Climate-Affected Australian Tropical Montane Cloud Forest Plants: Metabolomic Profiles, Isolated Phytochemicals, and Bioactivities
Abstract
1. Introduction
2. Climate-Affected Australian Tropical Montane Cloud Forest Plants and Their Medicinal Uses
| Botanical Name, Family, and Synonyms | Distribution | Life Form | Medicinal Uses | Metabolomics Profile Studied | Conservation Status (QLD) |
|---|---|---|---|---|---|
| Pteridophyta | |||||
| Dryopteridaceae | |||||
| Parapolystichum grayi (D.J.Jones) J.J.S. Gardner & Nagalingum Syn. Lastreopsis grayi D.L.Jones | Africa, the Neotropics, north-eastern Australia, Madagascar, Pacific Island, and southern Asia | Fern | NU | No | V |
| Parapolystichum tinarooense (Tindale) Labiak, Sundue & R.C.Moran Syn. Lastreopsis tinarooensis Tindale | Wet Tropics region (Australia) | Fern | NU | No | V |
| Hymenophyllaceae | |||||
| Hymenophyllum whitei Goy | Wet Tropics region (Australia) | Fern | NU | No | CR |
| Lindsaeaceae | |||||
| Lindsaea terrae-reginae K.U.Kramer | Wet Tropics region (Australia) | Fern | NU | No | E |
| Lycopodiaceae | |||||
| Phlegmariurus creber (Alderw.) A.R.Field & Bostock Syn. Huperzia crebra (Alderw.) Holub | Wet Tropics region (Australia), PNG, Hawaii | Epiphyte | Phlegmariurus/Huperzia species are traditionally used as vermifuge, purgative, and laxative [47]. | No | CR |
| Phlegmariurus delbrueckii (Herter) A.R.Field & Bostock Syn. Huperzia delbrueckii (Herter) Holub | Wet Tropics region (Australia) | Epiphyte | No | V | |
| Polypodiaceae | |||||
| Oreogrammitis albosetosa (F.M.Bailey) Parris Syn. Polypodium albosetosum F. M.Bailey | Wet Tropics region (Australia) | Fern | NU | No | V |
| Oreogrammitis leonardii (Parris) Parris Syn. Grammitis leonardii Parris | Wet Tropics region (Australia) | Fern | NU | No | V |
| Oreogrammitis reinwardtii Blume | Wet Tropics region (Australia), Sri Lanka, Philippines, Papua New Guinea, Solomon Islands, Malaysia | Fern | NU | No | V |
| Oreogrammitis wurunuran (Parris) Parris Syn. Grammitis wurunuran Parris | Wet Tropics region (Australia) | Fern | NU | No | SL |
| Magnoliophyta | |||||
| Apiaceae | |||||
| Trachymene geraniifolia F.M.Bailey | Wet Tropics region (Australia) | Herb | NU | No | NT |
| Apocynaceae | |||||
| Parsonsia bartlensis J.B.Williams | Wet Tropics region (Australia) | Climber | NU | No | V |
| Araliaceae | |||||
| Hydrocotyle miranda A.R.Bean & Henwood | Wet Tropics region (Australia) | Herb | Hydrocotyle species are used as anti-inflammatory herbs in Taiwanese folk medicines [48]. | No | V |
| Polyscias bellendenkerensis (F.M.Bailey) Philipson | Wet Tropics region (Australia) | Shrub | Polyscias species are traditionally used to treat ailments, such as malaria, obesity, and mental disorders [49]. | No | V |
| Polyscias willmottii (F.Muell.) Philipson | Wet Tropics region (Australia) | Tree | No | LC | |
| Araucariaceae | |||||
| Agathis atropurpurea B.Hyland | Australia | Tree | Agathis species are traditionally used to treat myalgia and headaches [50]. | Yes | LC |
| Arecaceae | |||||
| Linospadix apetiolatus Dowe & A.K.Irivine | Wet Tropics region (Australia) | Tree | NU | No | LC |
| Celastraceae | |||||
| Hypsophila halleyana F.Muell. | Wet Tropics region (Australia) | Shrub | NU | No | LC |
| Clusiaceae | |||||
| Garcinia brassii C.T.White | Wet Tropics region (Australia) | Tree | Infusions prepared from fruits of Garcinia species are traditionally used to treat dysentery, ulcers, and wounds [51]. | No | LC |
| Cunoniaceae | |||||
| Ceratopetalum corymbosum C.T.White | Wet Tropics region (Australia) | Tree | NU | No | V |
| Ceratopetalum hylandii Rozefelds & R.W.Barnes | Wet Tropics region (Australia) | Tree | NU | No | LC |
| Eucryphia wilkiei B.Hyland | Wet Tropics region (Australia) | Shrub | NU | Yes | CR |
| Ebenaceae | |||||
| Diospyros granitica Jessup | Wet Tropics region (Australia) | Tree | Diospyros species are used traditionally used as sedative, astringent, carminative, febrifuge, anti-hypertensive, vermifuge, antidiuretic, and to relieve constipation [52]. | No | NT |
| Elaeocarpaceae | |||||
| Elaeocarpus linsmithii Guymer | Wet Tropics region (Australia) | Tree | Elaeocarpus species are the source of popular spiritual beads (known as Rudraksha in Asia), which are used to treat various ailments, including mental/neurological disorders (stress, depression, anxiety, hypertension, epilepsy, migraine, and neuralgia), asthma, and also used as analgesic [53]. | No | LC |
| Elaeocarpus hylobroma Y.Baba & Crayn | Wet Tropics region (Australia) | Tree | No | LC | |
| Ericaceae | |||||
| Acrotriche baileyana (Domin) J.M.Powell | Wet Tropics region (Australia) | Shrub | NU | No | NT |
| Dracophyllum sayeri F.Muell | Wet Tropics region (Australia) | Tree | NU | No | V |
| Leucopogon malayanus subsp. novoguineensis (Sleumer) Pedley Syn. Styphelia malayana subsp. novoguineensis (Sleumer) Hislop, Crayn & Puente-Lel. | Wet Tropics region (Australia) | Shrub | NU | No | No |
| Rhododendron lochiae F.Muell. Syn. Rhododendron notiale, Craven | Wet Tropics region (Australia) | Shrub | Rhododendron species are used to prevent and treat many ailments, including respiratory disorders like asthma and bronchitis, dysentery, diarrhea, constipation, fever, cardiac disorders, and inflammation [54]. | No | No |
| Rhododendron viriosum Craven | Wet Tropics region (Australia) | Tree | No | LC | |
| Trochocarpa bellendenkerensis Domin | Wet Tropics region (Australia) | Tree | NU | No | LC |
| Escalloniaceae | |||||
| Polyosma reducta F.Muell. | Wet Tropics region (Australia) | Tree | NU | No | LC |
| Gesneriaceae | |||||
| Boea kinneari (F.Muell.) B.L.Burtt | Wet Tropics region (Australia) | Herb | NU | No | E |
| Lenbrassia australiana (C.T.White) G.W.Gillett | Wet Tropics region (Australia) | Shrub | NU | No | SL |
| Lamiaceae | |||||
| Prostanthera albohirta C.T.White | Mount Emerald, Wet Tropics region (Australia) | Shrub | Some Prostanthera species are used for topical applications to treat skin sores and infections [55,56]. | No | CR |
| Prostanthera athertoniana B.J.Conn & T.C.Wilson | Wet Tropics region (Australia) | Shrub | No | CR | |
| Lauraceae | |||||
| Cinnamomum propinquum F.M.Bailey | Wet Tropics region (Australia) | Tree | Cinnamomum species are most commonly used in traditional Chinese medicines to treat multiple disorders, including indigestion, microbial infections, and cough and cold [57]. | Yes | V |
| Cryptocarya bellendenkerana B.Hyland | Wet Tropics region (Australia) | Tree | NU | Yes | LC |
| Endiandra jonesii B.Hyland | Wet Tropics region (Australia) | Tree | Endiandra species are traditionally used to treat rheumatism, headache, dysentery, pulmonary disorders, and uterine tumours [58]. | No | V |
| Litsea granitica B.Hyland | Wet Tropics region (Australia) | Tree | Litsea species are used traditionally by Aboriginal communities to treat skin infections such as sores and scabies, and also used an antiseptic [59]. | No | V |
| Myrtaceae | |||||
| Leptospermum wooroonooran F.M.Bailey | Wet Tropics region (Australia) | Tree | Leptospermum species are traditionally used in Malaysia to relieve menstrual and stomach disorders [60,61]. | Yes | LC |
| Micromyrtus delicata A.R.Bean | Wet Tropics region (Australia) | Shrub | NU | No | E |
| Pilidiostigma sessile N.Snow | Wet Tropics region (Australia) | Shrub | NU | No | LC |
| Rhodamnia longisepala N.Snow & A.J.Ford | Wet Tropics region (Australia) | Shrub | Rhodamnia species are used traditionally in Indonesia to treat scars, toothache, and cough [62]. | No | CR |
| Syzygium fratris Craven | Wet Tropic region (Australia) | Shrub | NU | No | CR |
| Uromyrtus metrosideros (F.M.Bailey) A.J.Scott | Wet Tropics region (Australia) | Shrub | NU | Yes | LC |
| Orchidaceae | |||||
| Bulbophyllum lilianiae Rendle | Wet Tropics region (Australia) | Epiphyte | Bulbophyllum species are traditionally used to treat skin diseases, cardiovascular diseases, and rheumatism [63]. | No | LC |
| Bulbophyllum wadsworthii Dockrill Syn. Oxysepala wadsworthii (Dockrill) D.L.Jones & M.A.Clem. | Australia | Epiphyte | No | SL | |
| Bulbophyllum windsorense B.Gray & D.L.Jones Syn. Oxysepala windsorensis (B.Gray & D.L.Jones) D.L.Jones & M.A.Clem. | Wet Tropics region (Australia) | Epiphyte | No | V | |
| Dendrobium brevicaudum D.L.Jones & M.A.Clem. Syn. Dockrillia brevicauda (D.L.Jones & M.A.Clem.) M.A.Clem. & D.L.Jones | Wet Tropics region (Australia) | Herb, Epiphyte | Dendrobium species are used in traditional Chinese and Indian medicine systems as a source of tonic for longevity and also as an antipyretic, analgesic, astringent, and anti-inflammatory agent [64]. | No | No |
| Dendrobium carrii Rupp & C.T.White Syn. Australorchis carrii (Rupp & C.T.White) D.L.Jones & M.A.Clem. | Wet Tropics region (Australia) | Herb, Epiphyte | No | SL | |
| Dendrobium finniganense D.L.Jones Syn. Thelychiton finniganensis (D.L.Jones) M.A.Clem. & D.L.Jones | Wet Tropics region (Australia) | Herb, Epiphyte | No | SL | |
| Liparis fleckeri Nicholls | Wet Tropics region (Australia) | Lithophyte | Liparis species are traditionally used in Chinese medicine to treat inflammatory diseases, including haemoptysis, metrorrhagia, traumatic haemorrhage, and pneumonia; they are also used to stop bleeding from wounds and to detoxify snakebite [65]. | No | No |
| Octarrhena pusilla (F.M.Bailey) M.A.Clem. & D.L.Jones Syn. Octarrhena pusilla (F.M.Bailey) Dockrill | Wet Tropics region (Australia) | Epiphyte | NU | No | SL |
| Piperaceae | |||||
| Peperomia hunteriana P.I.Forst. | Wet Tropics region (Australia) | Herb | Peperomia species are traditionally used for treating pain and inflammation, gastric ulcers, asthma, and bacterial infections [66,67]. | No | LC |
| Podocarpaceae | |||||
| Prumnopitys ladei (F.M.Bailey) de Laub Syn. Stachycarpus ladei (Bailey) Gaussen, Podocarpus ladei F.M.Bailey | Endemic to Wet Tropics Australia | Tree | Fruits and bark of Prunmnopitys species are considered medicinal [68]. | Yes | No |
| Proteaceae | |||||
| Austromuellera valida B.Hyland | Endemic to Wet Tropics region | Tree | NU | No | V |
| Helicia lewisensis Foreman | Endemic to Wet Tropics region | Tree | Helicia species are used for treating mouth and skin sores and also kidney and gastric problems [59,69,70,71]. | No | V |
| Helicia recurva Foreman | Endemic to Wet Tropics region | Tree | No | No | |
| Hollandaea porphyrocarpa A.J.Ford & P.H.Weston Syn. Hollandaea sp. Pinnacle Rock Track (P.I.Forster PIF10714) | Endemic to Wet Tropics region | Shrub | NU | No | CR |
| Nothorites megacarpus (A.S.George & B.Hyland) P.H.Weston & A.R.Mast Syn. Orites megacarpa A.S.George & B.Hyland | Endemic to Wet Tropics region | Tree | NU | No | LC |
| Rubiaceae | |||||
| Aidia gyropetala A.J.Ford and Halford | Endemic to Wet Tropics region | Tree | Aidia species are used for treating body/muscle pains and pains due to gastric disorders [72]. | No | LC |
| Gynochthodes constipata (Halford & A.J.Ford) Razafim. & B.Bremer Syn. Morinda constipata Halford & A.J.Ford | Endemic to Wet Tropics region | Climber | Gynochthodes/Morinda species are traditionally used for treating diabetes, inflammation, cancer, psychiatric disorders, and microbial infections [73]. | No | LC |
| Gynochthodes podistra (Halford & A.J.Ford) Razafim. & B.Bremer Syn. Morinda podistra Halford & A.J.Ford | Endemic to Wet Tropics region | Climber | No | LC | |
| Ixora orophila C.T.White Syn. Psydrax montigena S.T.Reynolds & R.J.F.Hend. | Endemic to Wet Tropics region | Shrub | Ixora species are used in Ayurvedic medicine against leucorrhoea, hypertension, menstrual irregularities, sprains, bronchitis fever, sores, chronic ulcers, scabies, and skin diseases [74]. | No | No |
| Wendlandia connata C.T.White | Endemic to Wet Tropics region | Shrub | Wendlandia species are traditionally used for treating fever, dysentery, cough, hypertension, diabetes, constipation, inflammations, and hyperlipidemia [75]. | No | NT |
| Rutaceae | |||||
| Flindersia oppositifolia (F.Muell.) T.G.Hartley & Jessup | Wet Tropics region (Australia) | Tree | NU | Yes | V |
| Leionema ellipticum Paul G. Wilson | Endemic to Wet Tropics region | Shrub | NU | Yes | V |
| Zieria alata Duretto & P.I.Forst. | Endemic to Wet Tropics region | Shrub | NU | No | CR |
| Zieria madida Duretto & P.I.Forst. | Endemic to Wet Tropics region | Shrub | NU | No | CR |
| Santalaceae | |||||
| Korthalsella grayi Barlow | Endemic to Wet Tropics region | Herb | No | LC | |
| Sapindaceae | |||||
| Mischocarpus montanus C.T.White Syn. Mischocarpus pyriformis subsp. retusus (Radlk.) R.W.Ham, Mischocarpus retusus Radlk. | Wet Tropics region (Australia), New Guinea | Tree | NU | No | LC |
| Sapotaceae | |||||
| Pleioluma singuliflora (C.T.White & W.D.Francis) Swenson Syn. Planchonella singuliflora (C.T.White & W.D.Francis) P.Royen, Pouteria singuliflora (C.T.White & W.D.Francis) Baehni | Endemic to Wet Tropic region | Shrub | NU | No | LC |
| Sersalisia sessiliflora (C.T.White) Aubrév. Syn. Pouteria sylvatica Baehni, Lucuma sessiliflora C.T.White | Endemic to Wet Tropics region | Tree | NU | No | LC |
| Planchonella sp. Mt. Lewis (B.Hyland 14048) Qld Herbarium | Endemic to Wet Tropics region | Tree | Planchonella species have been used by Aboriginal medicine system to treat sores/sore throat and as an antiseptic for boils [59]. | No | No |
| Solanaceae | |||||
| Solanum dimorphispinum C.T.White | Endemic to Wet Tropics region | Shrub | Solanum species have been traditionally used against infectious diseases and also as anti-microbial agents and insecticidal against mosquitoes [76]. | No | LC |
| Solanum eminens A.R.Bean | Endemic to Wet Tropics region | Climber | No | LC | |
| Symplocaceae | |||||
| Symplocos bullata Jessup Syn. Symplocos sp. North Mary (B. Gray 2543) | Endemic to Wet Tropics region | Shrub | Symplocos species are traditionally known for treating diseases such as malaria, ulcers, leprosy, leucorrhea, menorrhagia, and gynecological disorders [77]. | No | LC |
| Symplocos graniticola Jessup | Endemic to Wet Tropics region | Shrub | No | V | |
| Symplocos oresbia Jessup Syn. Symplocos sp. Mt Finnigan (L.J. Brass 20129) | Endemic to Wet Tropics region | Shrub | No | NT | |
| Symplocos wooroonooran Jessup Syn. Symplocos stawellii var. montana C.T.White, Symplocos cochinchinensis var. montana (C.T.White) Noot | Endemic to Wet Tropics region | Shrub | No | NT | |
| Thymelaeaceae | |||||
| Phaleria biflora (C.T.White) Herber Syn. Oreodendron biflorum C.T.White | Endemic to Wet Tropics region | Tree | Phaleria species are used for treating stomachache, general pain, diarrhea, lowering glucose/cholesterol levels in blood, and also known for anti-cancer properties [78]. | No | V |
| Winteraceae | |||||
| Bubbia whiteana A.C.Sm. Syn. Zygogynum semecarpoides var. whiteanum Vink, Bubbia semecarpoides var. whiteana Vink | Endemic to Wet Tropics region | Shrub | NU | No | CR |
| Tasmannia sp. Mt Bellenden Ker (J.R.Clarkson 6571) | Wet Tropics region (Australia) | Shrub | Tasmania species are traditionally used for treating malaria, diarrhea, and cough [79]. | No | LC |
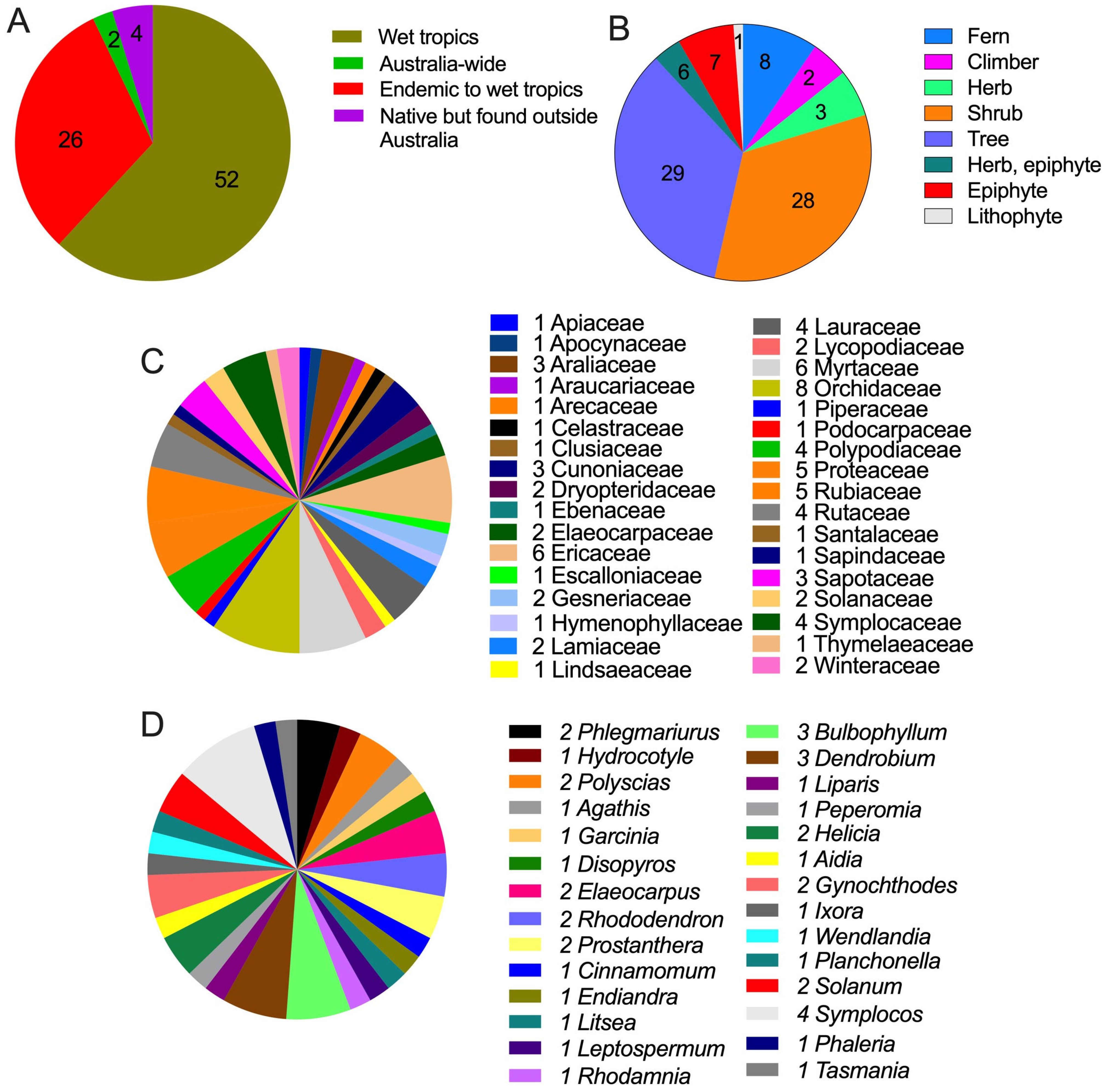

3. Metabolomic Profile of Climate-Affected Plants in WTWHA
4. Metabolomics Approaches, Tools, and Techniques Used in Plant Metabolomics

5. Phytochemicals Isolated from Climate-Affected Plants in WTWHA
6. Pharmacological Activities of Isolated Phytochemicals of Climate-Affected Plants in WTWHA
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnell, N.W.; Lowe, J.A.; Challinor, A.J.; Osborn, T.J. Global and regional impacts of climate change at different levels of global temperature increase. Clim. Chang. 2019, 155, 377–391. [Google Scholar] [CrossRef]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef] [PubMed]
- QLD. Climate Change in the Far North Queensland Region; Queensland Government: Queensland, Australia, 2019.
- Morris, R.J. Anthropogenic impacts on tropical forest biodiversity: A network structure and ecosystem functioning perspective. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Hoyle, G.L.; Sommerville, K.D.; Liyanage, G.S.; Worboys, S.; Guja, L.K.; Stevens, A.V.; Crayn, D.M. Seed banking is more applicable to the preservation of tropical montane flora than previously assumed: A review and cloud forest case study. Glob. Ecol. Conserv. 2023, 47, e02627. [Google Scholar] [CrossRef]
- Helmer, E.H.; Gerson, E.A.; Baggett, L.S.; Bird, B.J.; Ruzycki, T.S.; Voggesser, S.M. Neotropical cloud forests and páramo to contract and dry from declines in cloud immersion and frost. PLoS ONE 2019, 14, e0213155. [Google Scholar] [CrossRef] [PubMed]
- Costion, C.M.; Simpson, L.; Pert, P.L.; Carlsen, M.M.; John Kress, W.; Crayn, D. Will tropical mountaintop plant species survive climate change? Identifying key knowledge gaps using species distribution modelling in Australia. Biol. Conserv. 2015, 191, 322–330. [Google Scholar] [CrossRef]
- Karger, D.N.; Kessler, M.; Lehnert, M.; Jetz, W. Limited protection and ongoing loss of tropical cloud forest biodiversity and ecosystems worldwide. Nat. Ecol. Evol. 2021, 5, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Still, C.J.; Foster, P.N.; Schneider, S.H. Simulating the effects of climate change on tropical montane cloud forests. Nature 1999, 398, 608–610. [Google Scholar] [CrossRef]
- Foster, P. The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Sci. Rev. 2001, 55, 73–106. [Google Scholar] [CrossRef]
- Hu, J.; Riveros-Iregui, D.A. Life in the clouds: Are tropical montane cloud forests responding to changes in climate? Oecologia 2016, 180, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.E.; Bolitho, E.E.; Fox, S. Climate change in Australian tropical rainforests: An impending environmental catastrophe. Proc. R. Soc. B Biol. Sci. 2003, 270, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Le Saout, S.; Hoffmann, M.; Shi, Y.; Hughes, A.; Bernard, C.; Brooks, T.M.; Bertzky, B.; Butchart, S.H.; Stuart, S.N.; Badman, T.; et al. Conservation. Protected areas and effective biodiversity conservation. Science 2013, 342, 803–805. [Google Scholar] [CrossRef] [PubMed]
- UNESCO World Heritage Convention. Wet Tropics of Queensland. Available online: https://whc.unesco.org/en/list/486/ (accessed on 6 August 2023).
- Weber, E.T.; Catterall, C.P.; Locke, J.; Ota, L.S.; Prideaux, B.; Shirreffs, L.; Talbot, L.; Gordon, I.J. Managing a World Heritage Site in the Face of Climate Change: A Case Study of the Wet Tropics in Northern Queensland. Earth 2021, 2, 248–271. [Google Scholar] [CrossRef]
- Grossmann, G.; Krebs, M.; Maizel, A.; Stahl, Y.; Vermeer, J.E.M.; Ott, T. Green light for quantitative live-cell imaging in plants. J. Cell Sci. 2018, 131, 209270. [Google Scholar] [CrossRef]
- Awlia, M.; Alshareef, N.; Saber, N.; Korte, A.; Oakey, H.; Panzarová, K.; Trtílek, M.; Negrão, S.; Tester, M.; Julkowska, M.M. Genetic mapping of the early responses to salt stress in Arabidopsis thaliana. Plant J. 2021, 107, 544–563. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.S.; Brown, J.L.; Weber, J.J. An examination of climate-driven flowering-time shifts at large spatial scales over 153 years in a common weedy annual. Am. J. Bot. 2019, 106, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Cortijo, S.; Charoensawan, V.; Brestovitsky, A.; Buning, R.; Ravarani, C.; Rhodes, D.; van Noort, J.; Jaeger, K.E.; Wigge, P.A. Transcriptional regulation of the ambient temperature response by H2A. Z nucleosomes and HSF1 transcription factors in Arabidopsis. Mol. Plant 2017, 10, 1258–1273. [Google Scholar] [CrossRef] [PubMed]
- Sriden, N.; Charoensawan, V. Large-scale comparative transcriptomic analysis of temperature-responsive genes in Arabidopsis thaliana. Plant Mol. Biol. 2022, 110, 425–443. [Google Scholar] [CrossRef]
- Zhao, Y.; Antoniou-Kourounioti, R.L.; Calder, G.; Dean, C.; Howard, M. Temperature-dependent growth contributes to long-term cold sensing. Nature 2020, 583, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, R.; Xiang, C.; Zhang, R.; Wang, Q.; Wang, T.; Li, X.; Lu, X.; Gao, S.; Liu, Z.; et al. Transcriptomic and Physiological Analysis Reveal That alpha-Linolenic Acid Biosynthesis Responds to Early Chilling Tolerance in Pumpkin Rootstock Varieties. Front. Plant Sci. 2021, 12, 669565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.Y.; Li, C.X.; He, Y.; Hou, X.Y.; Ma, X.R. The Regulation of Adaptation to Cold and Drought Stresses in Poa crymophila Keng Revealed by Integrative Transcriptomics and Metabolomics Analysis. Front. Plant Sci. 2021, 12, 631117. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Alseekh, S.; Fernie, A.R. Plant secondary metabolic responses to global climate change: A meta-analysis in medicinal and aromatic plants. Glob. Chang. Biol. 2023, 29, 477–504. [Google Scholar] [CrossRef] [PubMed]
- Hodges, M.; Dellero, Y.; Keech, O.; Betti, M.; Raghavendra, A.S.; Sage, R.; Zhu, X.-G.; Allen, D.K.; Weber, A.P. Perspectives for a better understanding of the metabolic integration of photorespiration within a complex plant primary metabolism network. J. Exp. Bot. 2016, 67, 3015–3026. [Google Scholar] [CrossRef] [PubMed]
- Ncube, B.; Van Staden, J. Tilting plant metabolism for improved metabolite biosynthesis and enhanced human benefit. Molecules 2015, 20, 12698–12731. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Shahzad, R.; Gul, A.; Subthain, H.; Shen, S.; Lei, L.; Zheng, Z.; Zhou, J.; Lu, D.; Wang, S. Differentially evolved glucosyltransferases determine natural variation of rice flavone accumulation and UV-tolerance. Nat. Commun. 2017, 8, 1975. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Fernie, A.R. Leveraging natural variance towards enhanced understanding of phytochemical sunscreens. Trends Plant Sci. 2017, 22, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Boncan, D.A.T.; Tsang, S.S.; Li, C.; Lee, I.H.; Lam, H.-M.; Chan, T.-F.; Hui, J.H. Terpenes and terpenoids in plants: Interactions with environment and insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, H.N.; Rau, M.R.; Fett-Neto, A.G. Oxidative stress and production of bioactive monoterpene indole alkaloids: Biotechnological implications. Biotechnol. Lett. 2014, 36, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, M.; Rasmann, S. Variation in below-to aboveground systemic induction of glucosinolates mediates plant fitness consequences under herbivore attack. J. Chem. Ecol. 2020, 46, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Gargallo-Garriga, A.; Urban, O.; Klem, K.; Walker, T.W.N.; Holub, P.; Janssens, I.A.; Peñuelas, J. Ecometabolomics for a Better Understanding of Plant Responses and Acclimation to Abiotic Factors Linked to Global Change. Metabolites 2020, 10, 239. [Google Scholar] [CrossRef] [PubMed]
- Ma, A.; Qi, X. Mining plant metabolomes: Methods, applications, and perspectives. Plant Commun. 2021, 2, 100238. [Google Scholar] [CrossRef]
- Oh, S.-W.; Imran, M.; Kim, E.-H.; Park, S.-Y.; Lee, S.-G.; Park, H.-M.; Jung, J.-W.; Ryu, T.-H. Approach strategies and application of metabolomics to biotechnology in plants. Front. Plant Sci. 2023, 14, 1192235. [Google Scholar] [CrossRef] [PubMed]
- Colin, L.; Martin-Arevalillo, R.; Bovio, S.; Bauer, A.; Vernoux, T.; Caillaud, M.-C.; Landrein, B.; Jaillais, Y. Imaging the living plant cell: From probes to quantification. Plant Cell 2021, 34, 247–272. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.-S.; Huang, J.-Y. Bioimaging tools move plant physiology studies forward. Front. Plant Sci. 2022, 13, 976627. [Google Scholar] [CrossRef] [PubMed]
- Uslu, V.V.; Grossmann, G. The biosensor toolbox for plant developmental biology. Curr. Opin. Plant Biol. 2016, 29, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Bona, E.; Glick, B.R. Current Techniques to Study Beneficial Plant-Microbe Interactions. Microorganisms 2022, 10, 1380. [Google Scholar] [CrossRef]
- Belbin, L.; Wallis, E.; Hobern, D.; Zerger, A. The Atlas of Living Australia: History, current state and future directions. Biodivers. Data J. 2021, 9, e65023. [Google Scholar] [CrossRef] [PubMed]
- Zich, F.A.; Hyland, B.P.M.; Whiffin, T.; Kerrigan, R.A. Australian Tropical Rainforest Plants, 8th ed.; CSIRO: Canberra, Australia, 2020.
- Crayn, D.; Worboys, S. Personal communication, Australian Tropical Herbarium, James Cook University, Cairns, Australia, 2023.
- APC. Australian Plant Census IBIS database, Centre for Australian National Biodiversity Research, Council of Heads of Australasian Herbaria. 2024. Available online: https://www.anbg.gov.au/cpbr/program/hc/hc-APC.html (accessed on 17 December 2023).
- WFO. World Flora Online. 2023. Available online: http://www.worldfloraonline.org (accessed on 15 December 2023).
- CSIRO. CSIRO Annual Report 2010-11; ACT: Sydney, Australia, 2011; pp. 1–176.
- Roeble, E. Modelling the Vulnerability of Endemic Montane Flora to Climate Change in the Australian Wet Tropics. Ph.D. Thesis, Imperial College, London, UK, 2018. [Google Scholar]
- Armijos, C.; Gilardoni, G.; Amay, L.; Lozano, A.; Bracco, F.; Ramirez, J.; Bec, N.; Larroque, C.; Finzi, P.V.; Vidari, G. Phytochemical and ethnomedicinal study of Huperzia species used in the traditional medicine of Saraguros in Southern Ecuador; AChE and MAO inhibitory activity. J. Ethnopharmacol. 2016, 193, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, S.A.; El Hefnawy, H.M.; Azzam, S.M.; Aboutabl, E.A. Botanical and genetic characterization of Hydrocotyle umbellata L. cultivated in Egypt. Bull. Fac. Pharm. Cairo Univ. 2018, 56, 46–53. [Google Scholar] [CrossRef]
- Śliwińska, A.; Figat, R.; Zgadzaj, A.; Wileńska, B.; Misicka, A.; Nałęcz-Jawecki, G.; Pietrosiuk, A.; Sykłowska-Baranek, K. Polyscias filicifolia (Araliaceae) Hairy Roots with Antigenotoxic and Anti-Photogenotoxic Activity. Molecules 2021, 27, 186. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.T.; Liu, I.H.; Chang, S.T.; Wang, S.Y.; Chang, H.T. In Vitro and In Vivo Antimelanogenesis Effects of Leaf Essential Oil from Agathis dammara. Pharmaceutics 2023, 15, 2269. [Google Scholar] [CrossRef] [PubMed]
- Espirito Santo, B.; Santana, L.F.; Kato Junior, W.H.; de Araújo, F.O.; Bogo, D.; Freitas, K.C.; Guimarães, R.C.A.; Hiane, P.A.; Pott, A.; Filiú, W.F.O.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules 2020, 25, 4513. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Uddin, G.; Patel, S.; Khan, A.; Halim, S.A.; Bawazeer, S.; Ahmad, K.; Muhammad, N.; Mubarak, M.S. Diospyros, an under-utilized, multi-purpose plant genus: A review. Biomed. Pharmacother. 2017, 91, 714–730. [Google Scholar] [CrossRef] [PubMed]
- Sudradjat, S.E.; Timotius, K.H. Pharmacological properties and phytochemical components of Elaeocarpus: A comparative study. Phytomedicine Plus 2022, 2, 100365. [Google Scholar] [CrossRef]
- Nisar, M.; Ali, S.; Qaisar, M.; Gilani, S.N.; Shah, M.R.; Khan, I.; Ali, G. Antifungal activity of bioactive constituents and bark extracts of Rhododendron arboreum. Bangladesh J. Pharmacol. 2013, 8, 218–222. [Google Scholar] [CrossRef]
- Sadgrove, N.J.; Padilla-González, G.F.; Telford, I.R.H.; Greatrex, B.W.; Jones, G.L.; Andrew, R.; Bruhl, J.J.; Langat, M.K.; Melnikovova, I.; Fernandez-Cusimamani, E. Prostanthera (Lamiaceae) as a ‘Cradle of Incense’: Chemophenetics of Rare Essential Oils from Both New and Forgotten Australian ‘Mint Bush’ Species. Plants 2020, 9, 1570. [Google Scholar] [CrossRef] [PubMed]
- Lassak, E.V.; McCarthy, T. Australian Medicinal Plants; Methuen Australia Publisher: North Ryde, Australia, 1983. [Google Scholar]
- Wang, J.; Su, B.; Jiang, H.; Cui, N.; Yu, Z.; Yang, Y.; Sun, Y. Traditional uses, phytochemistry and pharmacological activities of the genus Cinnamomum (Lauraceae): A review. Fitoterapia 2020, 146, 104675. [Google Scholar] [CrossRef] [PubMed]
- Salleh, W.M.N.H.W.; Farediah, A.; Khong, H.Y.; Zulkifli, R. A Review of Endiandric Acid Analogues. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 844–856. [Google Scholar]
- Cock, I.E. Medicinal and aromatic plants—Australia. In Ethnopharmacology Section, Biological, Physiological and Health Sciences, Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO; EOLSS Publishers: Oxford, UK, 2011. [Google Scholar]
- Caputo, L.; Smeriglio, A.; Trombetta, D.; Cornara, L.; Trevena, G.; Valussi, M.; Fratianni, F.; De Feo, V.; Nazzaro, F. Chemical Composition and Biological Activities of the Essential Oils of Leptospermum petersonii and Eucalyptus gunnii. Front. Microbiol. 2020, 11, 518349. [Google Scholar] [CrossRef] [PubMed]
- Riley, M. Māori Healing and Herbal: New Zealand Ethnobotanical Sourcebook; Viking Sevenseas NZ: Wellington, New Zealand, 1994. [Google Scholar]
- Oktavia, D.; Pratiwi, S.D.; Munawaroh, S.; Hikmat, A.; Hilwan, I. The potential of medicinal plants from heath forest: Local knowledge from Kelubi Village, Belitung Island, Indonesia. Biodiversitas J. Biol. Divers. 2022, 23, 3553–3560. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Bouyahya, A.; El Menyiy, N.; El Omari, N.; Shahinozzaman, M.; Ara Haque Ovey, M.; Koirala, N.; Panthi, M.; Ertani, A.; et al. Ethnobotany, Phytochemistry, Biological Activities, and Health-Promoting Effects of the Genus Bulbophyllum. Evid Based Complement Altern. Med. 2022, 2022, 6727609. [Google Scholar] [CrossRef] [PubMed]
- Cakova, V.; Bonte, F.; Lobstein, A. Dendrobium: Sources of Active Ingredients to Treat Age-Related Pathologies. Aging Dis. 2017, 8, 827–849. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guo, X.; Nagle, D.G.; Zhang, W.-D.; Tian, X.-H. Genus Liparis: A review of its traditional uses in China, phytochemistry and pharmacology. J. Ethnopharmacol. 2019, 234, 154–171. [Google Scholar] [CrossRef] [PubMed]
- Ware, I.; Franke, K.; Hussain, H.; Morgan, I.; Rennert, R.; Wessjohann, L.A. Bioactive Phenolic Compounds from Peperomia obtusifolia. Molecules 2022, 27, 4363. [Google Scholar] [CrossRef] [PubMed]
- Al-Madhagi, W.M.; Mohd Hashim, N.; Awad Ali, N.A.; Alhadi, A.A.; Abdul Halim, S.N.; Othman, R. Chemical profiling and biological activity of Peperomia blanda (Jacq.) Kunth. PeerJ 2018, 6, 4839. [Google Scholar] [CrossRef] [PubMed]
- Inostroza-Blancheteau, C.; Sandoval, Y.; Reyes-Díaz, M.; Tighe-Neira, R.; González-Villagra, J. Phytochemical characterization and antioxidant properties of Prumnopitys andina fruits in different ripening stages in southern Chile. Chil. J. Agric. Res. 2022, 82, 285–293. [Google Scholar] [CrossRef]
- Tlau, L.; Lalawmpuii, L. Commonly used medicinal plants in N. Mualcheng, Mizoram, India. Sci. Vis. 2020, 20, 156–161. [Google Scholar] [CrossRef]
- Ray, S.; Saini, M.K. Impending threats to the plants with medicinal value in the Eastern Himalayas Region: An analysis on the alternatives to its non-availability. Phytomed. Plus 2022, 2, 100151. [Google Scholar] [CrossRef]
- Palombo, E.A.; Semple, S.J. Antibacterial activity of traditional Australian medicinal plants. J. Ethnopharmacol. 2001, 77, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Awang-Jamil, Z.; Basri, A.; Ahmad, N.; Taha, H. Phytochemical analysis, antimicrobial and antioxidant activities of Aidia borneensis leaf extracts. J. Appl. Biol. Biotechnol. 2019, 7, 92–97. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, R.A. Indian Morinda species: A review. Phytother. Res. 2020, 34, 924–1007. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Kurian, P.J. Ixora coccinea Linn.: Traditional uses, phytochemistry and pharmacology. Chin. J. Integr. Med. 2012, 18, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Maliha, F.; Hawlader, M.B.; Farzana, M.; Rashid, M.A. Ethnomedicinal uses, phytochemistry, pharmacology and toxicological aspects of genus Wendlandia: An overview. J. Bangladesh Acad. Sci. 2023, 47, 139–154. [Google Scholar] [CrossRef]
- Chidambaram, K.; Alqahtani, T.; Alghazwani, Y.; Aldahish, A.; Annadurai, S.; Venkatesan, K.; Dhandapani, K.; Thilagam, E.; Venkatesan, K.; Paulsamy, P.; et al. Medicinal Plants of Solanum Species: The Promising Sources of Phyto-Insecticidal Compounds. J. Trop. Med. 2022, 2022, 4952221. [Google Scholar] [CrossRef] [PubMed]
- Badoni, R.; Semwal, D.K.; Kothiyal, S.K.; Rawat, U. Chemical constituents and biological applications of the genus Symplocos. J. Asian. Nat. Prod. Res. 2010, 12, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Khairul Nizam Mazlan, M.; Firdaus Abdul Aziz, A.; Mohd Gazzali, A.; Amir Rawa, M.S.; Wahab, H.A. Phaleria macrocarpa (Scheff.) Boerl.: An updated review of pharmacological effects, toxicity studies, and separation techniques. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2023, 31, 874–888. [Google Scholar] [CrossRef]
- Mohanty, S. Bioactive Properties of Australian Native Fruits, Tasmannia Lanceolata and Terminalia Ferdinandiana: The Characterization of Their Active Compounds; Griffith University: Queensland, Australia, 2016. [Google Scholar]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet. 2023. Available online: http://www.plantsoftheworldonline.org/ (accessed on 28 December 2023).
- Jones, D.L.; Hopley, T.; Duffy, S.M. Australian Tropical Rainforest Orchids; Centre for Australian National Biodiversity Research (CPBR): Canberra, Australia, 2010.
- QLD. Nature Conservation Act 1992; Queensland Government: Queensland, Australia, 2017.
- Rivas-Ubach, A.; Pérez-Trujillo, M.; Sardans, J.; Gargallo-Garriga, A.; Parella, T.; Peñuelas, J. Ecometabolomics: Optimized NMR-based method. Methods Ecol. Evol. 2013, 4, 464–473. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Peñuelas, J.; Hódar, J.A.; Oravec, M.; Paša-Tolić, L.; Urban, O.; Sardans, J. We are what we eat: A stoichiometric and ecometabolomic study of caterpillars feeding on two pine subspecies of Pinus sylvestris. Int. J. Mol. Sci. 2018, 20, 59. [Google Scholar] [CrossRef] [PubMed]
- Allevato, D.M.; Kiyota, E.; Mazzafera, P.; Nixon, K.C. Ecometabolomic analysis of wild populations of Pilocarpus pennatifolius (Rutaceae) using unimodal analyses. Front. Plant Sci. 2019, 10, 436140. [Google Scholar] [CrossRef] [PubMed]
- Berini, J.L.; Brockman, S.A.; Hegeman, A.D.; Reich, P.B.; Muthukrishnan, R.; Montgomery, R.A.; Forester, J.D. Combinations of abiotic factors differentially alter production of plant secondary metabolites in five woody plant species in the boreal-temperate transition zone. Front. Plant Sci. 2018, 9, 389321. [Google Scholar] [CrossRef] [PubMed]
- Steinbauer, M.J.; Grytnes, J.-A.; Jurasinski, G.; Kulonen, A.; Lenoir, J.; Pauli, H.; Rixen, C.; Winkler, M.; Bardy-Durchhalter, M.; Barni, E. Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 2018, 556, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Lavola, A.; Julkunen-Tiitto, R.; Aphalo, P.; de la Rosa, T.; Lehto, T. The effect of UV-B radiation on UV-absorbing secondary metabolites in birch seedlings grown under simulated forest soil conditions. New Phytol. 1997, 137, 617–621. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef] [PubMed]
- Sallas, L.; Luomala, E.-M.; Utriainen, J.; Kainulainen, P.; Holopainen, J.K. Contrasting effects of elevated carbon dioxide concentration and temperature on Rubisco activity, chlorophyll fluorescence, needle ultrastructure and secondary metabolites in conifer seedlings. Tree Physiol. 2003, 23, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Večeřová, K.; Klem, K.; Veselá, B.; Holub, P.; Grace, J.; Urban, O. Combined Effect of Altitude, Season and Light on the Accumulation of Extractable Terpenes in Norway Spruce Needles. Forests 2021, 12, 1737. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejeryte, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.M.; Blande, J.D.; Souza, S.R.; Nerg, A.M.; Holopainen, J.K. Plant volatile organic compounds (VOCs) in ozone (O3) polluted atmospheres: The ecological effects. J. Chem. Ecol. 2010, 36, 22–34. [Google Scholar] [CrossRef]
- Schneider, G.F.; Coley, P.D.; Younkin, G.C.; Forrister, D.L.; Mills, A.G.; Kursar, T.A. Phenolics lie at the centre of functional versatility in the responses of two phytochemically diverse tropical trees to canopy thinning. J. Exp. Bot. 2019, 70, 5853–5864. [Google Scholar] [CrossRef] [PubMed]
- Pinasseau, L.; Vallverdu-Queralt, A.; Verbaere, A.; Roques, M.; Meudec, E.; Le Cunff, L.; Peros, J.P.; Ageorges, A.; Sommerer, N.; Boulet, J.C.; et al. Cultivar Diversity of Grape Skin Polyphenol Composition and Changes in Response to Drought Investigated by LC-MS Based Metabolomics. Front. Plant Sci. 2017, 8, 1826. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü. Uncovering the hidden facets of drought stress: Secondary metabolites make the difference. Tree Physiol. 2016, 36, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.F.; Yar, A.K.; Ullah, R.H.; Ali, B.G.; Ali, J.S.; Ahmad, J.S.; Fu, S. Impact of drought stress on active secondary metabolite production in Cichorium intybus roots. J. Appl. Env. Biol. Sci. 2017, 7, 39–43. [Google Scholar]
- Punia, H.; Tokas, J.; Malik, A.; Bajguz, A.; El-Sheikh, M.A.; Ahmad, P. Ascorbate-Glutathione Oxidant Scavengers, Metabolome Analysis and Adaptation Mechanisms of Ion Exclusion in Sorghum under Salt Stress. Int. J. Mol. Sci. 2021, 22, 13249. [Google Scholar] [CrossRef] [PubMed]
- Singiri, J.R.; Swetha, B.; Sikron-persi, N.; Grafi, G. Differential response to single and combined salt and heat stresses: Impact on accumulation of proteins and metabolites in dead pericarps of Brassica juncea. Int. J. Mol. Sci. 2021, 22, 7076. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Gilliham, M. Salinity tolerance of crops–what is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef]
- Goche, T.; Shargie, N.G.; Cummins, I.; Brown, A.P.; Chivasa, S.; Ngara, R. Comparative physiological and root proteome analyses of two sorghum varieties responding to water limitation. Sci. Rep. 2020, 10, 11835. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Mu, X.; Liu, J.; Li, B.; Liu, H.; Zhang, B.; Xiao, P. Plant metabolomics: A new strategy and tool for quality evaluation of Chinese medicinal materials. Chin. Med. 2022, 17, 45. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.; Kopka, J.; Moritz, T. Plant metabolomics coming of age. Physiol. Plant. 2008, 132, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.; Beale, M.; Fiehn, O.; Hardy, N.; Sumner, L.; Bino, R. Plant metabolomics: The missing link in functional genomics strategies. Plant Cell 2002, 14, 1437–1440. [Google Scholar] [CrossRef]
- Neilson, E.H.; Goodger, J.Q.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 18, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kesselmeier, J.; Staudt, M. Biogenic volatile organic compounds (VOC): An overview on emission, physiology and ecology. J. Atmos. Chem. 1999, 33, 23–88. [Google Scholar] [CrossRef]
- Pichersky, E.; Gang, D.R. Genetics and biochemistry of secondary metabolites in plants: An evolutionary perspective. Trends Plant Sci. 2000, 5, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp. Funct. Genom. 2001, 2, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Verpoorte, R. Sample preparation for plant metabolomics. Phytochem. Anal. 2010, 21, 4–13. [Google Scholar] [CrossRef]
- Garrison, M.S.; Irvine, A.K.; Setzer, W.N. Chemical composition of the resin essential oil from Agathis atropurpurea from North Queensland, Australia. Am. J. Essent. Oils Nat. Prod. 2016, 4, 4–5. [Google Scholar]
- Risner, D.; Marco, M.L.; Pace, S.A.; Spang, E.S. The Potential Production of the Bioactive Compound Pinene Using Whey Permeate. Processes 2020, 8, 263. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.L.D.; Jayaweera, S.; A. Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef] [PubMed]
- Rivas da Silva, A.C.; Lopes, P.M.; Barros de Azevedo, M.M.; Costa, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar] [CrossRef] [PubMed]
- Türkez, H.; Celik, K.; Toğar, B. Effects of copaene, a tricyclic sesquiterpene, on human lymphocytes cells in vitro. Cytotechnology 2014, 66, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Wollenweber, E.; Dörr, M.; Rozefelds, A.C.; Minchin, P.; Forster, P.I. Variation in flavonoid exudates in Eucryphia species from Australia and South America. Biochem. Syst. Ecol. 2000, 28, 111–118. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. The Leaf Oils of the Australian Species of Cinnamomum (Lauraceae). J. Essent. Oil Res. 2001, 13, 332–335. [Google Scholar] [CrossRef]
- Balahbib, A.; El Omari, N.; Hachlafi, N.E.L.; Lakhdar, F.; El Menyiy, N.; Salhi, N.; Mrabti, H.N.; Bakrim, S.; Zengin, G.; Bouyahya, A. Health beneficial and pharmacological properties of p-cymene. Food Chem. Toxicol. 2021, 153, 112259. [Google Scholar] [CrossRef] [PubMed]
- Han, N.R.; Moon, P.D.; Ryu, K.J.; Jang, J.B.; Kim, H.M.; Jeong, H.J. β-eudesmol suppresses allergic reactions via inhibiting mast cell degranulation. Clin. Exp. Pharmacol. Physiol. 2017, 44, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Tshering, G.; Pimtong, W.; Plengsuriyakarn, T.; Na-Bangchang, K. Anti-angiogenic effects of beta-eudesmol and atractylodin in developing zebrafish embryos. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 243, 108980. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.J.; Forster, P.I.; Goldsack, R.J. Coconut Laurels: The Leaf Essential Oils from Four Endemic Australian Cryptocarya Species: C. bellendenkerana, C. cocosoides, C. cunninghamii and C. lividula (Lauraceae). Nat. Prod. Commun. 2016, 11, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Vanitha, M.K. D-limonene: A multifunctional compound with potent therapeutic effects. J. Food Biochem. 2021, 45, e13566. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Tiyajamorn, T.; Bharathi, M.; Chaiyasut, C. A Narrative Review on the Bioactivity and Health Benefits of Alpha-Phellandrene. Sci. Pharm. 2022, 90, 57. [Google Scholar] [CrossRef]
- Ferraz, R.P.; Cardoso, G.M.; da Silva, T.B.; Fontes, J.E.; Prata, A.P.; Carvalho, A.A.; Moraes, M.O.; Pessoa, C.; Costa, E.V.; Bezerra, D.P. Antitumour properties of the leaf essential oil of Xylopia frutescens Aubl. (Annonaceae). Food Chem. 2013, 141, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Minh, P.T.H.; Tuan, N.T.; Van, N.T.H.; Bich, H.T.; Lam, D.T. Chemical Composition and Biological Activities of Essential Oils of Four Asarum Species Growing in Vietnam. Molecules 2023, 28, 1–13. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, J.; Song, D.; Duan, H.; Li, W.; Yang, X. Novel (E)-β-Farnesene Analogues Containing 2-Nitroiminohexahydro-1,3,5-triazine: Synthesis and Biological Activity Evaluation. Molecules 2016, 21, 825. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Punruckvong, A.; Bean, A.R.; Forster, P.I.; Lepschi, B.J.; Doran, J.C.; Rozefelds, A.C. Leaf essential oils of the genus Leptospermum (Myrtaceae) in eastern Australia. Part 7. Leptospermum petersonii, L. liversidgei and allies. Flavour Fragr. J. 2000, 15, 42–351. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, D.; Jung, S.H.; Lee, K.J.; Jin, H.; Kim, S.J.; Lee, H.M.; Kim, B.; Won, K.J. Sabinene Prevents Skeletal Muscle Atrophy by Inhibiting the MAPK-MuRF-1 Pathway in Rats. Int. J. Mol. Sci. 2019, 20, 4955. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Medeiros, D.; Nóbrega, J.; Silva, D.; Martins, E.; Barbosa-Filho, J.; et al. Terpinen-4-ol as an Antibacterial and Antibiofilm Agent against Staphylococcus aureus. Int. J. Mol. Sci. 2020, 21, 4531. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. The Essential Oils of the Australian Species of Uromyrtus (Myrtaceae). Flavour Fragr. J. 1996, 11, 133–138. [Google Scholar] [CrossRef]
- Ritmejeryte, E.; Ryan, R.Y.M.; Byatt, B.J.; Peck, Y.; Yeshi, K.; Daly, N.L.; Zhao, G.; Crayn, D.; Loukas, A.; Pyne, S.G.; et al. Anti-inflammatory properties of novel galloyl glucosides isolated from the Australian tropical plant Uromyrtus metrosideros. Chem. Biol. Interact. 2022, 368, 110124. [Google Scholar] [CrossRef]
- Hong, E.Y.; Kim, T.Y.; Hong, G.U.; Kang, H.; Lee, J.Y.; Park, J.Y.; Kim, S.C.; Kim, Y.H.; Chung, M.H.; Kwon, Y.I.; et al. Inhibitory Effects of Roseoside and Icariside E4 Isolated from a Natural Product Mixture (No-ap) on the Expression of Angiotensin II Receptor 1 and Oxidative Stress in Angiotensin II-Stimulated H9C2 Cells. Molecules 2019, 24, 414. [Google Scholar] [CrossRef] [PubMed]
- Yajima, A.; Oono, Y.; Nakagawa, R.; Nukada, T.; Yabuta, G. A simple synthesis of four stereoisomers of roseoside and their inhibitory activity on leukotriene release from mice bone marrow-derived cultured mast cells. Bioorg. Med. Chem. 2009, 17, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. Chemistry of the Australian Gymnosperms Part VIII. The Leaf Oil of Prumnopitys ladei (Podocarpaceae). J. Essent. Oil Res. 2006, 18, 212–214. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef] [PubMed]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A Sesquiterpene with Countless Biological Properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I. The Leaf Oils of the Australian Species of Flindersia (Rutaceae). J. Essent. Oil Res. 2005, 17, 388–395. [Google Scholar] [CrossRef]
- Robertson, L.P.; Hall, C.R.; Forster, P.I.; Carroll, A.R. Alkaloid diversity in the leaves of Australian Flindersia (Rutaceae) species driven by adaptation to aridity. Phytochemistry 2018, 152, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.P.; Duffy, S.; Wang, Y.; Wang, D.; Avery, V.M.; Carroll, A.R. Pimentelamines A-C, Indole Alkaloids Isolated from the Leaves of the Australian Tree Flindersia pimenteliana. J. Nat. Prod. 2017, 80, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Robertson, L.P.; Lucantoni, L.; Avery, V.M.; Carroll, A.R. Antiplasmodial Bis-Indole Alkaloids from the Bark of Flindersia pimenteliana. Planta Med. 2020, 86, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Resch, M.; Steigel, A.; Chen, Z.-L.; Bauer, R. 5-Lipoxygenase and Cyclooxygenase-1 Inhibitory Active Compounds from Atractylodes lancea. J. Nat. Prod. 1998, 61, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Mu, K.; Zhang, J.; Feng, X.; Zhang, D.; Li, K.; Li, R.; Yang, P.; Mao, S. Sedative-hypnotic effects of Boropinol-B on mice via activation of GABAA receptors. J. Pharm. Pharmacol. 2023, 75, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luo, L.; Yang, P.; Mu, K.; Yang, H.; Mao, S. Neuroprotection of boropinol-B in cerebral ischemia-reperfusion injury by inhibiting inflammation and apoptosis. Brain Res. 2023, 1798, 148132. [Google Scholar] [CrossRef]
- Liu, J.H.; Zschocke, S.; Reininger, E.; Bauer, R. Inhibitory effects of Angelica pubescens f. biserrata on 5-lipoxygenase and cyclooxygenase. Planta Med. 1998, 64, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Barbier de Reuille, P.; Routier-Kierzkowska, A.-L.; Kierzkowski, D.; Bassel, G.W.; Schüpbach, T.; Tauriello, G.; Bajpai, N.; Strauss, S.; Weber, A.; Kiss, A.; et al. MorphoGraphX: A platform for quantifying morphogenesis in 4D. eLife 2015, 4, 05864. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, R.; Das, P.; Mirabet, V.; Moscardi, E.; Traas, J.; Verdeil, J.-L.; Malandain, G.; Godin, C. Imaging plant growth in 4D: Robust tissue reconstruction and lineaging at cell resolution. Nat. Methods 2010, 7, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Perez de Souza, L.; Alseekh, S.; Scossa, F.; Fernie, A.R. Ultra-high-performance liquid chromatography high-resolution mass spectrometry variants for metabolomics research. Nat. Methods 2021, 18, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Jamtsho, T.; Yeshi, K.; Perry, M.J.; Loukas, A.; Wangchuk, P. Approaches, Strategies and Procedures for Identifying Anti-Inflammatory Drug Lead Molecules from Natural Products. Pharmaceuticals 2024, 17, 283. [Google Scholar] [CrossRef]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The future of NMR-based metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Bailey, N.J.; Johnson, H.E. Measuring the metabolome: Current analytical technologies. Analyst 2005, 130, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, J.I.; Hayes, A.; Mohammed, S.; Gaskell, S.J.; Oliver, S.G. An optimized protocol for metabolome analysis in yeast using direct infusion electrospray mass spectrometry. Phytochemistry 2003, 62, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.; Figueiredo, A.; Cordeiro, C.; Sousa Silva, M. FT-ICR-MS-based metabolomics: A deep dive into plant metabolism. Mass Spectrom. Rev. 2023, 42, 1535–1556. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.L.; Simons, B.L.; Young, J.B.; Hawkridge, A.M.; Muddiman, D.C. Performance characteristics of a new hybrid quadrupole time-of-flight tandem mass spectrometer (TripleTOF 5600). Anal. Chem. 2011, 83, 5442–5446. [Google Scholar] [CrossRef] [PubMed]
- Pelander, A.; Decker, P.; Baessmann, C.; Ojanperä, I. Evaluation of a high resolving power time-of-flight mass spectrometer for drug analysis in terms of resolving power and acquisition rate. J. Am. Soc. Mass Spectrom. 2011, 22, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ghaste, M.; Mistrik, R.; Shulaev, V. Applications of fourier transform ion cyclotron resonance (FT-ICR) and orbitrap based high resolution mass spectrometry in metabolomics and lipidomics. Int. J. Mol. Sci. 2016, 17, 816. [Google Scholar] [CrossRef] [PubMed]
- Glauser, G.; Veyrat, N.; Rochat, B.; Wolfender, J.-L.; Turlings, T.C. Ultra-high pressure liquid chromatography–mass spectrometry for plant metabolomics: A systematic comparison of high-resolution quadrupole-time-of-flight and single stage Orbitrap mass spectrometers. J. Chromatogr. A 2013, 1292, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-G.; Mohr, J.P.; Anderson, G.A.; Bruce, J.E. Application of frequency multiple FT-ICR MS signal acquisition for improved proteome research. Int. J. Mass Spectrom. 2021, 465, 116578. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, K.; Herzog, R.; Schwudke, D.; Metelmann-Strupat, W.; Bornstein, S.R.; Shevchenko, A. Bottom-up shotgun lipidomics by higher energy collisional dissociation on LTQ Orbitrap mass spectrometers. Anal. Chem. 2011, 83, 5480–5487. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, K.; Almeida, R.; Baumert, M.; Herzog, R.; Bornstein, S.R.; Shevchenko, A. Shotgun lipidomics on a LTQ Orbitrap mass spectrometer by successive switching between acquisition polarity modes. J. Mass Spectrom. 2012, 47, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.W.; Parker, D.; Beckmann, M.; Draper, J.; Goodacre, R. Fourier Transform Ion Cyclotron Resonance mass spectrometry for plant metabolite profiling and metabolite identification. Methods Mol. Biol. 2012, 860, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Barrow, M.P.; Burkitt, W.I.; Derrick, P.J. Principles of Fourier transform ion cyclotron resonance mass spectrometry and its application in structural biology. Analyst 2005, 130, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, K. Fundamentals of Mass Spectrometry; Springer: Berlin/Heidelberg, Germany, 2013; Volume 8. [Google Scholar]
- Folli, G.S.; Souza, L.M.; Araújo, B.Q.; Romão, W.; Filgueiras, P.R. Estimating the intermediate precision in petroleum analysis by (±) electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, 8861. [Google Scholar] [CrossRef] [PubMed]
- Hughey, C.A.; Rodgers, R.P.; Marshall, A.G. Resolution of 11000 compositionally distinct components in a single electrospray ionization Fourier transform ion cyclotron resonance mass spectrum of crude oil. Anal. Chem. 2002, 74, 4145–4149. [Google Scholar] [CrossRef] [PubMed]
- Allwood, J.W.; De Vos, R.C.; Moing, A.; Deborde, C.; Erban, A.; Kopka, J.; Goodacre, R.; Hall, R.D. Plant metabolomics and its potential for systems biology research: Background concepts, technology, and methodology. Methods Enzymol. 2011, 500, 299–336. [Google Scholar] [CrossRef] [PubMed]
- Shahbazy, M.; Moradi, P.; Ertaylan, G.; Zahraei, A.; Kompany-Zareh, M. FTICR mass spectrometry-based multivariate analysis to explore distinctive metabolites and metabolic pathways: A comprehensive bioanalytical strategy toward time-course metabolic profiling of Thymus vulgaris plants responding to drought stress. Plant Sci. 2020, 290, 110257. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.; Behnke, K.; Schnitzler, J.-P.; Kanawati, B.; Schmitt-Kopplin, P.; Polle, A. Pathway analysis of the transcriptome and metabolome of salt sensitive and tolerant poplar species reveals evolutionary adaption of stress tolerance mechanisms. BMC Plant Biol. 2010, 10, 150. [Google Scholar] [CrossRef] [PubMed]
- Kaling, M.; Kanawati, B.; Ghirardo, A.; Albert, A.; Winkler, J.B.; Heller, W.; Barta, C.; Loreto, F.; Schmitt-Kopplin, P.; Schnitzler, J.P. UV-B mediated metabolic rearrangements in poplar revealed by non-targeted metabolomics. Plant Cell Environ. 2015, 38, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.P.; Northen, T.R. Exometabolomics and MSI: Deconstructing how cells interact to transform their small molecule environment. Curr. Opin. Biotechnol. 2015, 34, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, V.; Olsson, L.; Nielsen, J. Metabolic footprinting in microbiology: Methods and applications in functional genomics and biotechnology. Trends Biotechnol. 2008, 26, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Kuzina, V.; Ekstrøm, C.T.; Andersen, S.B.; Nielsen, J.K.; Olsen, C.E.; Bak, S. Identification of defense compounds in Barbarea vulgaris against the herbivore Phyllotreta nemorum by an ecometabolomic approach. Plant Physiol. 2009, 151, 1977–1990. [Google Scholar] [CrossRef]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The role of reporting standards for metabolite annotation and identification in metabolomic studies. GigaScience 2013, 2, 2047–2217X. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Fu, X. Reprogramming of Plant Central Metabolism in Response to Abiotic Stresses: A Metabolomics View. Int. J. Mol. Sci. 2022, 23, 5716. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Ruscher, R.; Miles, K.; Crayn, D.; Liddell, M.; Wangchuk, P. Antioxidant and Anti-Inflammatory Activities of Endemic Plants of the Australian Wet Tropics. Plants 2022, 11, 2519. [Google Scholar] [CrossRef] [PubMed]
- Yeshi, K.; Wangchuk, P. Bush Medicinal Plants of the Australian Wet Tropics and Their Biodiscovery Potential. In Bioprospecting of Tropical Medicinal Plants; Arunachalam, K., Yang, X., Puthanpura Sasidharan, S., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 357–379. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; González-Sarrías, A.; Laparra-Llopis, J.M.; Schneider, C.; Espín, J.C. Targeting Mammalian 5-Lipoxygenase by Dietary Phenolics as an Anti-Inflammatory Mechanism: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7937. [Google Scholar] [CrossRef] [PubMed]
- Rådmark, O.; Samuelsson, B. 5-Lipoxygenase: Mechanisms of regulation1. J. Lipid Res. 2009, 50, S40–S45. [Google Scholar] [CrossRef] [PubMed]


| Botanical Name | Medicinal Uses | Number and Major Metabolites Identified | Isolated Compounds | Chemical Class | Biological Activities of Compounds |
|---|---|---|---|---|---|
| Agathis atropurpurea | Agathis species are traditionally used to treat myalgia and headaches [50]. | 27 metabolites; major metabolites are α-pinene, α-copaene, bicyclogermacrene, δ-cadinene, phyllocladane, and 16-kaurene [111] | NA | Terpenoid | Antimicrobial, antibacterial, antiviral, anti-cancer activity (α-pinene) [112,113,114], antioxidant activity (α-copaene) [115] |
| Eucryphia wilkiei | NU | 2 unknown metabolites [116] | NA | Flavonoid | NA |
| Cinnamomum propinquum | Cinnamomum species are most commonly used in traditional Chinese medicines to treat multiple disorders, including indigestion, microbial infections, and cough and cold [57]. | 40 metabolites; Major metabolites are p-cymene, α-pinene, and β-eudesmol [117] | NA | Terpenoid | Anti-cancer activity (p-cymene) [118], anti-allergic and anti-angiogenic effect (β-eudesmol) [119,120] |
| Cryptocarya bellendenkerana | NU | 39 metabolites; major metabolites are α-pinene, limonene, β-phellandrene, p-cymene, viridiflorene, E-β-farnesene, α-copaene, β-and α-selinene, δ-cadinene, bicyclogermacrene, calamenene, and cubeban-11-ol [121]. | NA | Antioxidant, antidiabetic, anticancer, anti-inflammatory (limonene) [122,123], anti-fungal (β-phellandrene) [124], antioxidant and antitumour properties (viridiflorene) [125,126], insect repellent (E-β-farnesene) [127] antioxidant activity (copaene) [115]. | |
| Leptospermum wooroonooran | Leptospermum species are traditionally used in Malaysia to relieve menstrual and stomach disorders [60,61]. | 45 metabolites; major metabolitesare α-pinene, β-pinene, sabinene, α-terpinene, γ-terpinene, terpinen-4-ol and α-terpineol [128] | NA | Reduce skeletal muscle atrophy (sabinene) [129], antibacterial and antibiofilm activities (terpinene-4-ol) [130] | |
| Uromyrtus metrosideros | NU | 27 metabolites; major metabolites are α-pinene, β-pinene, spathulenol and aromadendrene [131] | norbergenin, bergenin, (6S,9R)-roseoside, (4S)-α-terpineol 8-O-β-D-(6-O-galloyl) glucopyranoside, galloyl-lawsoniaside A, and uromyrtoside [132] | Benzopyran, Glucoside, | Anti-inflammatory (galloyl-lawsoniaside A) [132]; reduced hypertension and allergic reaction (roseoside) [133,134] |
| Prumnopitys ladei | Fruits and bark of Prunmnopitys species are considered medicinal [68]. | 44-metabolites; major compounds are α-pinene, limonene, verbenone, and p-cymene. β-caryophyllene, caryophyllene oxide, spathulenol, and α-humulene [135] | NA | Antimicrobial, anticarcinogenic, anti-inflammatory, antioxidant, and local anesthetic effects (β-caryophyllene) [136,137,138] | |
| Flindersia oppositifolia | NU | 37 metabolites; major compounds are β-caryophyllene and bicyclogermacrene [139]; Identified 8 alkaloids from leaf [140]. | pimentelamine A, pimentelamine B, pimentelamine C, 2-isoprenyl-N-N-dimethyltryptamine, 4-methylborreverine, borreverine, dimethylisoborreverine, quercitrin, and carpachromene [139]; harmalan, pimentelamine B, isoborreverine, skimmianine, kokusaginine, maculosidine, flindersiamine, 8-methoxy-N-methylflindersine [140]. | Terpene, Alkaloid | Antiplasmodial (pimentelamine C) [141,142] |
| Leionema ellipticum | NU | 3,4′,5-trimethoxyflavone-7-O-α-rhamnoside, boropinol-B, and osthol [143] | Flavonoid | Neuroprotective (boropinol-B) [144,145]; anti-inflammatory (osthol) [143,146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gempo, N.; Yeshi, K.; Crayn, D.; Wangchuk, P. Climate-Affected Australian Tropical Montane Cloud Forest Plants: Metabolomic Profiles, Isolated Phytochemicals, and Bioactivities. Plants 2024, 13, 1024. https://doi.org/10.3390/plants13071024
Gempo N, Yeshi K, Crayn D, Wangchuk P. Climate-Affected Australian Tropical Montane Cloud Forest Plants: Metabolomic Profiles, Isolated Phytochemicals, and Bioactivities. Plants. 2024; 13(7):1024. https://doi.org/10.3390/plants13071024
Chicago/Turabian StyleGempo, Ngawang, Karma Yeshi, Darren Crayn, and Phurpa Wangchuk. 2024. "Climate-Affected Australian Tropical Montane Cloud Forest Plants: Metabolomic Profiles, Isolated Phytochemicals, and Bioactivities" Plants 13, no. 7: 1024. https://doi.org/10.3390/plants13071024
APA StyleGempo, N., Yeshi, K., Crayn, D., & Wangchuk, P. (2024). Climate-Affected Australian Tropical Montane Cloud Forest Plants: Metabolomic Profiles, Isolated Phytochemicals, and Bioactivities. Plants, 13(7), 1024. https://doi.org/10.3390/plants13071024










