Antidiabetic Properties of the Root Extracts of Dandelion (Taraxacum officinale) and Burdock (Arctium lappa)
Abstract
:1. Introduction
2. Results
2.1. Extract Analysis
2.1.1. Identification of Inulin
2.1.2. Determination of Total Phenolic Content (TPC)
2.1.3. Determination of Tannin Level
2.1.4. Determination of Total Polysaccharide Content (TP)
2.2. Determination of Antioxidant Activities of Extracts by Using DPPH (2,2-diphenyl-1-picrylhydrazyl) Assay
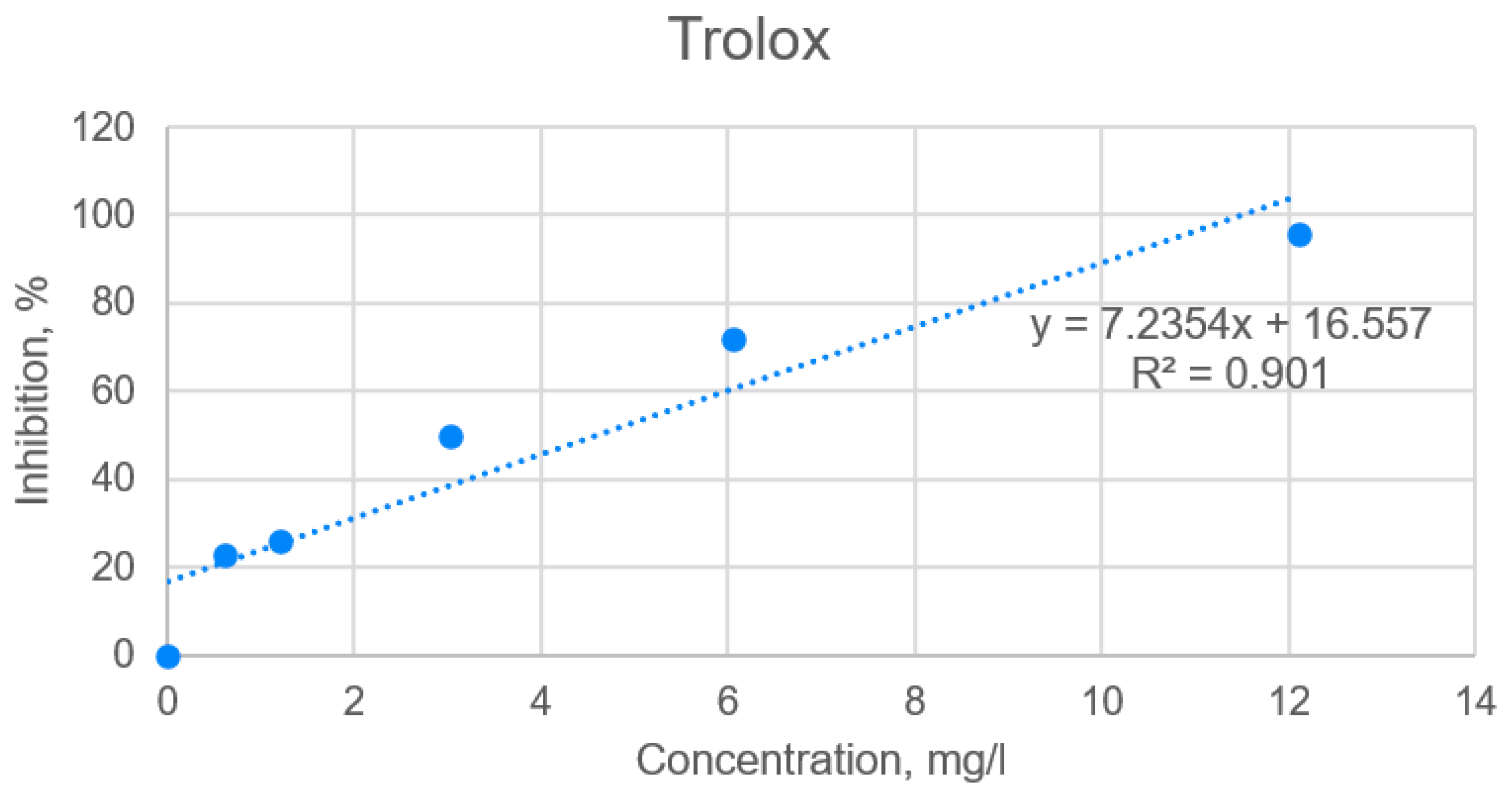
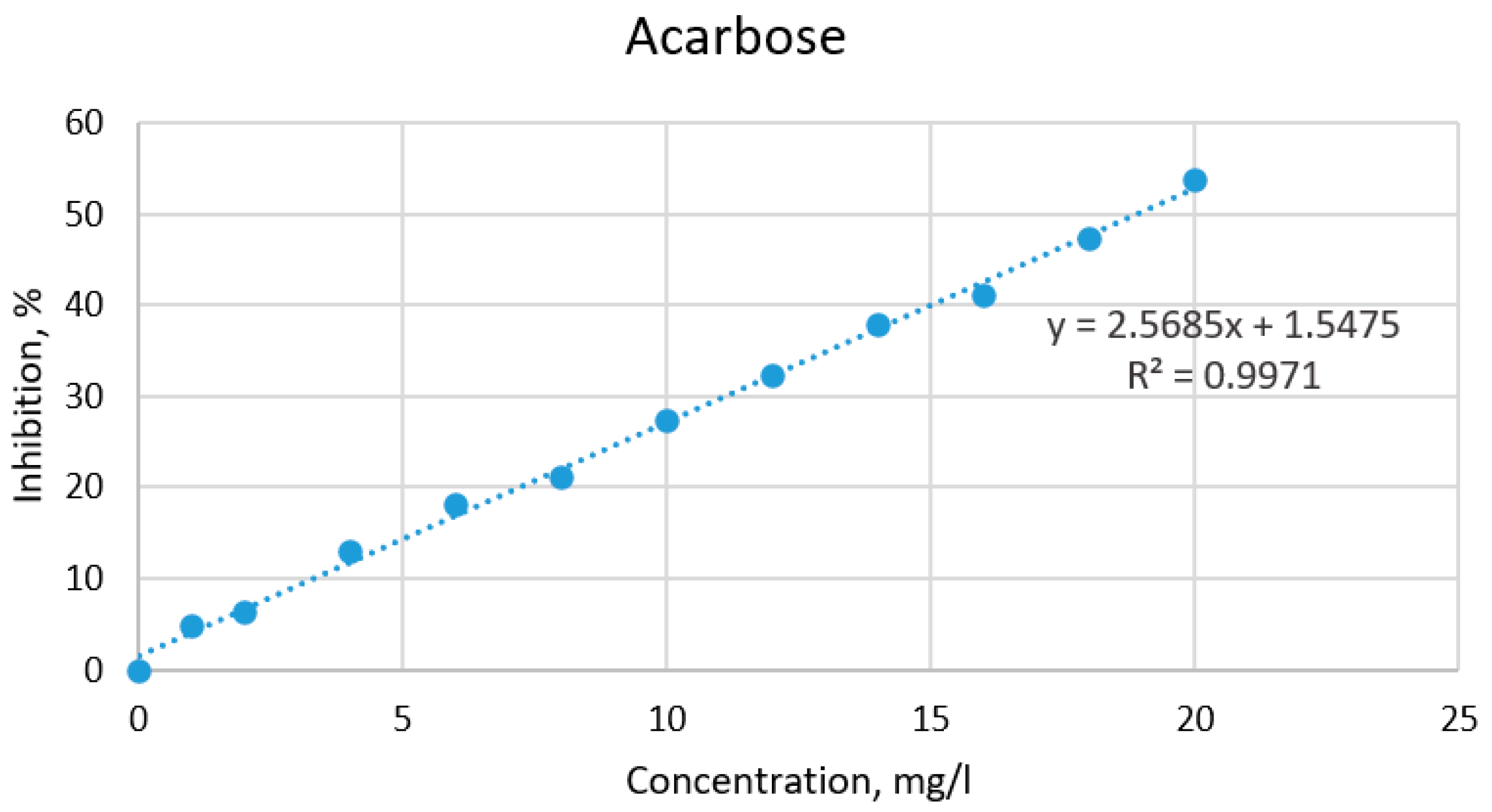
2.3. Hypoglycemic Properties of the Extracts Based on α-Amylase Activity
2.4. Qualitative Analysis of Extracts by Liquid Chromatography–Mass Spectrometry (LC-MS)
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
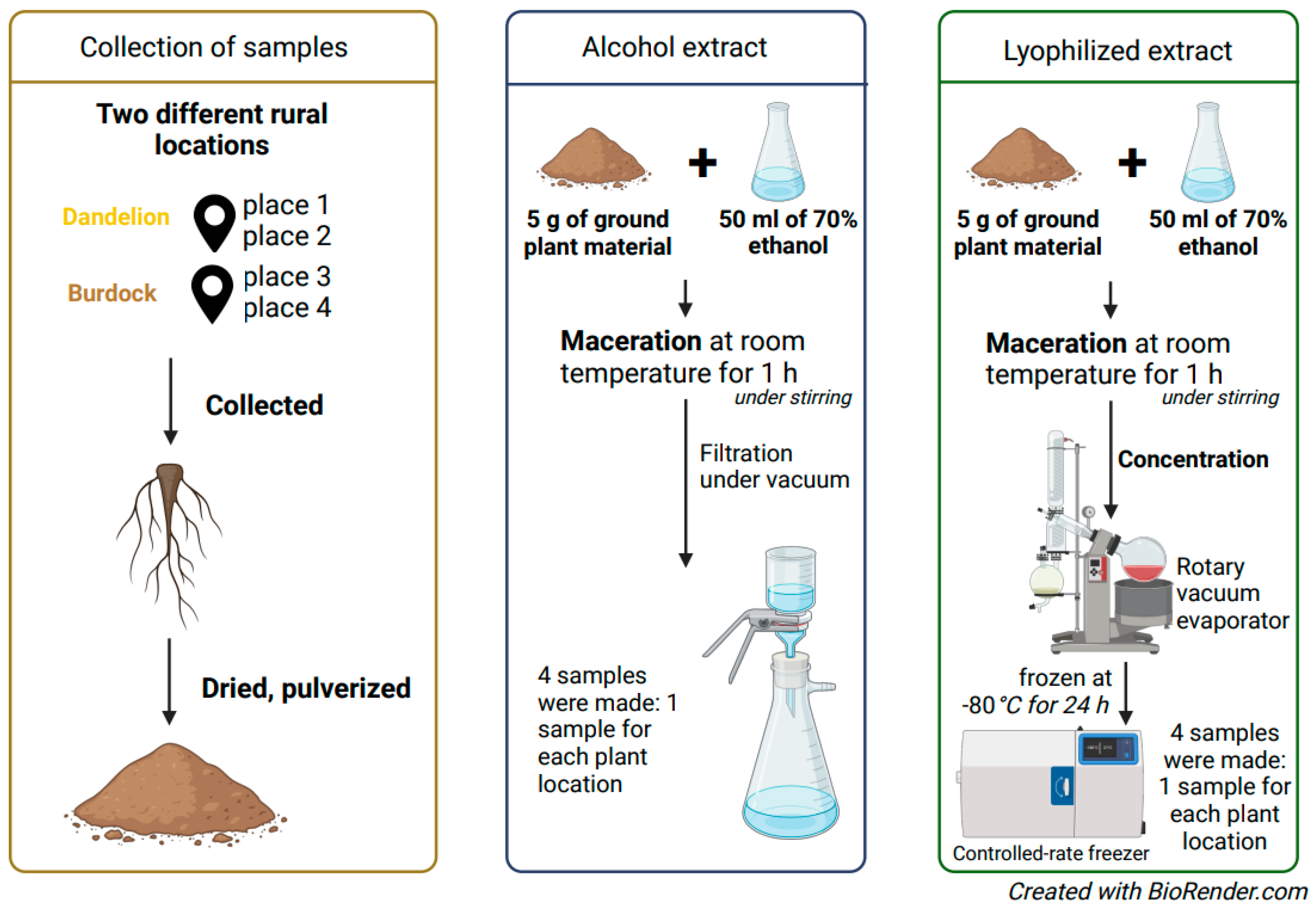
4.2. Plant Material
4.3. Preparation of Ethyl Alcohol and Lyophilizate Extracts
4.4. Extract Analysis
4.4.1. Identification of Inulin
4.4.2. Determination of Total Phenolic Content (TPC)
4.4.3. Determination of Tannin Level
4.4.4. Determination of Total Polysaccharide Content (TP)
4.4.5. Determination of Antioxidant Activities of Extracts by Using DPPH (2,2-diphenyl-1-picrylhydrazyl) Assay
4.4.6. Hypoglycemic Properties of the Extracts Based on α-Amylase Activity
4.5. Qualitative Analysis of Extracts by Liquid Chromatography–Mass Spectrometry (LC-MS)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ojo, O.A.; Ibrahim, H.S.; Rotimi, D.E.; Ogunlakin, A.D.; Ojo, A.B. Diabetes mellitus: From molecular mechanism to pathophysiology and pharmacology. Med. Nov. Technol. Devices 2023, 19, 100247. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; nternational Diabetes Federation: Brussels, Belgium, 2021; Available online: https://www.diabetesatlas.org (accessed on 12 December 2023).
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; Fernandes da Rocha, J.D.; Ohlrogge, A.W.; Malanda, B. Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Research and Clinical Practice. IDF Diabetes Atlas 2018, 138, 271–281. [Google Scholar]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, G.; Lee, H.; Hoang, T.; Chae, H. Glucose-lowering effect of Gryllus bimaculatus powder on streptozotocin-induced diabetes through the AKT/mTOR pathway. Food Sci. Nutr. 2020, 8, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Ersan, Y.; Dilek Ozsahin, A.; Ihsan Ozturk, A.; Ozkan, Y. Consequences of the Combined α-tocopherol, Ascorbic Acid and α-lipoic Acid on the Glutathione, Cholesterol and Fatty Acid Composition in Muscle and Liver of Diabetic Rats. Iran J. Basic Med. Sci. 2013, 16, 165–172. [Google Scholar] [PubMed]
- Chander, A.P.; Reddy, R.A.; Puchchakayala, G. Hypoglycemic and Antidiabetic Activity of Glochidion velutinum on Streptozotocin-Nicotinamide Induced Type 2 Diabetic Rats. Eur. J. BiolSci. 2011, 3, 126–130. [Google Scholar]
- Bindu, J.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech 2019, 9, 4. [Google Scholar]
- Przeor, M. Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals 2022, 15, 65. [Google Scholar] [CrossRef]
- Ndip, R.N.; Tanih, N.F.; Kuete, V. Antidiabetes Activity of African Medicinal Plants. In Medicinal Plant Research in Africa: Pharmacology and Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 753–786. [Google Scholar]
- Arya, A.; Nyamathulla, S.; Noordin, M.I.; Mohd, M.A. Antioxidant and Hypoglycemic Activities of Leaf Extracts of Three Popular Terminalia Species. J. Chem. 2012, 9, 883–892. [Google Scholar]
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products—History and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Yosri, N.; Alsharif, S.M.; Xiao, J.; Musharraf, S.G.; Zhao, C.; Saeed, A.; Gao, R.; Said, N.S.; Di Minno, A.; Daglia, M.; et al. Arctium lappa (Burdock): Insights from ethnopharmacology potential, chemical constituents, clinical studies, pharmacological utility and nanomedicine. Biomed. Pharmacother. 2023, 158, 114104. [Google Scholar] [CrossRef]
- González-Castejón, M.; Visioli, F.; Rodriguez-Casado, A. Diverse biological activities of dandelion. Nutr. Rev. 2012, 70, 534–547. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Koryakina, L.P.; Vladimirov, L.N. Bitter Gentian Teas: Nutritional and Phytochemical Profiles, Polysaccharide Characterisation and Bioactivity. Molecules 2015, 20, 20014–20030. [Google Scholar] [CrossRef]
- Monmai, C.; Park, S.H.; You, S.; Park, W.J. Immuno-enhancement effect of polysaccharide extracted from Stichopus japonicus on cyclophosphamide-induced immunosuppression mice. Food Sci. Biotechnol. 2017, 27, 565–573. [Google Scholar] [CrossRef]
- Su, J.; Liu, X.; Li, H.; Cheng, X.; Shi, S.; Li, N.; Wu, J.; Xu, Y.; Liu, R.; Tian, X.; et al. Hypoglycaemic effect and mechanism of an RG-II type polysaccharide purified from Aconitum coreanum in diet-induced obese mice. Int. J. Biol. Macromol. 2020, 149, 359–370. [Google Scholar] [CrossRef]
- Patel, D.K.; Kumar, R.; Laloo, D.; Hemalatha, S. Diabetes mellitus: An overview on its pharmacological aspects and reported medicinal plants having antidiabetic activity. Asian Pac. J. Trop. Biomed. 2012, 2, 411–420. [Google Scholar] [CrossRef]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 Diabetes Mellitus: A Review of Multi-Target Drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- Akhtar, W.; Ali, G.; Ashraf, N.; Fatima, I.; Kayani, W.K.; Shaheen, H.; Ghoneim, M.M.; Abdelgawad, M.A.; Khames, A. Efficiency of Multiple Extraction Solvents on Antioxidant, Cytotoxic, and Phytotoxic Potential of Taraxacum officinale (L.) Weber ex F.H. Wigg. from Poonch Valley, Azad Kashmir, Pakistan. Evid.-Based Complement. Altern. Med. 2022, 2022, 5118553. [Google Scholar] [CrossRef]
- Moayyed, M.; Eftekharian, A.; Fereiduni, M.; Akbary, P. Research Article: Effect of solvent type on phytochemical properties of burdock (Arctium lappa) extract and their effect on some pathogenic bacteria strains in rainbow trout, Oncorhynchus mykiss. IJFS 2023, 22, 493–510. [Google Scholar]
- Zhang, Z.; Zhang, L.; Xu, H. Effect of Astragalus polysaccharide in treatment of diabetes mellitus: A narrative review. J. Tradit. Chin. Med. 2019, 39, 133–138. [Google Scholar] [PubMed]
- Hu, F.; Li, X.; Zhao, L.; Feng, S.; Wang, C. Antidiabetic properties of purified polysaccharide from Hedysarum polybotrys. Can. J. Physiol. Pharmacol. 2010, 88, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Birkeland, E.; Gharagozlian, S.; Birkeland, K.I.; Valeur, J.; Måge, I.; Rud, I.; Aas, A.M. Prebiotic effect of inulin-type fructans on faecal microbiota and short-chain fatty acids in type 2 diabetes: A randomised controlled trial. Eur. J. Nutr. 2020, 59, 3325–3338. [Google Scholar] [CrossRef] [PubMed]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological Function of Plant Tannin and Its Application in Animal Health. Front Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Shori, A.B. Inclusion of phenolic compounds from different medicinal plants to increase α-amylase inhibition activity and antioxidants in yogurt. J. Taibah Univ. Sci. 2020, 14, 1000–1008. [Google Scholar] [CrossRef]
- Ali, H.; Houghton, P.J.; Soumyanath, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Daou, M.; Elnaker, N.A.; Ochsenkühn, M.A.; Amin, S.A.; Yousef, A.F.; Yousef, L.F. In vitro α-glucosidase inhibitory activity of Tamarix nilotica shoot extracts and fractions. PLoS ONE 2022, 17, e0264969. [Google Scholar] [CrossRef]
- Ansari, P.; Flatt, P.R.; Harriott, P.; Abdel-Wahab, Y.H.A. Insulin secretory and antidiabetic actions of Heritiera fomes bark together with isolation of active phytomolecules. PLoS ONE 2022, 17, e0264632. [Google Scholar] [CrossRef]
- Doan, H.V.; Riyajan, S.; Iyara, R.; Chudapongse, N. Antidiabetic activity, glucose uptake stimulation and α-glucosidase inhibitory effect of Chrysophyllum cainito L. stem bark extract. BMC Complement. Altern. Med. 2018, 18, 267. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Oxidative Stress and Stress-Activated Signaling Pathways: A Unifying Hypothesis of Type 2 Diabetes. Endocr. Rev. 2002, 23, 599–622. [Google Scholar] [CrossRef]
- Caturano, A.; D’Angelo, M.; Mormone, A.; Russo, V.; Mollica, M.P.; Salvatore, T.; Galiero, R.; Rinaldi, L.; Vetrano, E.; Marfella, R.; et al. Oxidative Stress in Type 2 Diabetes: Impacts from Pathogenesis to Lifestyle Modifications. Curr. Issues Mol. Biol. 2023, 45, 6651–6666. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Sun, Y. Structure and biological activities of the polysaccharides from the leaves, roots and fruits of Panax ginseng C.A. Meyer: An overview. Carbohydr. Polym. 2011, 85, 490–499. [Google Scholar] [CrossRef]
- Ooi, V.E.; Liu, F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Yan, L.; Zhang, Q.; Zhu, J.; Huang, N.; Wang, Z. Chemical compositions and antioxidant activities of polysaccharides from the sporophores and cultured products of Armillaria mellea. Molecules 2015, 20, 5680–5697. [Google Scholar] [CrossRef]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863. [Google Scholar] [CrossRef]
- Wang, L.; Yang, H.; Huang, H.; Zhang, C.; Zuo, H.X.; Xu, P.; Niu, Y.M.; Wu, S.S. Inulin-type fructans supplementation improves glycemic control for the prediabetes and type 2 diabetes populations: Results from a GRADE-assessed systematic review and dose-response meta-analysis of 33 randomized controlled trials. J. Transl. Med. 2019, 17, 410. [Google Scholar] [CrossRef]
- Segar, H.M.; Gani, S.A.; Khayat, M.E.; Rahim, M.B.H.A. Antioxidant and Antidiabetic Properties of Pectin Extracted from Pomegranate (Punica granatum) Peel. J. Biochem. Microbiol. Biotechnol. 2023, 11, 35–40. [Google Scholar] [CrossRef]
- Lu, N.; Wei, J.; Gong, X.; Tang, X.; Zhang, X.; Xiang, W.; Liu, S.; Luo, C.; Wang, X. Preventive Effect of Arctium lappa Polysaccharides on Acute Lung Injury through Anti-Inflammatory and Antioxidant Activities. Nutrients 2023, 15, 4946. [Google Scholar] [CrossRef]
- Li, M.; Zhang, H.; Hu, X.; Liu, Y.; Liu, Y.; Song, M.; Wu, R.; Wu, J. Isolation of a New Polysaccharide from Dandelion Leaves and Evaluation of Its Antioxidant, Antibacterial, and Anticancer Activities. Molecules 2022, 27, 7641. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Ledesma, F.R.; Sánchez-Moreno, V.E.; Vera, E.; Ciobotă, V.; Jentzsch, P.V.; Jaramillo, L.I. Extraction of Inulin from Andean Plants: An Approach to Non-Traditional Crops of Ecuador. Molecules 2020, 25, 5067. [Google Scholar] [CrossRef] [PubMed]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and Characterization of Inulin-Type Fructans from Artichoke Wastes and Their Effect on the Growth of Intestinal Bacteria Associated with Health. BioMed. Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.; Jansen, O.; Oles, C.J.; Lazar, E.Z.; Rader, J.I. Precipitation of inulins and oligoglucoses by ethanol and other solvents. Food Chem. 2003, 81, 125–132. [Google Scholar] [CrossRef]
- Puupponen-Pimiä, R.; Nohynek, L.; Meier, C.; Kähkönen, M.; Heinonen, M.; Hopia, A.; Oksman-Caldentey, K.M. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001, 90, 494–507. [Google Scholar] [CrossRef]
- Vattem, D.A.; Ghaedian, R.; Shetty, K. Enhancing health benefits of berries through phenolic antioxidant enrichment: Focus on cranberry. Asia Pac. J. Clin. Nutr. 2005, 14, 120–130. [Google Scholar]
- Aba, P.E.; Asuzu, I.U. Mechanisms of actions of some bioactive anti-diabetic principles from phytochemicals of medicinal plants: A review. Indian J. Nat. Prod. Resour. 2018, 9, 85–96. [Google Scholar]
- Okuda, T. Systematics and Health Effects of Chemically Distinct Tannins in Medicinal Plants. Phytochemistry 2005, 66, 2012–2031. [Google Scholar] [CrossRef]
- Chung, K.T.; Wong, T.Y.; Wei, C.I.; Huang, Y.W.; Lin, Y. Tannins and human health: A review. Crit. Rev. Food Sci. Nutr. 1998, 38, 421–464. [Google Scholar] [CrossRef] [PubMed]
- Szczurek, A. Perspectives on Tannins. Biomolecules 2021, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.-J.; Liu, Y.-Z.; Yang, H.; Lin, Z.-W.; Sun, H.-D. Hydrolyzable Tannins and Related Polyphenols from Eucalyptus globulus. J. Asian Nat. Prod. Res. 2000, 2, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Singla, E.; Dharwal, V.; Naura, A.S. Gallic Acid Protects against the COPD-Linked Lung Inflammation and Emphysema in Mice. Inflamm. Res. 2020, 69, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Shree, A.; Islam, J.; Vafa, A.; Mohammad Afzal, S.; Sultana, S. Gallic Acid Prevents 1, 2-Dimethylhydrazine Induced Colon Inflammation, Toxicity, Mucin Depletion, and Goblet Cell Disintegration. Environ. Toxicol. 2020, 35, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Eddouks, M. The Promising Role of Plant Tannins as Bioactive Antidiabetic Agents. Curr. Med. Chem. 2019, 26, 4852–4884. [Google Scholar] [CrossRef]
- Antasionasti, I.; Datu, O.S.; Lestari, U.S.; Abdullah, S.S.; Jayanto, I. Correlation Analysis of Antioxidant Activities with Tannin, Total Flavonoid, and Total Phenolic Contents of Nutmeg (Myristica Fragrans Houtt) Fruit Precipitated by Egg White. Borneo J. Pharm. 2021, 4, 301–310. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Heidari, H.; Oroojan, A.A.; Mirzavandi, F.; Nasr Esfehani, K.; Dehghan Mohammadi, Z. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna J. Phytomed. 2017, 7, 169–179. [Google Scholar] [PubMed]
- Yuan, P.-C.; Shao, T.-L.; Han, J.; Liu, C.-Y.; Wang, G.-D.; He, S.-G.; Xu, S.-X.; Nian, S.-H.; Chen, K.-S. Burdock fructooligosaccharide as an α-glucosidase inhibitor and its antidiabetic effect on high-fat diet and streptozotocin-induced diabetic mice. J. Funct. Foods 2021, 86, 104703. [Google Scholar] [CrossRef]
- Kritsak, M.; Stechyshyn, I.; Pavliuk, B.; Konovalenko, S. Analysis of patients’ rehabilitation results after surgical treatment of diabetes complications. Pol. Merkur. Lek. 2021, 49, 269–272. [Google Scholar]
- Skyler, J.S.; Bakris, G.L.; Bonifacio, E.; Darsow, T.; Eckel, R.H.; Groop, L.; Groop, P.-H.; Handelsman, Y.; Insel, R.A.; Mathieu, C.; et al. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef]
- Reis, C.E.G.; Dórea, J.G.; da Costa, T.H.M. Effects of coffee consumption on glucose metabolism: A systematic review of clinical trials. J. Tradit. Complement. Med. 2018, 9, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Lecoultre, V.; Carrel, G.; Egli, L.; Binnert, C.; Boss, A.; MacMillan, E.L.; Kreis, R.; Boesch, C.; Darimont, C.; Tappy, L. Coffee consumption attenuates short-term fructose-induced liver insulin resistance in healthy men. Am. J. Clin. Nutr. 2014, 99, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, S.; Sarriá, B.; Mateos, R.; Bravo-Clemente, L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: Results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur. J. Nutr. 2019, 58, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Spínola, V.; Castilho, P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro). Phytochemistry 2017, 143, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Celik, S.; Erdogan, S.; Tuzcu, M. Caffeic acid phenethyl ester (CAPE) exhibits significant potential as an antidiabetic and liver-protective agent in streptozotocin-induced diabetic rats. Pharmacol. Res. 2009, 60, 270–276. [Google Scholar] [CrossRef]
- Chao, C.Y.; Mong, M.C.; Chan, K.C.; Yin, M.C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef]
- Bezerra, R.M.N.; Veiga, L.F.; Caetano, A.C.; Rosalen, P.L.; Amaral, M.E.C.; Palanch, A.C.; de Alencar, S.M. Caffeic acid phenethyl ester reduces the activation of the nuclear factor κB pathway by high-fat diet-induced obesity in mice. Metabolism 2012, 61, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Singh, S.V.; Jaiswal, K.; Kumar, R.; Pandey, A.K. Modulatory effect of caffeic acid in alleviating diabetes and associated complications. World J. Diabetes 2023, 14, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, Z.; Chang, C. Chlorogenic acid intake guidance: Sources, health benefits, and safety. Asia Pac. J. Clin. Nutr. 2022, 31, 602–610. [Google Scholar] [PubMed]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic acid improves late diabetes through adiponectin receptor signaling pathways in db/db mice. PLoS ONE 2015, 10, e0120842. [Google Scholar] [CrossRef] [PubMed]
- Yadikar, N.; Ahmet, A.; Zhu, J.; Bao, X.; Yang, X.; Han, H.; Rozi, P. Exploring the mechanism of citric acid for treating glucose metabolism disorder induced by hyperlipidemia. J. Food Biochem. 2022, 46, e14404. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, Q.; He, X.; Gao, X.; Wu, L.; Xiao, M.; Cai, W.; Liu, B.; Zeng, F. Antioxidant and anti-aging activities of Laminaria japonica polysaccharide in Caenorhabditis elegans based on metabonomic analysis. Int. J. Biol. Macromol. 2022, 221, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Muhamed, S.A.; Moussa, E.M.; Aboasy, N.K.; Gaweesh, Y.Y. Effect of 1% malic acid spray on diabetes mellitus-induced xerostomia: A randomized clinical trial. Oral Dis. 2024, 30, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Radhakrishnan, S.; Angayarkanni, N.; Sulochana, K.N. Antidiabetic effect of free amino acids supplementation in human visceral adipocytes through adiponectin-dependent mechanism. Indian J. Med. Res. 2019, 149, 41–46. [Google Scholar]
- Errichiello, F.; D’Amato, M.; Gambuti, A.; Moio, L.; Pastore, A.; Al-Hmadi, H.; Stornaiuolo, M.; Serino, E.; Taglialatela-Scafati, O.; Forino, M. Oleanolic acid: A promising antidiabetic metabolite detected in Aglianico grape pomace. J. Funct. Foods 2023, 104, 105548. [Google Scholar] [CrossRef]
- Harini, R.; Pugalendi, K.V. Antihyperglycemic effect of protocatechuic acid on streptozotocin-diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 79–91. [Google Scholar] [CrossRef]
- Comerford, K.B.; Pasin, G. Emerging Evidence for the Importance of Dietary Protein Source on Glucoregulatory Markers and Type 2 Diabetes: Different Effects of Dairy, Meat, Fish, Egg, and Plant Protein Foods. Nutrients 2016, 8, 446. [Google Scholar] [CrossRef]
- Najafi, F.; Mohseni, P.; Pasdar, Y.; Niknam, M.; Izadi, N. The association between dietary amino acid profile and the risk of type 2 diabetes: Ravansar non-communicable disease cohort study. BMC Public Health 2023, 23, 2284. [Google Scholar] [CrossRef] [PubMed]
- García-Carrasco, B.; Fernandez-Dacosta, R.; Dávalos, A.; Ordovás, J.M.; Rodriguez-Casado, A. In vitro Hypolipidemic and Antioxidant Effects of Leaf and Root Extracts of Taraxacum Officinale. Med. Sci. 2015, 3, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Kania-Dobrowolska, M.; Baraniak, J. Dandelion (Taraxacum officinale L.) as a Source of Biologically Active Compounds Supporting the Therapy of Co-Existing Diseases in Metabolic Syndrome. Foods 2022, 11, 2858. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal Plants; World Health Organization: Geneva, Switzerland, 2003; pp. 5–78.
- Ma, X.; Wu, H.; Liu, L.; Yao, Q.; Wang, S.; Zhan, R.; Xing, S.; Zhou, Y. Polyphenolic compounds and antioxidant properties in mango fruits. Sci. Hortic. 2011, 129, 102–107. [Google Scholar] [CrossRef]
- Maimulyanti, A.; Prihadi, A.R.; Mellisani, B.; Nurhidayati, I.; Putri FA, R.; Puspita, F.; Widarsih, R. Green Extraction Technique to Separate Tannin from Coffee Husk Waste Using Natural Deep Eutectic Solvent (Nades). Rasayan J. Chem. 2023, 16, 2002–2008. [Google Scholar] [CrossRef]
- Ćujić, N.; Šavikin, K.; Janković, T.; Pljevljakušić, D.; Zdunić, G.; Ibrić, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Cacique, A.P.; Barbosa, S.; de Pinho, G.P.; Silvério, F.O. Maceration extraction conditions for determining the phenolic compounds and the antioxidant activity of Catharanthus roseus (L.) G. Don. Ciênc. Agrotec. 2020, 44, e017420. [Google Scholar] [CrossRef]
- Kehili, M.; Sayadi, S.; Frikha, F.; Zammel, A.; Allouche, N. Optimization of lycopene extraction from tomato peels industrial by-product using maceration in refined olive oil. Food Bioprod. Process. 2019, 117, 321–328. [Google Scholar] [CrossRef]
- The Russian Federation Ministry of Health. State Pharmacopoeia of Russian Federation, XIV and II ed., Volume IV, 2018. Federal Electronic Medical Library. Available online: https://femb.ru/record/pharmacopea14 (accessed on 25 October 2023).
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.-P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant Activity of Plant Extracts Containing Phenolic Compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- European Council; European Directorate for the Quality of Medicines & HealthCare (EDQM). Tannins in Herbal Drugs. In European Pharmacopoeia, 8th ed.; European Council: Strasbourg, France, 2013; Chapter 2.8.14. [Google Scholar]
- Muniandy, P.; Shori, A.B.; Baba, A.S. Influence of green, white and black tea addition on the antioxidant activity of probiotic yogurt during refrigerated storage. Food Packag. Shelf Life 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Sekhon-Loodu, S.; Rupasinghe, H.P.V. Evaluation of Antioxidant, Antidiabetic and Antiobesity Potential of Selected Traditional Medicinal Plants. Front. Nutr. 2019, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Bashmil, Y.M.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. LC-MS/MS-QTOF Screening and Identification of Phenolic Compounds from Australian Grown Herbs and Their Antioxidant Potential. Antioxidants 2021, 10, 1770. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.N.; French, D.; Jannetto, P.J.; Rappold, B.A.; Clarke, W.A. Liquid chromatography-tandem mass spectrometry for clinical diagnostics. Nat. Rev. Methods Primers 2022, 2, 96. [Google Scholar] [CrossRef] [PubMed]


| Plant Sample | TPC (mg GSE/g of EA Extract), ± SE | TPC (mg GSE/g of LE Extract), ± SE |
|---|---|---|
| Burdock from “Viļani” (BV) | 21.23 ± 0.07 a | 100.97 ± 0.49 b |
| Burdock from “Būdiņas” (BB) | 14.36 ± 0.04 a | 69.73 ± 0.39 b |
| Dandelion from “Kaļķis” (DK) | 4.61 ± 0.03 a | 27.72 ± 0.57 b |
| Dandelion from “Vecpiebalga” (DV) | 4.51 ± 0.03 a | 26.35 ± 0.25 b |
| Plant Sample | DPPH IC50 (mg/L) for AE | DPPH IC50 (mg/L) for LE | DPPH IC50 (mg/L) for Trolox |
|---|---|---|---|
| Burdock from “Viļani” (BV) | 27.95 a | 1.33 b | 4.62 |
| Burdock from “Būdiņas” (BB) | 25.89 a | 0.77 b | |
| Dandelion from “Kaļķis” (DK) | 236.32 a | 9.52 b | |
| Dandelion from “Vecpiebalga” (DV) | 150.54 a | 7.00 b |
| Plant Sample | IC50 (mg/mL) for AE | IC50 (mg/mL) for LE | IC50 (mg/mL) for Acarbose |
|---|---|---|---|
| Burdock from “Viļani” (BV) | 135.09 a | 79.18 b | 18.86 |
| Burdock from “Būdiņas” (BB) | 106.67 a | 57.94 b | |
| Dandelion from “Kaļķis” (DK) | 205.35 a | 142.07 b | |
| Dandelion from “Vecpiebalga” (DV) | 450.11 a | 186.43 b |
| No. | tR (min) | Tentative Compound | Class/Type | Proposed Formula | Ion Mode | Measured m/z | Error (ppm) | MS/MS |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.52 | L-Histidine | Amino acid | C6H9N3O2 | [M+H]+ | 156.08 | 0.21 | 138.05; 110.02; 84.04; 73.03 |
| 2 | 0.52 | L-Lysine | Amino acid | C6H14N2O2 | [M+H]+ | 147.11 | 0.26 | 130.05; 101.07; 84.05 |
| 3 | 0.53 | Arginine | Amino acid | C6H14N4O2 | [M+H]+ | 175.12 | −0.65 | 158.09; 130.09; 116.07; 70.07 |
| 4 | 0.71 | (+)-Valine | Amino acid | C5H11NO2 | [M+H]+ | 118.09 | −0.08 | 72.08 |
| 5 | 0.94 | L-Tyrosine | Amino acid | C9H11NO3 | [M+H]+ | 182.08 | −0.13 | 165.05; 147.04; 136.08; 123.04 |
| 6 | 0.99 | L-Leucine/Isoleucine | Amino acid | C6H13NO2 | [M+H]+ | 132.10 | −0.09 | 116.03; 86.09; 69.07 |
| 7 | 1.59 | (±)-Phenylalanine | Amino acid | C9H11NO2 | [M+H]+ | 166.09 | −0.20 | 120.09 |
| 8 | 3.63 | L-Tryptophan | Amino acid | C11H12N2O2 | [M+H]+ | 205.10 | −0.21 | 188.07; 87.04 |
| 9 | 1.94 | Pantothenic acid | Amino acid and derivatives | C9H17NO5 | [M+H]+ | 220.12 | 0.00 | 202.11; 184.10; 90.06 |
| 10 | 0.62 | Caffeic acid | Phenylic acid | C9H8O4 | [M+H]+ | 181.05 | 0.20 | 163.04 |
| 11 | 5.28 | Neochlorogenic acid | Phenylic acid | C16H18O9 | [M+H]+ | 355.10 | −0.44 | 163.04 |
| 12 | 5.63 | Chlorogenic acid | Phenylic acid | C16H18O9 | [M+H]+ | 355.10 | −0.44 | 163.04 |
| 13 | 11.20 | 3,4-Dicaffeoylquinic acid | Phenylic acid | C25H24O12 | [M+H]+ | 517.13 | −0.39 | 163.04 |
| 14 | 11.54 | 3,5-Dicaffeoylquinic acid | Phenylic acid | C25H24O12 | [M+H]+ | 517.13 | −0.39 | 163.04 |
| 15 | 12.37 | 4,5-Dicaffeoylquinic acid | Phenylic acid | C25H24O12 | [M+H]+ | 517.13 | −0.15 | 163.04 |
| 16 | 0.62 | Trigonelline | Alkaloids | C7H7NO2 | [M+H]+ | 138.05 | 0.24 | 128.11 |
| 17 | 11.90 | Eremanthin | Guaianolides | C15H18O2 | [M+H]+ | 231.14 | −0.22 | 213.13; 185.13; 175.07 |
| 18 | 19.62 | Campholenic aldehyde | Monocyclic monoterpenoids | C10H16O | [M+H]+ | 153.13 | −0.06 | 135.12; 109.10; 107.09; 97.06 |
| 19 | 21.50 | Arctinone A | Oligothiophenes | C13H10O2S2 | [M+H]+ | 263.02 | −0.11 | 263.02; 245.01; 217.02; 205.02 |
| No. | tR (min) | Tentative Compound | Class/Type | Proposed Formula | Ion Mode | Measured m/z | Error (ppm) | MS/MS |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.59 | Disaccharide | Carbohydrates | C12H22O11 | [M-H]− | 341.11 | −0.81 | 179.05; 89.02; 59.01 |
| 2 | 0.68 | Malic acid | Hydroxy acid | C4H6O5 | [M-H]− | 133.01 | −0.33 | 133.01; 115.00; 71.01 |
| 3 | 1.51 | Galloyl glucose | Tannins | C13H16O10 | [M-H]− | 331.07 | 0.48 | 313.06; 168.01; 125.02 |
| 4 | 2.08 | Protocatechuic acid 4-glucoside | Phenolic glycosides | C13H16O9 | [M-H]− | 315.07 | 0.14 | 315.07; 153.02; 152.01; 109.03. 108.02 |
| 5 | 3.27 | 3-Methoxy-4-hydroxyphenylglycol glucuronide | Phenolic glycosides | C15H20O10 | [M-H]− | 359.10 | −0.97 | 197.05; 182.02; 153.06; 138.03 |
| 6 | 5.28 | Neochlorogenic acid | Phenylic acid | C16H18O9 | [M-H]− | 353.09 | −0.98 | 191.05 |
| 7 | 5.40 | n-Caffeoylquinic acid | Phenylic acid | C16H18O9 | [M-H]− | 353.09 | −1.07 | 191.05 |
| 8 | 5.76 | Chlorogenic acid | Phenylic acid | C16H18O9 | [M-H]− | 353.09 | −0.98 | 191.05 |
| 9 | 8.38 | p-coumaric acid | Phenylic acid | C9H8O3 | [M-H]− | 163.04 | 0.00 | 119.05 |
| 10 | 11.25 | 3,4-Dicaffeoylquinic acid | Phenylic acid | C25H24O12 | [M-H]− | 515.12 | −0.46 | 353.09; 191.06 |
| 11 | 11.57 | 3,5-Dicaffeoylquinic acid | Phenylic acid | C25H24O12 | [M-H]− | 515.12 | −0.70 | 353.09; 191.06 |
| 12 | 12.42 | 4,5-Dicaffeoylquinic acid | Phenylic acid | C25H24O12 | [M-H]− | 515.12 | −0.70 | 353.09; 191.06 |
| 13 | 15.16 | Caffeic acid ethyl ester | Coumaric acids and derivatives | C11H12O4 | [M-H]− | 207.07 | −0.79 | 208.07; 179.03; 161.02 |
| 14 | 6.46 | Protocatechuic acid | Benzoic acid and derivatives | C7H6O4 | [M-H]− | 153.02 | 0.12 | 153.02; 135.01; 109.03 |
| 15 | 4.28 | Salicylic acid | Benzoic acid and derivatives | C7H6O3 | [M-H]− | 137.02 | −0.04 | 93.03 |
| 16 | 4.76 | Salicylic acid glucoside | Benzoic acid and derivatives | C13H16O8 | [M-H]− | 299.08 | 0.00 | 137.02; 93.03 |
| 17 | 27.85 | Oleanolic acid | Pentacyclic triterpene | C30H48O3 | [M-H]− | 455.35 | −0.43 | 455.35 |
| No. | Tentative Compound | Class/Type | Plant Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LE of BB | AE of BB | LE of BV | AE of BV | LE of DK | AE of DK | LE of DV | AE of DV | |||
| 1 | L-histidine | Amino acid | X | X | X | X | n.d. | X | n.d. | n.d. |
| 2 | L-lysine | Amino acid | X | X | X | X | n.d. | n.d. | n.d. | X |
| 3 | Arginine | Amino acid | X | X | X | X | X | X | X | X |
| 4 | Caffeic acid | Phenylic acid | X | X | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 5 | Trigonelline | Alkaloids | X | X | X | n.d. | n.d. | n.d. | n.d. | n.d. |
| 6 | (+)-Valine | Amino acid | X | X | X | X | X | X | X | X |
| 7 | L-tyrosine | Amino acid | X | X | X | X | n.d. | X | n.d. | n.d. |
| 8 | L-leucine/isoleucine | Amino acid | X | X | X | X | n.d. | X | X | X |
| 9 | (±)-Phenylalanine | Amino acid | X | X | X | X | X | X | X | X |
| 10 | Pantothenic acid | Amino acid and derivatives | n.d. | n.d. | n.d. | X | n.d. | n.d. | n.d. | X |
| 11 | L-tryptophan | Amino acid | X | X | X | X | n.d. | X | X | X |
| 12 | Neochlorogenic acid | Phenylic acid | X | X | X | X | n.d. | X | X | X |
| 13 | Chlorogenic acid | Phenylic acid | X | X | X | X | n.d. | X | X | X |
| 14 | 3,4-Dicaffeoylquinic acid | Phenylic acid | X | n.d. | X | X | n.d. | n.d. | n.d. | n.d. |
| 15 | 3,5-Dicaffeoylquinic acid | Phenylic acid | X | X | X | X | n.d. | X | n.d. | X |
| 16 | Eremanthin | Guaianolides | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | X |
| 17 | 4,5-Dicaffeoylquinic acid | Phenylic acid | X | X | X | X | n.d. | X | n.d. | n.d. |
| 18 | Campholenic aldehyde | Monocyclic monoterpenoids | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | X |
| 19 | Arctinone A | Oligothiophenes | n.d. | X | n.d. | X | n.d. | n.d. | n.d. | n.d. |
| No. | Tentative Compound | Class/Type | Plant Sample | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LE of BB | AE of BB | LE of BV | AE of BV | LE of DK | AE of DK | LE of DV | AE of DV | |||
| 1 | Disaccharide | Carbohydrates | X | X | X | X | X | X | X | X |
| 2 | Malic acid | Hydroxy acid | n.d. | X | X | X | X | X | X | X |
| 3 | Galloyl glucose | Tannins | n.d. | X | n.d. | X | n.d. | X | n.d. | X |
| 4 | Protocatechuic acid 4-glucoside | Phenolic glycosides | n.d. | X | n.d. | X | X | X | X | X |
| 5 | 3-Methoxy-4-hydroxyphenylglycol glucuronide | Phenolic glycosides | n.d. | X | X | X | X | X | n.d. | X |
| 6 | Salicylic acid | Benzoic acid and derivatives | n.d. | X | n.d. | X | X | X | X | X |
| 7 | Salicylic acid glucoside | Benzoic acid and derivatives | n.d. | n.d. | n.d. | n.d. | n.d. | X | n.d. | X |
| 8 | Neochlorogenic acid | Phenylic acid | X | X | X | X | X | X | X | X |
| 9 | n-Caffeoylquinic acid | Phenylic acid | X | X | X | X | X | X | X | X |
| 10 | Chlorogenic acid | Phenylic acid | X | X | X | X | X | X | X | X |
| 11 | Protocatechuic acid | Benzoic acid and derivatives | n.d. | n.d. | n.d. | n.d. | n.d. | X | n.d. | n.d. |
| 12 | p-coumaric acid | Phenylic acid | n.d. | X | n.d. | X | n.d. | n.d. | n.d. | n.d. |
| 13 | 3,4-Dicaffeoylquinic acid | Phenylic acid | X | X | X | X | n.d. | n.d. | X | X |
| 14 | 3,5-Dicaffeoylquinic acid | Phenylic acid | X | X | X | X | X | X | X | X |
| 15 | 4,5-Dicaffeoylquinic acid | Phenylic acid | X | X | X | X | X | X | X | X |
| 16 | Caffeic acid ethyl ester | Coumaric acids and derivatives | n.d. | n.d. | n.d. | X | n.d. | n.d. | n.d. | n.d. |
| 17 | Oleanolic acid | Pentacyclic triterpene | X | X | n.d. | X | n.d. | n.d. | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolotova, D.; Teterovska, R.; Bandere, D.; Lauberte, L.; Niedra, S. Antidiabetic Properties of the Root Extracts of Dandelion (Taraxacum officinale) and Burdock (Arctium lappa). Plants 2024, 13, 1021. https://doi.org/10.3390/plants13071021
Zolotova D, Teterovska R, Bandere D, Lauberte L, Niedra S. Antidiabetic Properties of the Root Extracts of Dandelion (Taraxacum officinale) and Burdock (Arctium lappa). Plants. 2024; 13(7):1021. https://doi.org/10.3390/plants13071021
Chicago/Turabian StyleZolotova, Daria, Renāte Teterovska, Dace Bandere, Liga Lauberte, and Santa Niedra. 2024. "Antidiabetic Properties of the Root Extracts of Dandelion (Taraxacum officinale) and Burdock (Arctium lappa)" Plants 13, no. 7: 1021. https://doi.org/10.3390/plants13071021
APA StyleZolotova, D., Teterovska, R., Bandere, D., Lauberte, L., & Niedra, S. (2024). Antidiabetic Properties of the Root Extracts of Dandelion (Taraxacum officinale) and Burdock (Arctium lappa). Plants, 13(7), 1021. https://doi.org/10.3390/plants13071021






