Igarashi, and Kenji Miura. 2024. "Comparative Evaluation of Transient Protein Expression Efficiency in Tissues across Soybean Varieties Using the Tsukuba System" Plants 13, no. 6: 858. https://doi.org/10.3390/plants13060858





