Updating the Knowledge on the Secretory Machinery of Hops (Humulus lupulus L., Cannabaceae)
Abstract
:1. Introduction
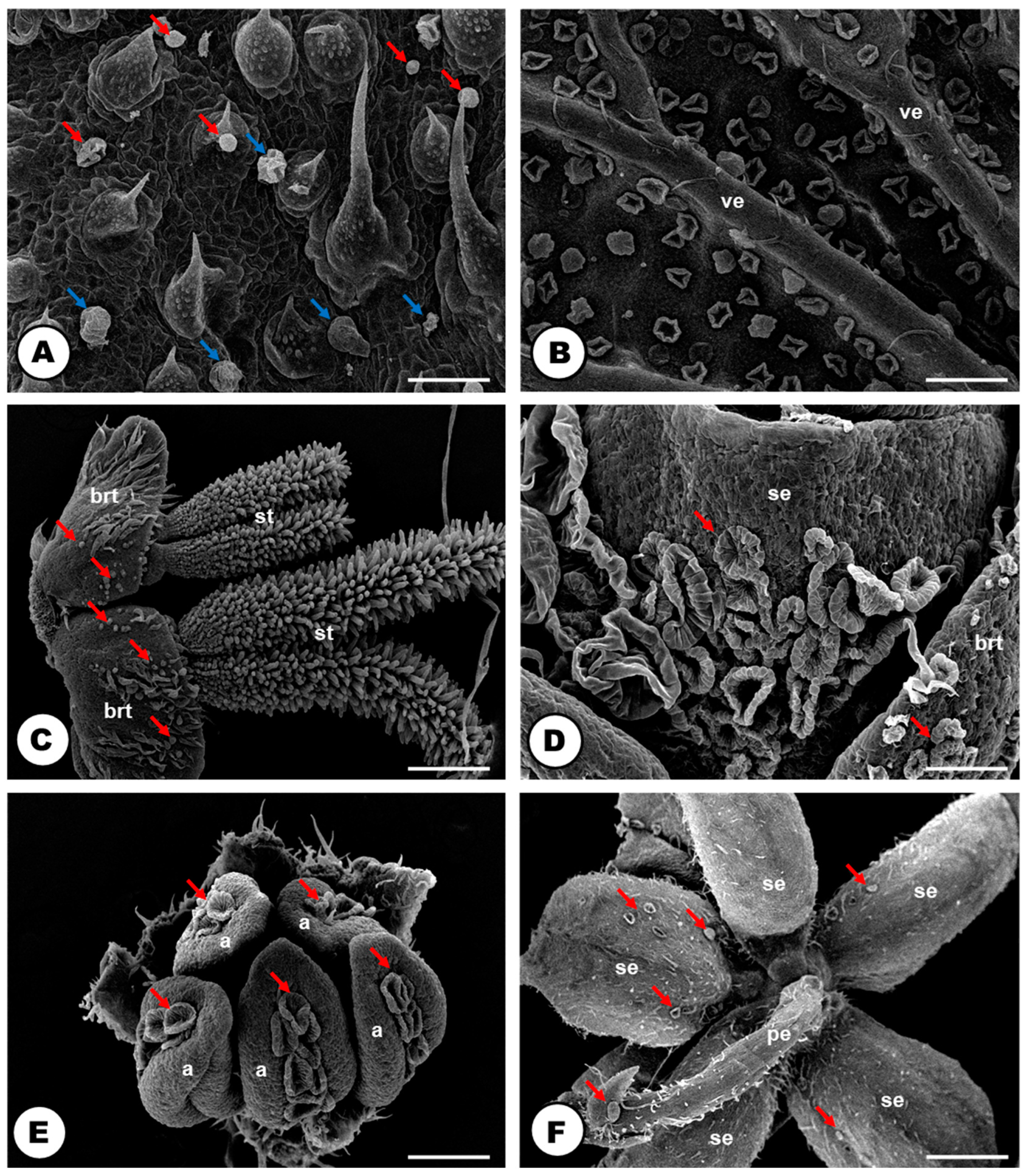
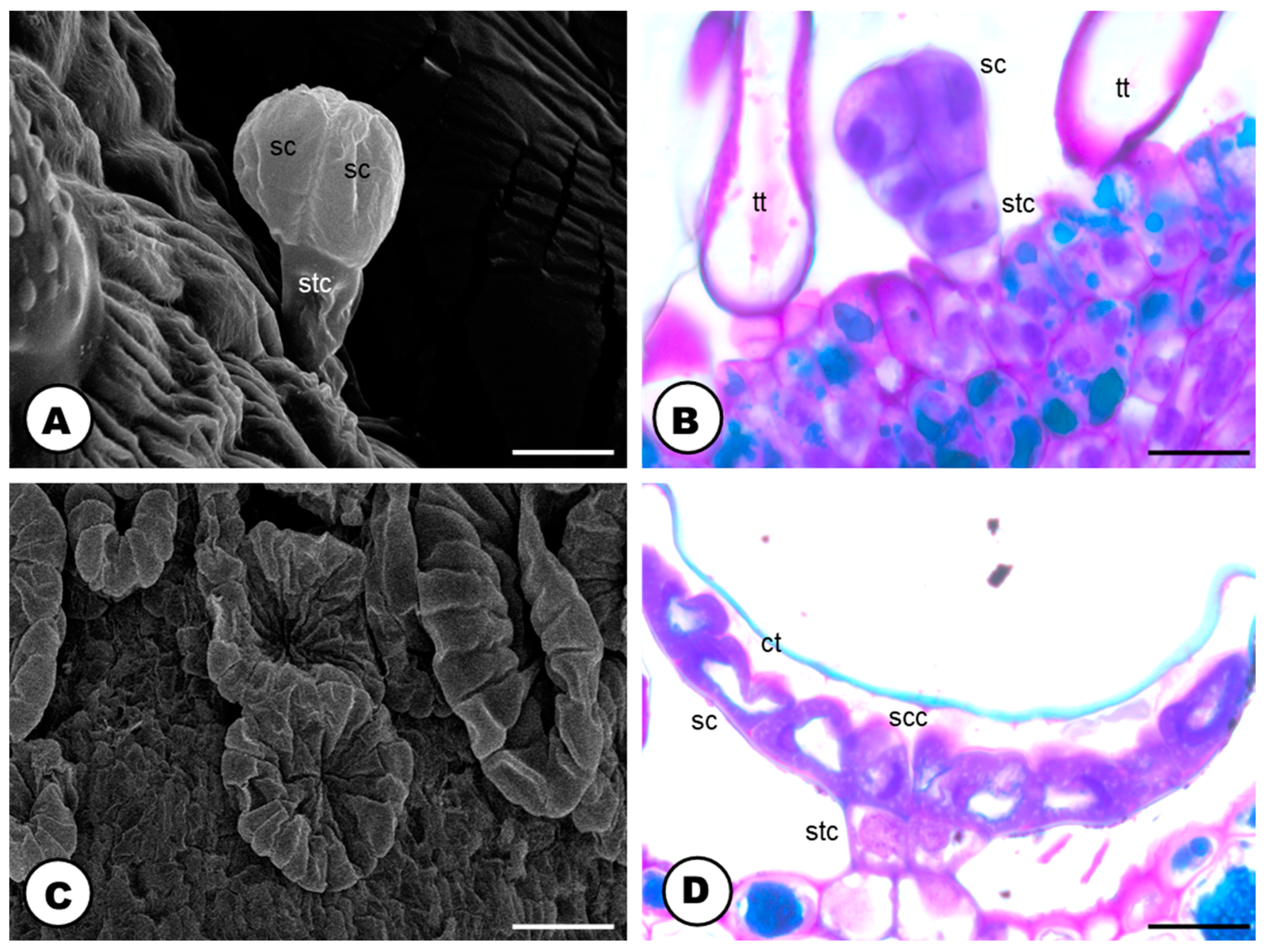
2. Results
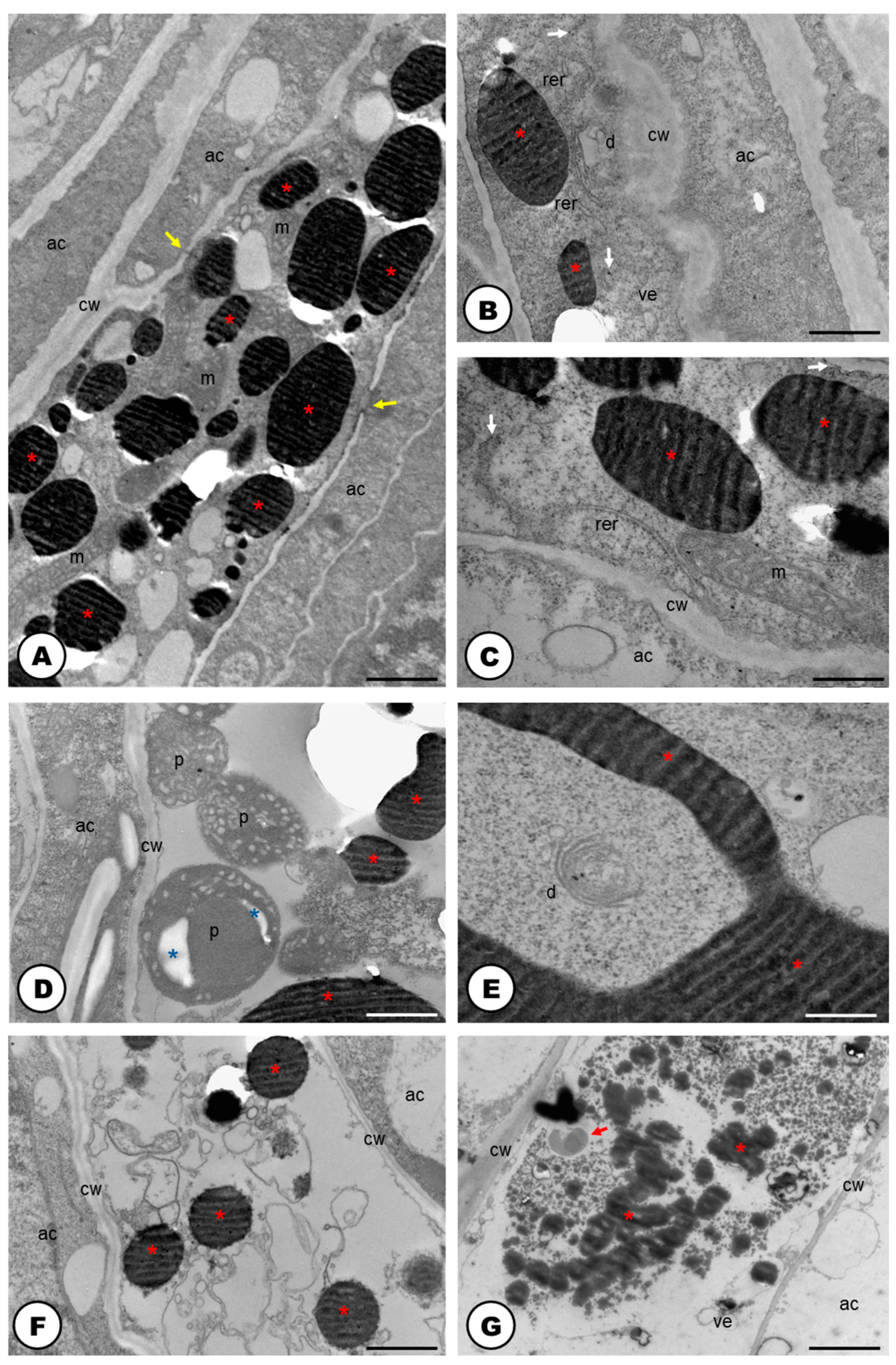
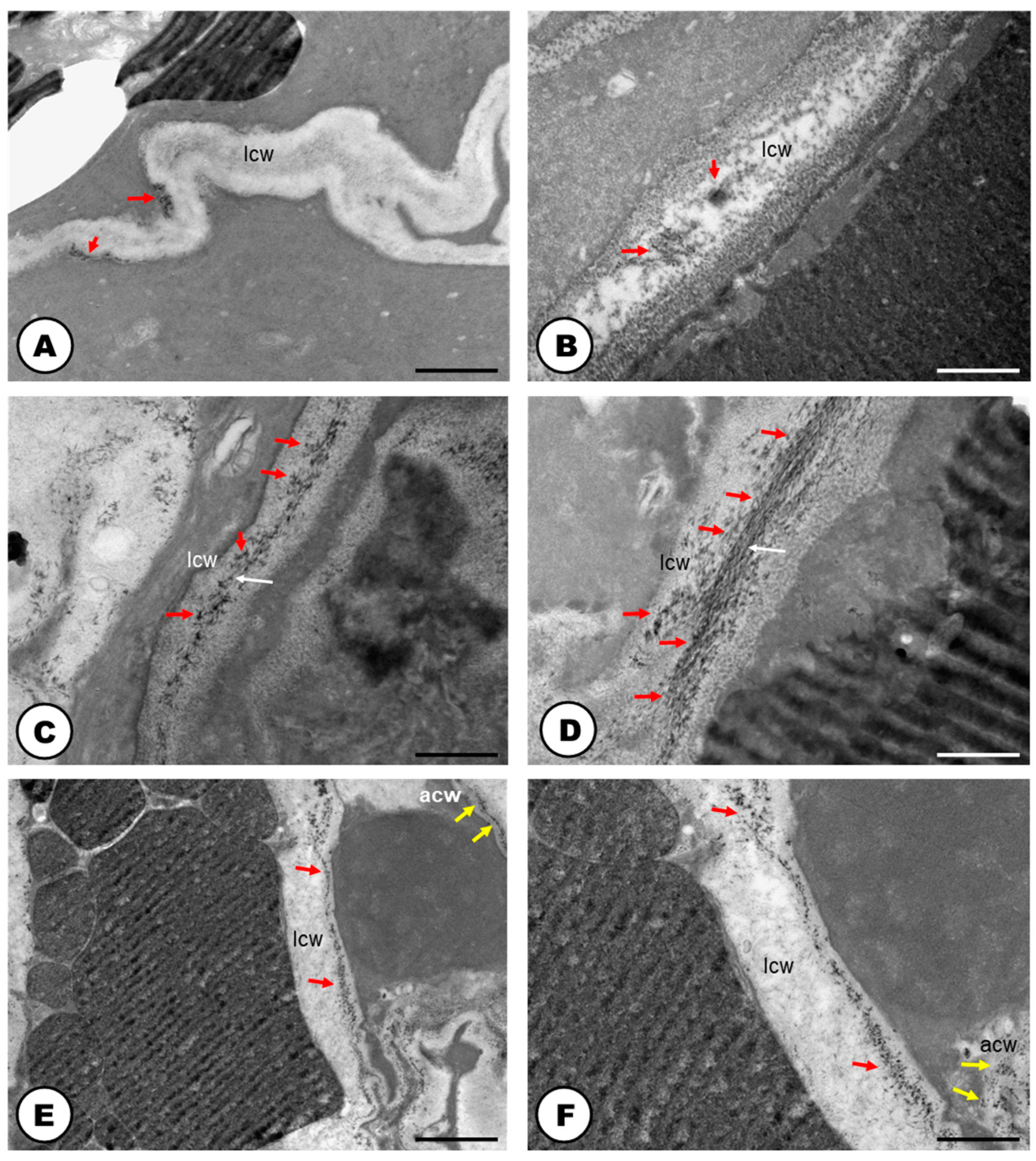
2.1. Laticifer System
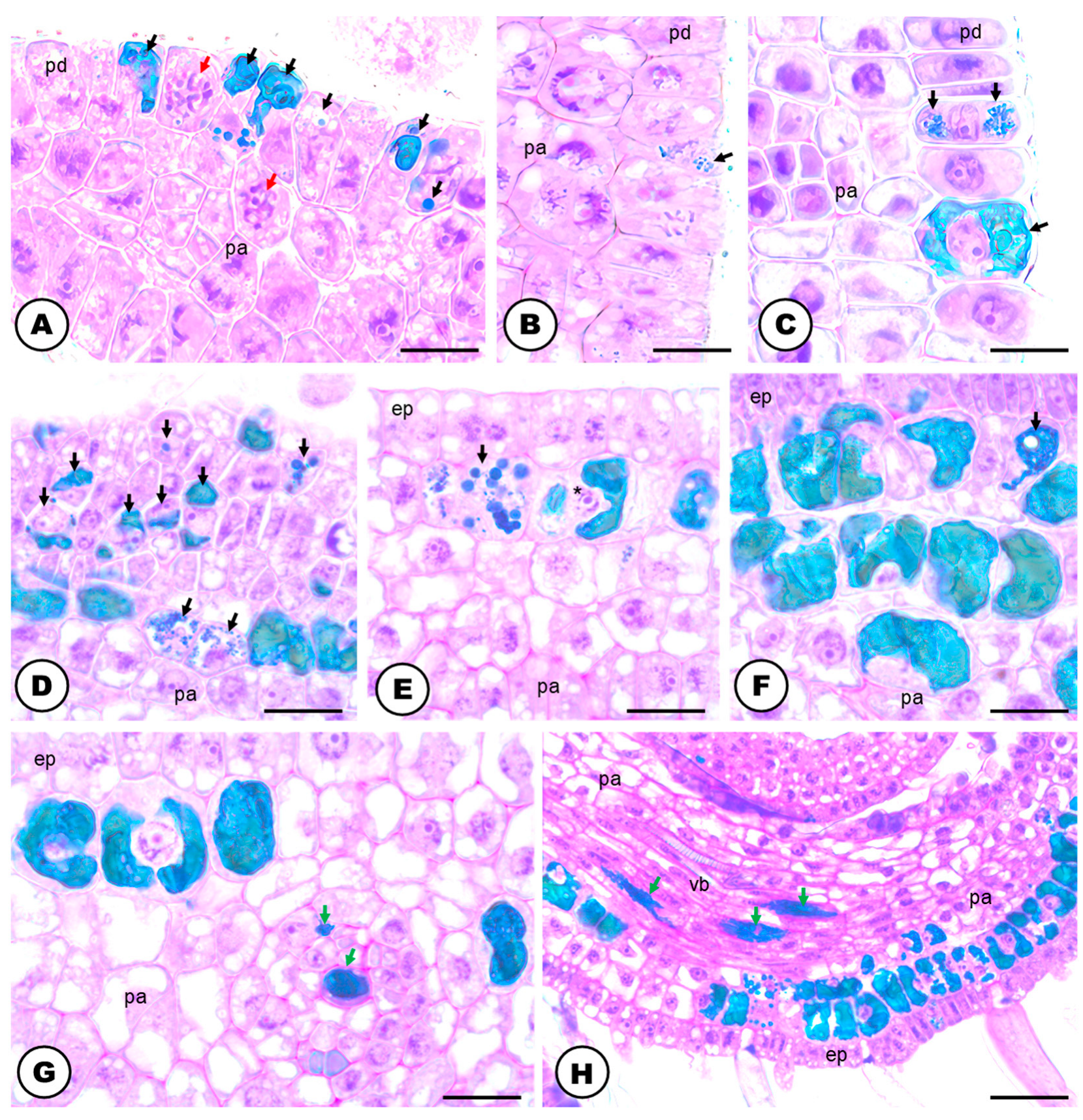
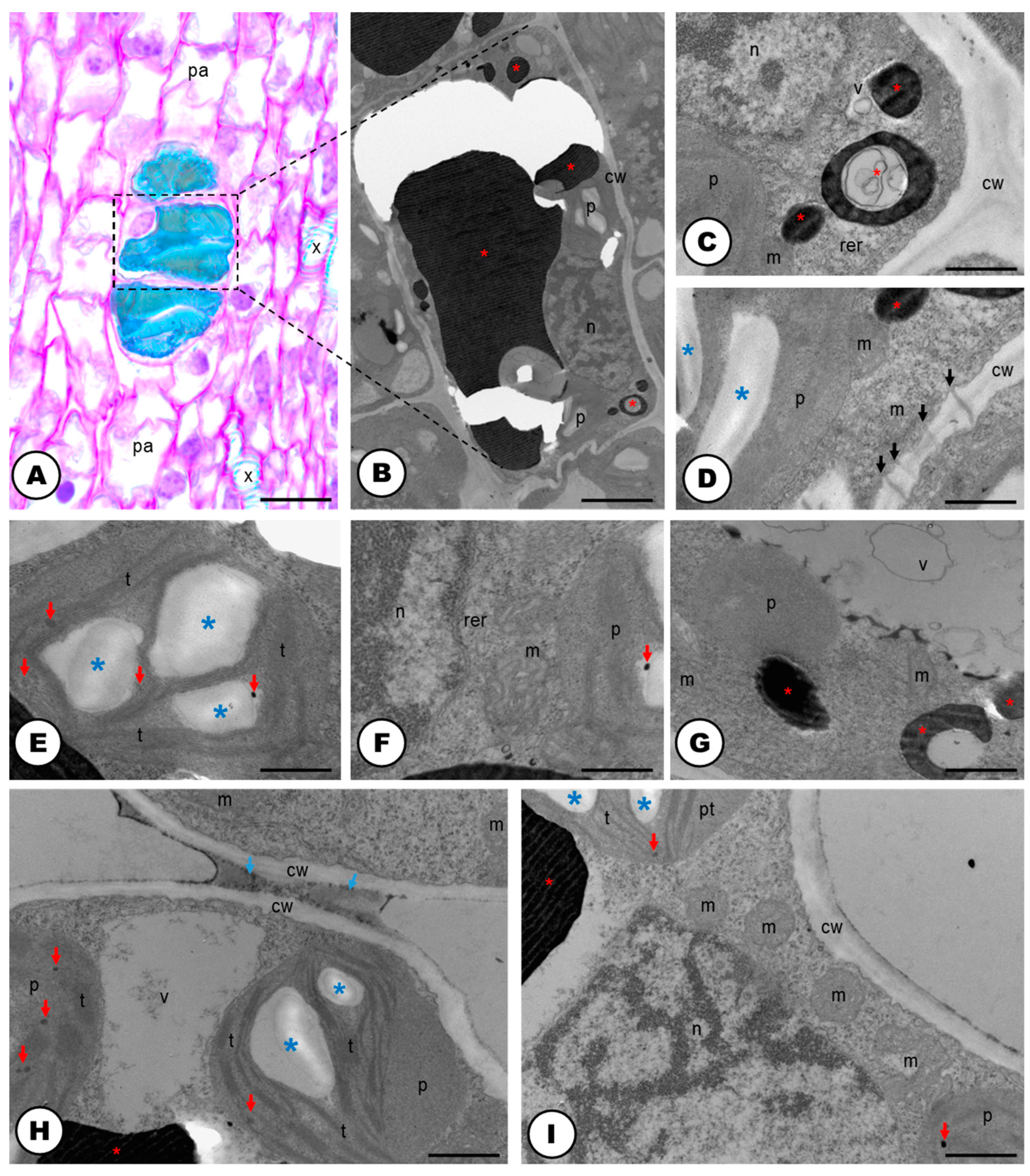
2.2. Secretory Idioblasts
2.3. Glandular Trichomes
3. Discussion
3.1. The Laticifer System in Humulus lupulus is Articulated, Unbranched and Crosses Almost All Organs of the Plant Body
3.2. The Latex of Humulus lupulus Seems to be Chemically Consistent to other Cannabaceae Species
3.3. Phenolic-Secreting Cells of Humulus lupulus
3.4. Humulus lupulus Show an Extensive Cover of Glandular Trichomes
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahn, A. Secretory Tissues in Plants; Academic Press: London, UK; New York, NY, USA, 1979; ISBN 9780122476501. [Google Scholar]
- Furr, M.; Mahlberg, P.G. Histochemical Analyses of Laticifers and Glandular Trichomes in Cannabis sativa. J. Nat. Prod. 1981, 44, 153–159. [Google Scholar] [CrossRef]
- Ashton, C.H. Pharmacology and Effects of Cannabis: A Brief Review. Br. J. Psychiatry 2001, 178, 101–106. [Google Scholar] [CrossRef]
- Happyana, N.; Agnolet, S.; Muntendam, R.; Van Dam, A.; Schneider, B.; Kayser, O. Analysis of Cannabinoids in Laser-Microdissected Trichomes of Medicinal Cannabis sativa Using LCMS and Cryogenic NMR. Phytochemistry 2013, 87, 51–59. [Google Scholar] [CrossRef]
- Hill, M.N.; Patel, S.; Campolongo, P.; Tasker, J.G.; Wotjak, C.T.; Bains, J.S. Functional Interactions between Stress and the Endocannabinoid System: From Synaptic Signaling to Behavioral Output. J. Neurosci. 2010, 30, 14980–14986. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, P.; Zavatti, M. Pharmacognostic and Pharmacological Profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Van Cleemput, M.; Cattoor, K.; De Bosscher, K.; Haegeman, G.; De Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-Derived Bitter Acids as Multipotent Bioactive Compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef]
- Sugiyama, R.; Oda, H.; Kurosaki, F. Two Distinct Phase of Glandular Trichome Development in Hop (Humulus lupulus L.). Plant Biotechnol. 2006, 23, 493–496. [Google Scholar] [CrossRef]
- Trindade, A.F.G.; Cassago, A.L.L.; Da Costa, F.B. Lúpulo: A planta peculiar que está conquistando o Brasil e impulsionando a indústria cervejeira. In A Flora: Divulgação Científica em Farmacognosia; Sociedade Brasileira de Farmacognosia: Rio de Janeiro, Brazil, 2023; Volume 3, pp. 5–8. ISBN 2764-7501. [Google Scholar]
- Colen, L.; Swinnen, J. Economic Growth, Globalisation and Beer Consumption. J. Agric. Econ. 2016, 67, 186–207. [Google Scholar] [CrossRef]
- Zugravu, C.-A.; Bohiltea, R.-E.; Salmen, T.; Pogurschi, E.; Otelea, M.R. Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products. Antioxidants 2022, 11, 241. [Google Scholar] [CrossRef]
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and Present Use, and Future Potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- Chiancone, B.; Guarrasi, V.; Leto, L.; Del Vecchio, L.; Calani, L.; Ganino, T.; Galaverni, M.; Cirlini, M. Vitro-Derived Hop (Humulus lupulus L.) Leaves and Roots as Source of Bioactive Compounds: Antioxidant Activity and Polyphenolic Profile. Plant Cell Tiss. Org. 2023, 153, 295–306. [Google Scholar] [CrossRef]
- Kyrou, I.; Christou, A.; Panagiotakos, D.; Stefanaki, C.; Skenderi, K.; Katsana, K.; Tsigos, C. Effects of a Hops (Humulus lupulus L.) Dry Extract Supplement on Self-Reported Depression, Anxiety and Stress Levels in Apparently Healthy Young Adults: A Randomized, Placebo-Controlled, Double-Blind, Crossover Pilot Study. Hormones 2017, 16, 171–180. [Google Scholar] [CrossRef]
- da Silva Nobrega, J.C.; de Araújo Batista, A.V.; da Silva, O.S.; de Belchior, V.C.S.; de Almeida Lacerda, W.; de Belchior, S.M.S. Plantas medicinais no tratamento de ansiedade e depressão: Uma revisão. Res. Soc. Dev. 2022, 11, e5511124024. [Google Scholar] [CrossRef]
- Min, B.; Ahn, Y.; Cho, H.; Kwak, W.; Jo, K.; Suh, H.J. Chemical Compositions and Sleep-promoting Activities of Hop (Humulus lupulus L.) Varieties. J. Food Sci. 2023, 88, 2217–2228. [Google Scholar] [CrossRef]
- Koriem, K.M.M. An Overview on Chemical Constituents, Medicinal Applications, Pharmacological Activity, Toxicology, Metabolism and Pharmacokinetics of Strobilus Lupuli. Biointerface Res. Appl. Chem. 2021, 12, 4613–4625. [Google Scholar] [CrossRef]
- Xiao-Lei, S.; Tian-Shuang, X.; Yi-Ping, J.; Na-Ni, W.; Ling-Chuan, X.; Ting, H.; Hai-Liang, X. Humulus lupulus L. Extract and Its Active Constituent Xanthohumol Attenuate Oxidative Stress and Nerve Injury Induced by Iron Overload via Activating AKT/GSK3β and Nrf2/NQO1 Pathways. J. Nat. Med. 2023, 77, 12–27. [Google Scholar] [CrossRef]
- Bouback, T.A.; Aljohani, A.M.; Albeshri, A.; Al-Talhi, H.; Moatasim, Y.; Gaballah, M.; Badierah, R.; Albiheyri, R.; Al-Sarraj, F.; Ali, M.A. Antiviral Activity of Humulus lupulus (HOP) Aqueous Extract against MERS-CoV and SARS-CoV-2: In-Vitro and in-Silico Study. Biotechnol. Biotechnol. Equip. 2023, 37, 167–179. [Google Scholar] [CrossRef]
- Wilson, D.G. Plant Remains from the Graveney Boat and the Early History of Humulus lupulus L. New Phytol. 1975, 75, 627–648. [Google Scholar] [CrossRef]
- Duke, J.A. Handbook of Energy Crops; Center for New Crops & Plants Products, Purdue University: Lafayette, IN, USA, 1983. [Google Scholar]
- Delyser, D.Y.; Kasper, W.J. Hopped Beer: The Case For Cultivation. Econ. Bot. 1994, 48, 166–170. [Google Scholar] [CrossRef]
- Edwardson, J.R. Hops—Their Botany, History, Production and Utilization. Econ. Bot. 1952, 6, 160–175. [Google Scholar] [CrossRef]
- Hagel, J.; Yeung, E.; Facchini, P. Got Milk? The Secret Life of Laticifers. Trends Plant Sci. 2008, 13, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Livingston, S.J.; Quilichini, T.D.; Booth, J.K.; Wong, D.C.J.; Rensing, K.H.; Laflamme-Yonkman, J.; Castellarin, S.D.; Bohlmann, J.; Page, J.E.; Samuels, A.L. Cannabis Glandular Trichomes Alter Morphology and Metabolite Content during Flower Maturation. Plant J. 2020, 101, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Holzner, G.; Lermer, J.K. Die trichromatischen Gebilde der Hopfenpflanze. Zeitschr. Ges. Brauwesen. 1893, 16, 103–106. [Google Scholar]
- Runner, D.K. The Structure and Development of the Storage Root of Humulus lupulus L. Master’s Thesis, Oregon State College, Corvallis, OR, USA, 1950. [Google Scholar]
- Miller, R.H. Morphology of Humulus lupulus. II. Secondary Growth In The Root And Seedling Vascularization. Am. J. Bot. 1959, 46, 269–277. [Google Scholar] [CrossRef]
- Мelnychuk, M.D.; Yakubenko, B.Y.; Likhanov, A.F. Structure, Development and Function of the Secretory System of Humulus lupulus L. (Cannabaceae). Ukr. Bot. J. 2013, 70, 342–350. [Google Scholar]
- Wang, G.; Tian, L.; Aziz, N.; Broun, P.; Dai, X.; He, J.; King, A.; Zhao, P.X.; Dixon, R.A. Terpene Biosynthesis in Glandular Trichomes of Hop. Plant Physiol. 2008, 148, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Wang, G.; Yang, D.S.; Tang, Y.; Broun, P.; Marks, M.D.; Sumner, L.W.; Dixon, R.A.; Zhao, P.X. TrichOME: A Comparative Omics Database for Plant Trichomes. Plant Physiol. 2009, 152, 44–54. [Google Scholar] [CrossRef]
- Srečec, S.; Zechner-Krpan, V.; Marag, S.; Špoljarić, I.; Kvaternjak, I.; Mršić, G. Morphogenesis, Volume and Number of Hop (Humulus lupulus L.) Glandular Trichomes, and Their Influence on Alpha-Acid Accumulation in Fresh Bracts of Hop Cones. Acta Bot. Croat. 2011, 70, 1–8. [Google Scholar] [CrossRef]
- Patzak, J.; Krofta, K.; Henychová, A.; Nesvadba, V. Number and Size of Lupulin Glands, Glandular Trichomes of Hop (Humulus lupulus L.), Play a Key Role in Contents of Bitter Acids and Polyphenols in Hop Cone. Int. J. Food Sci. Technol. 2015, 50, 1864–1872. [Google Scholar] [CrossRef]
- Champagne, A.; Boutry, M. A Comprehensive Proteome Map of Glandular Trichomes of Hop (Humulus lupulus L.) Female Cones: Identification of Biosynthetic Pathways of the Major Terpenoid-related Compounds and Possible Transport Proteins. Proteomics 2017, 17, 1600411. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Salomk, M.; Pais, S. Glandular Trichomes of Humulus lupulus var. Brewer’s Gold: Ontogeny and Histochemical Characterization of the Secretion. Nord. J. Bot. 1988, 8, 349–359. [Google Scholar] [CrossRef]
- Santagostini, L.; Caporali, E.; Giuliani, C.; Bottoni, M.; Ascrizzi, R.; Araneo, S.R.; Papini, A.; Flamini, G.; Fico, G. Humulus lupulus L. cv. Cascade Grown in Northern Italy: Morphological and Phytochemical Characterization. Plant Biosyst. 2020, 154, 316–325. [Google Scholar] [CrossRef]
- Campos, O.P.; Leme, F.M.; Fortuna, G.C.; Gomes, J.A.D.O.; Neves, C.S.; Arruda, R.D.C.D.O.; Bonfim, F.P.G. Morphological Characteristics, Trichomes, and Phytochemistry of Inflorescences of Humulus lupulus L: Comparison of Cropping Systems and Varieties. Aust. J. Crops Sci. 2023, 17, 263–274. [Google Scholar] [CrossRef]
- Oliveira, M.M.; Pais, M.S. Glandular Trichomes of Humulus lupulus var. Brewer’s Gold (Hops): Ultrastructural Aspects of Peltate Trichomes. J. Submicr. Cytol. Path. 1990, 22, 241–248. [Google Scholar]
- Kim, E.-S.; Mahlberg, P.G. Early Development of the Secretory Cavity of Peltate Glands in Humulus lupulus L. (Cannabaceae). Mol. Cells 2000, 10, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Tung-kwang, F. Observations on the Morphology and Anatomy in the Rhizome of Hop (Humulus lupulus L.). J. Integr. Plant. Biol. 1957, 6, 297–317. [Google Scholar]
- Sheldrake, A.R. Cellulase in Latex and Its Possible Significance in Cell Differentiation. Planta 1969, 89, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Nessler, C.L.; Mahlberg, P.G. Cytochemical Localization of Cellulase Activity in Articulated, Anastomosing Laticifers of Papaver somniferum L. (Papaveraceae). Am. J. Bot. 1981, 68, 730–732. [Google Scholar] [CrossRef]
- Pilatzke-Wunderlich, I.; Nessler, C.L. Expression and Activity of Cell-Wall-Degrading Enzymes in the Latex of Opium Poppy, Papaver somniferum L. Plant Mol. Biol. 2001, 45, 567–576. [Google Scholar] [CrossRef]
- Marinho, C.R.; Teixeira, S.P. Cellulases and Pectinases Act Together on the Development of Articulated Laticifers in Ficus montana and Maclura tinctoria (Moraceae). Protoplasma 2019, 256, 1093–1107. [Google Scholar] [CrossRef]
- Liang, S.; Wang, H.; Yang, M.; Wu, H. Sequential Actions of Pectinases and Cellulases during Secretory Cavity Formation in Citrus Fruits. Trees 2009, 23, 19–27. [Google Scholar] [CrossRef]
- Yu, G.-H.; Guo, G.-Q.; Nie, X.-W.; Zheng, G.C. Cytochemical Localization of Pectinase Activity in Pollen Mother Cells of Tobacco During Meiotic ProphaseⅠand Its Relation to the Formation of Secondary Plasmodesmata and Cytoplasmic Channels. J. Integr. Plant Biol. 2004, 46, 1443. [Google Scholar]
- Wang, X.-Y.; Guo, G.-Q.; Nie, X.-W.; Zheng, G.-C. Cytochemical Localization of Cellulase Activity in Pollen Mother Cells of David Lily during Meiotic Prophase I and Its Relation to Secondary Formation of Plasmodesmata. Protoplasma 1998, 204, 128–138. [Google Scholar] [CrossRef]
- Giordani, R. Dislocation du Plasmalemme et Liberation de Vesicules Parietales Lors de la Degradation des Parois Terminales Durant la Differenciation des Laticiferes Articules. Biol. Cell 1980, 38, 231–234. [Google Scholar]
- Demarco, D.; Castro, M.D.M. Laticíferos Articulados Anastomosados em Espécies de Asclepiadeae (Asclepiadoideae, Apocynaceae) e Suas Implicações Ecológicas. Rev. Bras. Bot. 2008, 31, 701–713. [Google Scholar] [CrossRef]
- Teixeira, S.P.; Marinho, C.R.; Leme, F.M. Chapter Two—Structural Diversity and Distribution of Laticifers. In Advances in Botanical Research; Nawrot, R., Ed.; Latex, Laticifers and Their Molecular Components; Academic Press: Cambridge, MA, USA, 2020; Volume 93, pp. 27–54. [Google Scholar]
- Marinho, C.R.; Teixeira, S.P. Novel Reports of Laticifers in Moraceae and Urticaceae: Revisiting Synapomorphies. Plant Syst. Evol. 2019, 305, 13–31. [Google Scholar] [CrossRef]
- Leme, F.M.; Borella, P.H.; Marinho, C.R.; Teixeira, S.P. Expanding the Laticifer Knowledge in Cannabaceae: Distribution, Morphology, Origin, and Latex Composition. Protoplasma 2020, 257, 1183–1199. [Google Scholar] [CrossRef] [PubMed]
- Guérin, M.P. Les Urticées: Cellules à mucilage, laticifères et canaux sécréteurs. Bull. Société Bot. Fr. 1923, 70, 125–136. [Google Scholar] [CrossRef]
- Metcalfe, C.R. Distribution of Latex in the Plant Kingdom. Econ. Bot. 1967, 21, 115–127. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy: Meristems, Cells, and Tissues of the Plant Body: Their Structure, Function, and Development, 1st ed.; Wiley: Hoboken, NJ, USA, 2006; ISBN 9780471738435. [Google Scholar]
- Mesquita, J.F.; Santos Dias, J.D. Ultrastructural and cytochemical study of the laticifers of Cannabis sativa L. Bol. Soc. Brot. 1984, 57, 337–356. [Google Scholar]
- Marinho, C.R.; Martucci, M.E.P.; Gobbo-Neto, L.; Teixeira, S.P. Chemical Composition and Secretion Biology of the Floral Bouquet in Legume Trees (Fabaceae). Bot. J. Linn. Soc. 2018, 187, 5–25. [Google Scholar] [CrossRef]
- Wilson, K.J.; Mahlberg, P.G. Ultrastructure of Developing and Mature Nonarticulated Laticifers in the Milkweed Asclepias syriaca L. (Asclepiadaceae). Am. J. Bot. 1980, 67, 1160–1170. [Google Scholar] [CrossRef]
- Lee, K.B.; Mahlberg, P.G. Ultrastructure and Development of Nonarticulated Laticifers in Seedlings of Euphorbia maculata L. J. Plant Biol. 1999, 42, 57–62. [Google Scholar] [CrossRef]
- Gama, T.D.S.S.; Rubiano, V.S.; Demarco, D. Laticifer Development and Its Growth Mode in Allamanda blanchetii A. DC. (Apocynaceae). J. Torrey Bot. Soc. 2017, 144, 303–312. [Google Scholar] [CrossRef]
- Jacob, J.L.; Prevot, J.C. CHAPTER 6 - Metabolism of the Laticiferous System and Its Biochemical Regulation. In Developments in Crop Science; Sethuraj, M.R., Mathew, N.M., Eds.; Natural Rubber; Elsevier: Amsterdam, The Netherlands, 1992; Volume 23, pp. 116–136. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Papini, A. Ultrastructure of Autophagy in Plant Cells: A Review. Autophagy 2013, 9, 1922–1936. [Google Scholar] [CrossRef] [PubMed]
- Machado, S.R.; Rodrigues, T.M. Autophagy and Vacuolar Biogenesis during the Nectary Development. Planta 2019, 250, 519–533. [Google Scholar] [CrossRef]
- Gonçalves, M.P.; Mercadante-Simões, M.O.; Ribeiro, L.M. Ontogeny of Anastomosed Laticifers in the Stem Apex of Hancornia speciosa (Apocynaceae): A Topographic Approach. Protoplasma 2018, 255, 1713–1724. [Google Scholar] [CrossRef]
- Marques, H.K.O.; Figueiredo, M.G.F.; de Souza Pio, W.S.; Ribeiro, L.M.; de Azevedo, I.F.P.; Duarte, L.P.; de Sousa, G.F.; de Aguilar, M.G.; Mercadante-Simões, M.O. Laticifer Ontogenesis and the Chemical Constituents of Marsdenia zehntneri (Apocynaceae) Latex in a Semiarid Environment. Planta 2022, 257, 19. [Google Scholar] [CrossRef]
- Inamdar, J.A.; Murugan, V.; Subramanian, R.B. Ultrastructure of Non-Articulated Laticifers in Allamanda violacea. Ann. Bot. 1988, 62, 583–588. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, D.; Zhang, H.; Wang, M.; Li, P.; Fang, X.; Cai, X. Detection of Autophagy Processes during the Development of Nonarticulated Laticifers in Euphorbia kansui Liou. Planta 2018, 247, 845–861. [Google Scholar] [CrossRef]
- Fang, X.; Zhang, Y.; Wang, M.; Li, P.; Zhang, Q.; Si, J.; Wei, B.; Miao, Y.; Tian, L.; Cai, X. Lysosome and Proteasome Pathways Are Distributed in Laticifers of Euphorbia helioscopia L. Physiol. Plantarum 2019, 166, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Moghe, G.D.; Frank, M.H. Growing a Glue Factory: Open Questions in Laticifer Development. Curr. Opin. Plant Biol. 2021, 64, 102096. [Google Scholar] [CrossRef]
- Canaveze, Y.; Mastroberti, A.A.; Mariath, J.E.D.A.; Machado, S.R. Cytological Differentiation and Cell Wall Involvement in the Growth Mechanisms of Articulated Laticifers in Tabernaemontana catharinensis A.DC. (Apocynaceae). Protoplasma 2019, 256, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.V.; Freitas, C.D.T.; Morais, F.S.; Prado, E.; Medina, M.C.; Demarco, D. Plant Latex and Latex-Borne Defense. In Advances in Botanical Research; Nawrot, R., Ed.; Latex, Laticifers and Their Molecular Components; Academic Press: Cambridge, MA, USA, 2020; Volume 93, pp. 1–25. ISBN 9780081029954. [Google Scholar]
- Lewinsohn, T.M. The Geographical Distribution of Plant Latex. Chemoecology 1991, 2, 64–68. [Google Scholar] [CrossRef]
- Prado, E.; Demarco, D. Laticifers and Secretory Ducts: Similarities and Differences. In Ecosystem Services and Global Ecology; IntechOpen: London, UK, 2018; ISBN 9781789237399. [Google Scholar]
- De Barros, T.C.; Teixeira, S.P. Morphology and Ontogeny of Tannin-Producing Structures in Two Tropical Legume Trees. Botany 2014, 92, 513–521. [Google Scholar] [CrossRef]
- Foster, A.S. Plant Idioblasts: Remarkable Examples of Cell Specialization. Protoplasma 1956, 46, 184–193. [Google Scholar] [CrossRef]
- Gregory, M.; Baas, P. A Survey of Mucilage Cells in Vegetative Organs of the Dicotyledons. Israel J. Bot. 1989, 38, 125–174. [Google Scholar] [CrossRef]
- Fortuna-Perez, A.P. Estudos Anatômicos e Genéticos Subsidiando a Taxonomia No Complexo Zornia diphylla (L.) Pers. (Leguminosae, Papilionoideae, Aeschynomeneae). Master’s Thesis, Universidade Estadual de Campinas, Campinas, Brasil, 2005. [Google Scholar]
- Rio, M.C.S.; Kinoshita, L.S.; Castro, M.M. Anatomia foliar como subsídio para a taxonomia de espécies de Forsteronia G. Mey. (Apocynaceae) dos cerrados paulistas. Braz. J. Bot. 2005, 28, 713–726. [Google Scholar] [CrossRef]
- Marquiafável, F.S.; Canavaci, G.M.C.B.; Teixeira, S.P. Idioblastos secretores em espécies brasileiras de Indigofera L. (Leguminosae, Papilionoideae). Rev. Bras. Biociências 2007, 5, 354–356. [Google Scholar]
- Silva, F.S.; Mastroberti, A.A.; Mariath, J.E.A. Aspectos anatômico-funcionais das células epidérmicas de pínulas de Adiantum raddianum Presl. (Pteridaceae). Rev. Bras. Biociências 2007, 5, 831–833. [Google Scholar]
- Castro, M.M.; Demarco, D. Phenolic Compounds Produced by Secretory Structures in Plants: A Brief Review. Nat. Prod. Commun. 2008, 3, 1934578X0800300. [Google Scholar] [CrossRef]
- De Luna, B.N.; Defaveri, A.C.A.E.; Sato, A.; Bizzo, H.R.; Freitas, M.D.F.; Barros, C.F. Leaf Secretory Tissues in Myrsine coriacea and Myrsine venosa (Primulaceae): Ontogeny, Morphology, and Chemical Composition of Essential Oils. Botany 2014, 92, 757–766. [Google Scholar] [CrossRef]
- Bento, J.P.S.P.; Rosa, M.P.G.; Sartori, Â.L.B. Discolobium and Riedeliella (Fabaceae–Faboideae–Dalbergieae Clade): Leaflet Anatomy, Secretory Structures and Their Systematic Implications. Bot. J. Linn. Soc. 2023, 201, 415–427. [Google Scholar] [CrossRef]
- Commoner, B.; Zucker, M. Cellular differentiation: An experimental approach. In Growth and Differentiation in Plants; Loomis, W.E., Ed.; The Iowa State College Press: Ames, IA, USA, 1953; pp. 339–392. [Google Scholar]
- Muravnik, L.E. The Structural Peculiarities of the Leaf Glandular Trichomes: A Review. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Ramawat, K.G., Ekiert, H.M., Goyal, S., Eds.; Reference Series in Phytochemistry; Springer International Publishing: Cham, Switzerland, 2021; pp. 63–97. ISBN 9783030301859. [Google Scholar]
- Livingston, S.J.; Bae, E.J.; Unda, F.; Hahn, M.G.; Mansfield, S.D.; Page, J.E.; Samuels, A.L. Cannabis Glandular Trichome Cell Walls Undergo Remodeling to Store Specialized Metabolites. Plant Cell Physiol. 2021, 62, 1944–1962. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.B.; Stevens, R. Composition and Biogenesis of Essential Oil of Hops. J. Inst. Brew. 1962, 68, 420–427. [Google Scholar] [CrossRef]
- Nascimento, I.C.; Leme, F.M.; Teixeira, S.P. Morphological Diversity of Glandular Trichomes in Urticalean Rosids. Acta Bot. Bras. 2022, 36, e2022abb0061. [Google Scholar] [CrossRef]
- Tobe, H.; Takaso, T. Trichome micromorphology in Celtidaceae and Ulmaceae (Urticales). Acta Phytotax. Geobot. 1997, 47, 153–168. [Google Scholar] [CrossRef]
- Schnetzler, B.N.; Teixeira, S.P.; Marinho, C.R. Trichomes That Secrete Substances of a Mixed Nature in the Vegetative and Reproductive Organs of Some Species of Moraceae. Acta Bot. Bras. 2017, 31, 392–402. [Google Scholar] [CrossRef]
- Løe, G.; Toräng, P.; Gaudeul, M.; Ågren, J. Trichome Production and Spatiotemporal Variation in Herbivory in the Perennial Herb Arabidopsis lyrata. Oikos 2007, 116, 134–142. [Google Scholar] [CrossRef]
- Small, E.; Naraine, S.G.U. Size Matters: Evolution of Large Drug-Secreting Resin Glands in Elite Pharmaceutical Strains of Cannabis sativa (Marijuana). Genet. Resour. Crop Evol. 2016, 63, 349–359. [Google Scholar] [CrossRef]
- Naraine, S.G.U.; Small, E. Germplasm Sources of Protective Glandular Leaf Trichomes of Hop (Humulus lupulus). Genet. Resour. Crop Evol. 2017, 64, 1491–1497. [Google Scholar] [CrossRef]
- Verzele, M. 100 Years Of Hop Chemistry And Its Relevance To Brewing. J. Inst. Brew. 1986, 92, 32–48. [Google Scholar] [CrossRef]
- Saito, T.; Hirosawa, T.; Horiuchi, S.; Murakami, A.; Matsushima, H. A Study of SEM Examination on Fresh Hop (Humulus lupulus L.) Peltate Glandular Trichomes. J. Electron Microsc. 1995, 44, 39–44. [Google Scholar] [CrossRef]
- Nagel, J.; Culley, L.K.; Lu, Y.; Liu, E.; Matthews, P.D.; Stevens, J.F.; Page, J.E. EST Analysis of Hop Glandular Trichomes Identifies an O -Methyltransferase That Catalyzes the Biosynthesis of Xanthohumol. Plant Cell 2008, 20, 186–200. [Google Scholar] [CrossRef]
- Mori, Y. Mechanism of Humulone and Lupulone Synthesis in the Hop Cone 1 : Free Amino Acid in the Developing Cones of Hops. Bull. Brew. Sci. 1961, 6, 19–22. [Google Scholar]
- Johansen, D.A. Plant Microtechnique, 1st ed.; McGraw-Hill Publishing Company, Ltd.: London, UK, 1940. [Google Scholar]
- Lillie, R.D. Histopathological Technic and Practical Histochemistry, 3rd ed.; McGraw-Hill Publishing Company, Ltd.: New York, NY, USA, 1965. [Google Scholar]
- O’Brien, T.P.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Vidal, B.D.C. Dichroism in collagen bundles stained with Xylidine-Ponceau 2R. Ann. Histochem. 1970, 15, 289–296. [Google Scholar]
- Jensen, W.A. Botanical Histochemistry: Principles and Practice; Freeman, William and Jensen: San Francisco, CA, USA, 1962. [Google Scholar]
- Pearse, A.G.E. Histochemistry: Theoretical and Applied, 3rd ed.; JA Churchill, Ltd.: London, UK, 1968. [Google Scholar]
- David, R.; Carde, J.P. Coloration différentielle dês inclusions lipidique et terpeniques dês pseudophylles du Pin maritime au moyen du reactif Nadi. C. R. Hebd. Seances Acad. Sci. 1964, 258, 1338–1340. [Google Scholar]
- Jayabalan, M.; Shah, J.J. Histochemical Techniques to Localize Rubber in Guayule (Parthenium argentatum GRAY). Stain Technol. 1986, 61, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Mace, M.E.; Howell, C.R. Histochemistry and identification of condensed tannin precursors in roots of cotton seedlings. Can. J. Bot. 1974, 52, 2423–2426. [Google Scholar] [CrossRef]
- Ventrella, M.C.; Almeida, A.L.; Nery, L.A.; Coelho, V.P.M. Métodos Histoquímicos Aplicados às Sementes [Recurso Eletrônico]; UFV: Viçosa, Brazil, 2013. [Google Scholar]
- Karnovsky, M.J. A formaldehyde–glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 1965, 27, 137–138. [Google Scholar]
- Venable, J.H.; Coggeshall, R. A simplified lead citrate stain for use in electron microscopy. J. Cell Biol. 1965, 25, 407–408. [Google Scholar] [CrossRef]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef]
- Bal, A.K. Cellulase. In Electron Microscopy of Enzymes; Hayat, M.A., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1974; pp. 68–79. [Google Scholar]
- Nolan, R.A.; Bal, A.K. Cellulase Localization in Hyphae of Achlya ambisexualis. J. Bacteriol. 1974, 117, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Allen, R.D.; Nessler, C.L. Cytochemical Localization of Pectinase Activity in Laticifers of Nerium oleander L. Protoplasma 1984, 119, 74–78. [Google Scholar] [CrossRef]



| Reagents | Target Compound | Latex | Idioblast Content |
|---|---|---|---|
| Toluidine blue O (purple) | Mucilage | − | − |
| Toluidine blue O (green) | Phenolics | + | + |
| Xylidine Ponceau | Protein bodies | + | + |
| Sudan IV | Total lipids | + | + |
| PAS reagent | Polysaccharides | + | + |
| Wagner’s reagent | Alkaloids | + | + |
| Nadi reagent | Terpenes | + | + |
| Oil O Red | Rubber particles | + | − |
| Ferric chloride | Non-structural phenolics | + | + |
| Vanillin hydrochloric acid | Tannins | + | − |
| Lugol’s solution | Starch | + | + |
| Plant Organ | Laticifer System | Glandular Trichomes | Phenolic Idioblasts |
|---|---|---|---|
| Stem | − | ||
| Cortex | + | − | |
| Medula | − | − | |
| Petiole | + | + | + |
| Leaf (adaxial side) | |||
| Leaf blade | − | + | + |
| Central vein | + | + | − |
| Parallel veins | + | + | + |
| Leaf (abaxial side) | |||
| Leaf blade | − | + | + |
| Central vein | + | − | − |
| Parallel veins | + | − | + |
| Staminate inflorescence | |||
| Peduncle | + | + | + |
| Bracts | + | + | + |
| Perianth | + | + | + |
| Filament | − | + | + |
| Anthers | |||
| Connective | − | + | + |
| Thecae | − | − | + |
| Pistillate inflorescence | |||
| Peduncle | + | + | + |
| Bracts | + | + | + |
| Sepals | + | + | + |
| Ovary | − | − | + |
| Stigma | − | − | + |
| Root | − | ||
| Cortex | − | + | |
| Medula | − | + |
| Target Compounds | Humulus lupulus | Cannabis sativa | Celtis pubescens | Pteroceltis tatarinowii | Trema micrantha |
|---|---|---|---|---|---|
| Phenolics | + | + | − | − | − |
| Non-structural phenolics | + | + | − | − | − |
| Tannins | − | ? | − | ? | − |
| Proteins | + | + | + | + | + |
| Total lipids | + | + | + | + | + |
| Terpenes | + | ? | + | ? | + |
| Rubber particles | + | ? | ? | ? | ? |
| Polysaccharides | + | + | + | + | + |
| Starch | + | − | + | + | + |
| Alkaloids | + | ? | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, F.P.; Iwamoto, L.; Piva, V.H.; Teixeira, S.P. Updating the Knowledge on the Secretory Machinery of Hops (Humulus lupulus L., Cannabaceae). Plants 2024, 13, 864. https://doi.org/10.3390/plants13060864
Ramos FP, Iwamoto L, Piva VH, Teixeira SP. Updating the Knowledge on the Secretory Machinery of Hops (Humulus lupulus L., Cannabaceae). Plants. 2024; 13(6):864. https://doi.org/10.3390/plants13060864
Chicago/Turabian StyleRamos, Felipe Paulino, Lucas Iwamoto, Vítor Hélio Piva, and Simone Pádua Teixeira. 2024. "Updating the Knowledge on the Secretory Machinery of Hops (Humulus lupulus L., Cannabaceae)" Plants 13, no. 6: 864. https://doi.org/10.3390/plants13060864








