Agrobacterium-Mediated Transformation of the Dwarf Soybean MiniMax
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- Experimental Design
- Reagents
- Plant Materials and Growth Conditions
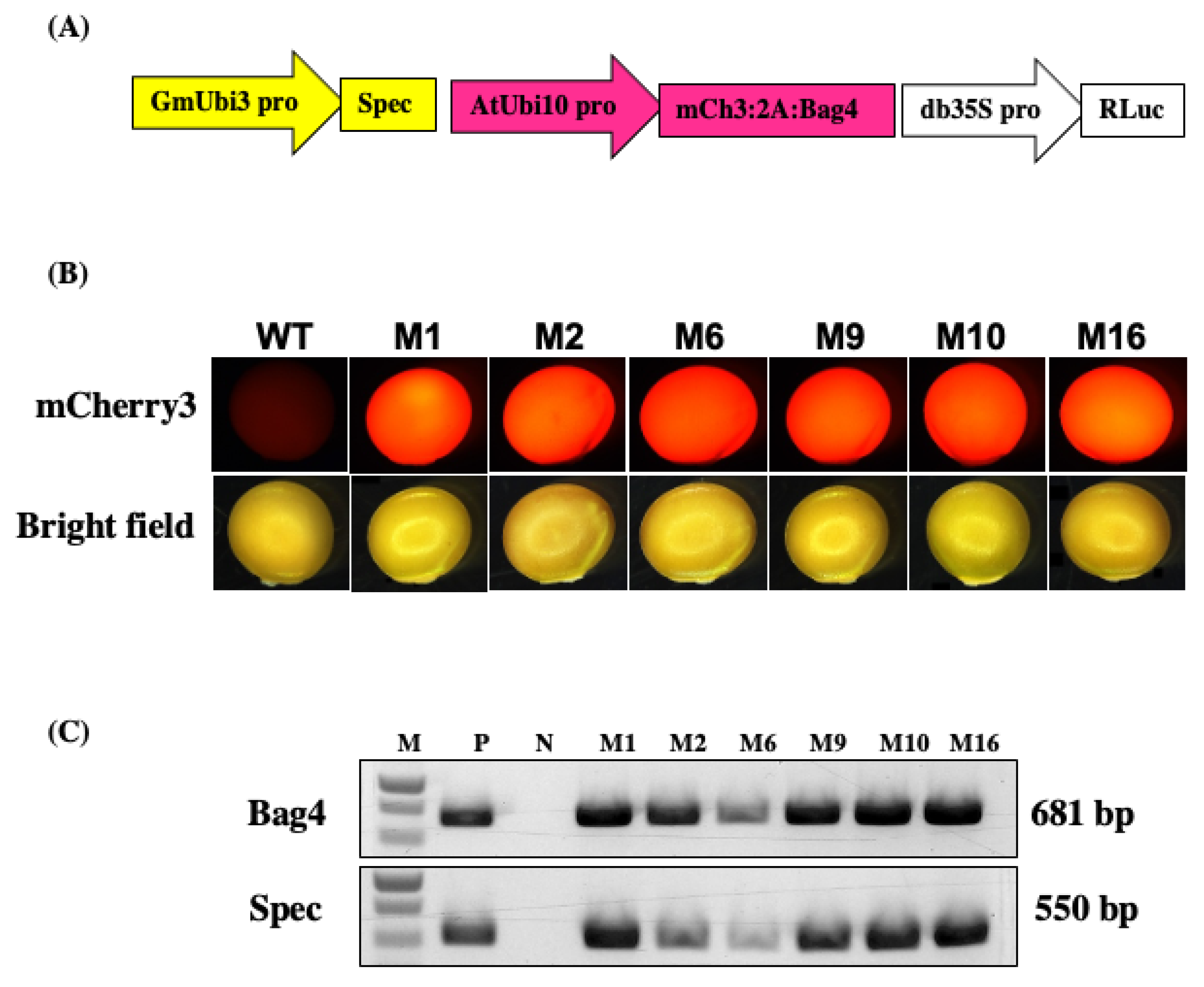
- Construct genotype and expected phenotype
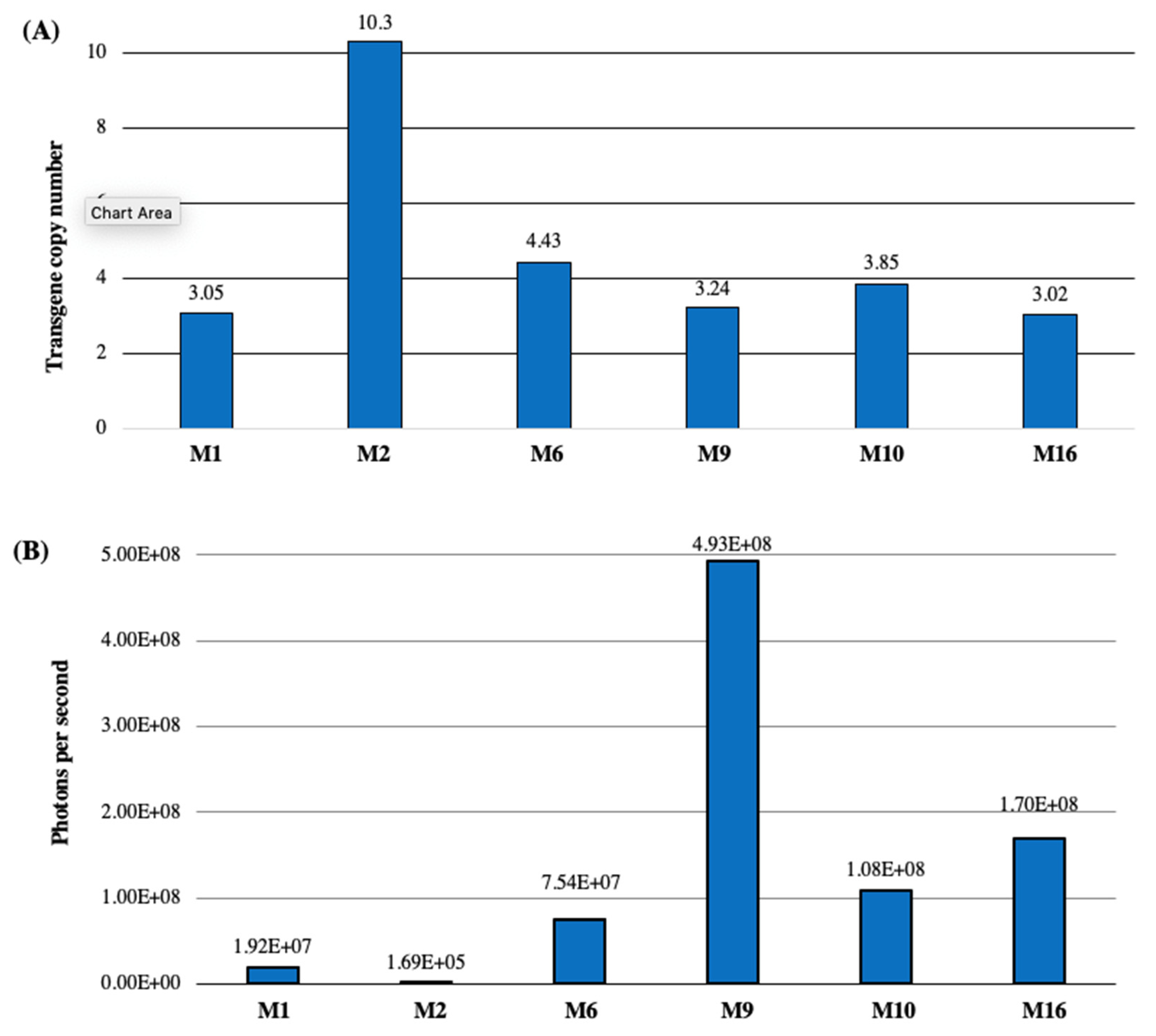
- Molecular analysis of the transformants
- Procedure
- Detailed transformation steps and time required
- i.
- Place mature soybean seeds in a sterile 100 × 20 mm Petri dish with about 150 seeds per dish. Write the seed name on Petri dish. Caution: Do not exceed approximately 200 seeds per Petri dish, as a larger number of seeds results in incomplete surface sterilization.
- ii.
- In the fume hood, put the Petri dish with seeds in a 15 mL round bottom culture tube sterilization container and open the lid. Place a 250 mL beaker inside the sterilization container and add 100 mL of bleach containing 5.25% sodium hypochlorite.
- iii.
- Add 4.5 mL 12N HCl to the beaker containing bleach and close the sterilization container immediately. The initial reaction will produce bubbles (chlorine gas).
- iv.
- Allow the container to vent for 20 h to complete the reaction, eliminate the chlorine gas, and sterilize the seeds. Seeds should be surface-sterilized completely to avoid contamination.
- v.
- Close the Petri dish in the sterilization container and transfer it to a laminar flow hood. Open the lid and allow to sit for 30 min or more. Close the Petri dish, seal with Parafilm, and store at 4 °C. Sterilized seeds can be stored at 4 °C in dry conditions for six months to a year.
- vi.
- In a laminar flow hood, add sterile water into 100 × 25 mm Petri dish containing 150–200 sterilized seeds until seeds are covered. Close and seal Petri dish with parafilm.
- vii.
- Store at room temperature (22–25 °C) overnight (~20 h).
- viii.
- In a laminar flow hood, transfer the soaked seeds to a sterile Petri dish. Use a scalpel blade to make a longitudinal cut along the hilum to separate the cotyledons.
- ix.
- Remove the seed coat and keep the embryo containing half-seed explants in the Petri dish. Cover with lid. Do not let explants dry out and turn brown; this will decrease the susceptibility of explants to Agrobacterium infection.
- x.
- Start Agrobacterium preparation 2 days before transformation. Streak Agrobacterium, in this case GAANTRY strain JGT44 (Figure 3A), on an LB plate containing 200 mg/mL gentamicin. Incubate the plate for 1 d at 28 °C.
- xi.
- Inoculate a single colony of Agrobacterium from the plate into 3 mL LB liquid medium containing 100 mg/mL gentamicin. Culture at 28 °C with shaking (250 rpm) for 36 h.
- xii.
- Spin the agrobacterium culture at 3600 rpm for 10 min at room temperature. Remove the supernatant, resuspend the pellet in liquid IM medium, and adjust the OD (650 nm) to 0.6 with IM medium.
- xiii.
- Gently shake the resulting infection medium at 60 rpm at room temperature for 2 h before use.
- xiv.
- Prepare co-culture medium plates. After autoclaving co-culture medium, add GA3 0.25 mg/L, BAP 1.67 mg/L, AS 40 mg/L, cysteine 400 mg/L and DTT 154.2 mg/L. Pour into 100 × 15 mm Petri plates. Overlay plate with sterile filter paper to reduce agrobacteria overgrowth after the medium is solidified.
- xv.
- Transfer prepared half-seed explants into Agrobacterium resuspension liquid tube (around 100 explants per 50 mL tube), and cover with Parafilm. Make 2–4 holes in the parafilm and place under vacuum (200 mm Hg) (Stratagene, Vacuum Control Station) for 10 min. Remove from vacuum and incubate at room temperature for an additional 10 min.
- xvi.
- After co-culture gently remove excess infection media with pipet. Transfer infected explants onto co-cultivation medium using sterile forceps, with the flat, adaxial side touching the filter paper, allowing roughly 6–9 explants per Petri dish plate. Do not transfer liquid with explants to co-cultivation plates, to avoid overgrowth of agrobacterium.
- xvii.
- Wrap the plates with Parafilm and place at 24 °C under an 18/6 photoperiod for 5 days.
- xviii.
- After 5 days co-cultivation, place the explants on spectinomycin selection shoot induction medium (SI, 6–9 explants per plate). Explants should be oriented with the nodal end of the cotyledon embedded in the medium and the regeneration regions flush to the surface, with the flat side up at a 30–45° angle.
- xix.
- Wrap each plate with microporous surgical tape and incubate at 24 °C, 18/6 photoperiod for 14 days. Explants should be transferred to fresh SI every 14 days.
- xx.
- After 28 days on SI, transfer the explants to fresh shoot elongation medium (SE), seal each plate with microporous surgical tape, and incubate at 24 C, 18/6 photoperiod.
- xxi.
- Transfer the tissue to fresh SE to maintain cultures. Seal each plate with microporous surgical tape and incubate at 24 °C, 18/6 photoperiod, under the same conditions described above. Repeat every 14 days.
- xxii.
- When shoots surviving in spectinomycin selection medium reach at least 3 cm, excise them from the shoot pad. Using sterile forceps, transfer green shoots to 100 × 25 sterile Petri dish plates containing root initiation medium (RM) without selection. Wrap the plate with microporous surgical tape and incubate in growth chamber at 24 °C, 18/6 photoperiod, for 2–3 weeks. Shoots should continue developing in root initiation medium with spectinomycin selection. If plants appear weak, transfer to RM without transgenic selective agent (spectinomycin) to help shoot rooting in root step.
- xxiii.
- Roots should start appearing after a week. When roots grow longer than 3 cm, and the shoots 4–5 cm, remove the plants from deep Petri dish with forceps. Wash briefly with running tap water to remove traces of Bacto-agar.
- xxiv.
- Fill new pots with the ¾ Sunshine Mix 1: ¼ Peat Moss mix and moisten the soil with FOOP Garden solution. Make a hole in the soil (depth depending upon the root length) and place the regenerated shoot into the hole. Compact the soil around the shoot slightly.
- xxv.
- Place containers with shoots into a growth chamber at 24 °C, with 18/6 photoperiod, covered with plastic dome for 1–2 weeks. Shoots should show some growth before being transferred to greenhouse conditions.
- xxvi.
- FOOP is added to the soil upon transfer to solid medium and plants are misted weekly, according to the manufacturer’s instructions.
- xxvii.
- After being in the growth chamber for 1–2 weeks, plants are transferred to big pots (6” H × 5” L × 8” D) and moved to the greenhouse with a 16/8 light–dark photoperiod at 24 °C.
- xxviii.
- Harvest T1 seeds. After around 2 months, the mature T1 seeds can be harvested, Dry at 30 °C for 3–10 days.
- xxix.
- Separate mCherry3 positive transgenic T1 seeds under a Leica MZ16F florescence stereomicroscope at 1X magnification (Figure 3B). Plate the mCherry3 positive seeds in big pots containing the Sunshine Mix #1, with 2 plants per pot. Grow in a greenhouse with a 16/8 light–dark photoperiod at 24 °C.
- xxx.
- After around 3 months, the mature T2 seeds can be harvested. Dry at 30 °C for 3–10 days.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matthews, B.F.; MacDonald, M.H.; Song, Q.J.; Cregan, P.B.; Lewers, K.S. Registration of ‘MiniMax’ Soybean. J. Plant Regist. 2007, 1, 97–98. [Google Scholar] [CrossRef]
- Klink, V.P.; MacDonald, M.H.; Martins, V.E.; Park, S.-C.; Kim, K.-H.; Baek, S.-H.; Matthews, B.F. MiniMax, a new diminutive Glycine max genotype with a rapid life cycle, embryogenic potential and transformation capabilities. Plant Cell Tissue Organ. Cult. 2008, 92, 183–195. [Google Scholar] [CrossRef]
- Kocsy, G.; Simon-Sarkadi, L.; Galiba, G.; de Ronde, J.A. Transformation of Soybean and Transgenic Lines in Basic and Applied Research. Transgenic Plant J. 2007, 1, 129–144. [Google Scholar]
- Loganathan, L.; Maruthasalam, S.; Shiu, L.Y.; Lien, W.C.; Hsu, W.H.; Lee, P.F.; Yu, C.W.; Lin, C.H. Regeneration of soybean (Glycine max, L. Merrill) through direct somatic embryogenesis from the immature embryonic shoot tip. Vitr. Cell Dev. Biol. Plant 2010, 46, 265–273. [Google Scholar] [CrossRef][Green Version]
- Paes de Melo, B.; Lourenço-Tessutti, I.T.; Morgante, C.V.; Santos, N.C.; Pinheiro, L.B.; de Jesus Lins, C.B.; Silva, M.C.M.; Macedo, L.L.P. Fontes EPB and Grossi-de-Sa MF Soybean Embryonic Axis Transformation: Combining Biolistic and Agrobacterium-Mediated Protocols to Overcome Typical Complications of In Vitro Plant Regeneration. Front. Plant Sci. 2020, 11, 1228. [Google Scholar] [CrossRef]
- Hathwaik, L.T.; Thomson, J.G.; Thilmony, R. Gene Assembly in Agrobacterium via Nucleic Acid Transfer Using Recombinase Technology (GAANTRY). Methods Mol. Biol. 2021, 2238, 3–17. [Google Scholar]
- Paz, M.M.; Martinez, J.C.; Kalvig, A.B.; Fonger, T.M.; Wang, K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006, 25, 206–213. [Google Scholar] [CrossRef]
- De Oliveria, M.L.; Thomson, J.G.; Stover, E. High-efficiency propagation of mature ‘Washington Navel’ orange and juvenile ‘Carrizo’ citrange using axillary shoot proliferation. HortTechnology 2016, 26, 278–286. [Google Scholar] [CrossRef]
- Hesami, M.; Naderi, R.; Tohidfar, M. Modeling and optimizing Medium composition for Shoot Regeneration of Chrysanthemum via Radial Basis function-non- dominated Sorting Genetic Algorithm-II (RBf-nSGAii). Sci. Rep. 2019, 9, 18237. [Google Scholar] [CrossRef]
- Collier, R.; Dasgupta, K.; Xing, Y.-P.; Hernandez, B.T.; Shao, M.; Rohozinski, D.; Kovak, E.; Lin, J.; de Oliveira, M.L.P.; Stover, E.; et al. Accurate measurement of transgene copy number in crop plants using droplet digital PCR. Plant J. 2017, 90, 1014–1025. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Dobránszki, J.; Ross, S. Phloroglucinol in plant tissue culture. Vitr. Cell Dev. Biol. Plant 2013, 49, 1–16. [Google Scholar] [CrossRef]
- Sarkar, D.; Naik, P.S. Phloroglucinol enhances growth and rate of axillary shoot proliferation in potato shoot tip cultures in vitro. Plant Cell Tissue Organ. Cult. 2000, 60, 139–149. [Google Scholar] [CrossRef]
- Siwach, P.; Gill, A.R. Enhanced shoot multiplication in Ficus religiosa, L. in the presence of adenine sulphate, glutamine and phloroglucinol. Physiol. Mol. Biol. Plants 2011, 17, 271–280. [Google Scholar] [CrossRef] [PubMed]
- De Klerk., G.-J.; Guan, H.; Huisman, P.; Marinova, S. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9′. Plant Growth Regul. 2011, 63, 175–185. [Google Scholar] [CrossRef]
- Tan, S.N.; Tee, C.S.; Wong, H.L. Multiple shoot bud induction and plant regeneration studies of Pongamia pinnata. Plant Biotech. 2018, 35, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Tallón, C.I.; Porras, I.; Pérez-Tornero, O. Efficient propagation and rooting of three citrus rootstocks using different plant growth regulators. Vitr. Cell Dev. Biol. Plant 2012, 48, 488–499. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Gulyás, A.; Magyar-Tábori, K.; Wang, M.-R.; Wang, Q.-C.; Dobránszki, J. In vitro tissue culture of apple and other Malus species: Recent advances and applications. Planta 2019, 249, 975–1006. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.; Castillo, A. Mass propagation of Vaccinium corymbosum in bioreactors. Agrociencia 2009, XIII, 1–8. [Google Scholar] [CrossRef]
- Ross, S.; Castillo, A. Micropropagación de Achyrocline flaccida (Weinm.) DC. en medios de cultivo líquidos. Agrociencia 2010, XIV, 1–7. [Google Scholar] [CrossRef]
- Ross, S.; Grasso, R. In vitro propagation of ‘Guayabo del país’ (Acca sellowiana (Berg.) Burret). Fruit. Veg. Cereal Sci. Biotech. 2010, 4, 83–87. [Google Scholar]
- Wang, Q. Factors affecting rooting of microcuttings of the pear rootstock BP10030. Sci. Hortic. 1991, 45, 209–213. [Google Scholar] [CrossRef]
- Sharifian, S.; Vahdati, K.; Mirmasoumi, M.; Ghaem, S.A. Assessment of phloroglucinol effect on rooting of tissue cultured Persian walnut. Acta Hort. 2009, 812, 189–195. [Google Scholar] [CrossRef]
- Ainsley, P.J.; Collins, G.G.; Sedgley, M. In vitro rooting of almond (Prunus dulcis Mill.). Vitr. Cell Dev. Biol. Plant 2001, 37, 778–785. [Google Scholar] [CrossRef]
- Dawson, J.; Pandey, S.; Yu, Q.; Schaub, P.; Wüst, F.; Moradi, A.B.; Dovzhenko, O.; Palme, K.; Welsch, R. Determination of protoplast growth properties using quantitative single-cell tracking analysis. Plant Methods 2022, 18, 64. [Google Scholar] [CrossRef] [PubMed]


| Replicate No. | Explant No. | Regeneration Shoots | Rooting Plants | mCherry3 Expressing Plants | Greenhouse Survival Plants | PCR Positive Plants | Transgenic Rate % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | No. | No. | Rate % | No. | Rate % | No. | Rate % | No. | Rate % | No. | Rate % |
| 1 | 206 | 61 | 29.6 | 58 | 28.2 | 49 | 23.8 | 10 | 4.85 | 9 | 4.37 |
| 2 | 203 | 57 | 28.1 | 45 | 22.2 | 43 | 21.2 | 7 | 3.45 | 7 | 3.45 |
| 3 | 190 | 63 | 33.2 | 49 | 25.8 | 46 | 24.2 | 9 | 4.74 | 8 | 4.21 |
| Total | 599 | 181 | 30.2 | 152 | 25.4 | 138 | 23.0 | 26 | 4.34 | 24 | 4.01 |
| Medium | Composition (Basic) | Added after Autoclaving (Final Concentrations) |
|---|---|---|
| CCM | 1/10 Gamborg B5 basal medium, 1 mL Gamborg vitamin (1 mg/mL), 30 g Sucrose, 3.9 g MES, 4.25 g Bacto-agar, pH to 5.4 with KOH. | GA3 0.25 mg/L, BAP 1.67 mg/L, AS 40 mg/L, L-Cysteine 400 mg/L, and DTT 154.2 mg/L |
| IM | 1/10 Gamborg B5 basal medium, 1 mL Gamborg vitamin (1mg/mL), 30 g Sucrose, 3.9 g MES, pH to 5.4 with KOH. | GA3 0.25 mg/L, BAP 1.67 mg/L, AS 40 mg/L |
| SIW | Gamborg B5 medium with vitamins, 30 g Sucrose, 0.59 g MES, pH to 5.7 with KOH. | BAP 1.67 mg/L, ticarcillin 200 mg/L |
| SI | Gamborg B5 medium with vitamins, 30 g Sucrose, 0.59 g MES, 7 g Bacto-agar, pH to 5.7 with KOH. | BAP 1.67 mg/L, ticarcillin 200 mg/L, PG 1.0 mg/L |
| SE | MS medium with vitamins, 30 g Sucrose, 0.59 g MES, 7 g Bacto-agar, pH to 5.7 with KOH. | Asparagine 50 mg/L, L-Pyroglutamic Acid 100 mg/L, IAA 1 mg/L, GA3 0.5 mg/L, ZR 1 mg/L, Ticarcillin 200 mg/L, PG 1.0 mg/L |
| RM1 | MS medium with vitamins, 20 g Sucrose, 0.59 g MES, 7 g Bacto-agar, pH to 5.6 with KOH. | AS 50 mg/L, L-Pyroglutamic Acid 100 mg/L, Ticarcillin 200 mg/L, IBA 1mg/L, PG 1.0 mg/L |
| RM2 | MS medium with vitamins, 20 g Sucrose, 0.59 g MES, 7 g Bacto-agar, pH to 5.6 with KOH | AS 50 mg/L, L-Pyroglutamic Acid 100 mg/L, ticarcillin 200 mg/L, IBA 1mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, M.; McCue, K.F.; Thomson, J.G. Agrobacterium-Mediated Transformation of the Dwarf Soybean MiniMax. Plants 2024, 13, 1013. https://doi.org/10.3390/plants13071013
Shao M, McCue KF, Thomson JG. Agrobacterium-Mediated Transformation of the Dwarf Soybean MiniMax. Plants. 2024; 13(7):1013. https://doi.org/10.3390/plants13071013
Chicago/Turabian StyleShao, Min, Kent F. McCue, and James G. Thomson. 2024. "Agrobacterium-Mediated Transformation of the Dwarf Soybean MiniMax" Plants 13, no. 7: 1013. https://doi.org/10.3390/plants13071013
APA StyleShao, M., McCue, K. F., & Thomson, J. G. (2024). Agrobacterium-Mediated Transformation of the Dwarf Soybean MiniMax. Plants, 13(7), 1013. https://doi.org/10.3390/plants13071013






