Comparative Phytoprofiling of Achillea millefolium Morphotypes: Assessing Antioxidant Activity, Phenolic and Triterpenic Compounds Variation across Different Plant Parts
Abstract
:1. Introduction
2. Results
2.1. Phenolic and Triterpenic Profiles of Two Morphotypes: Inflorescences, Leaves and Stems
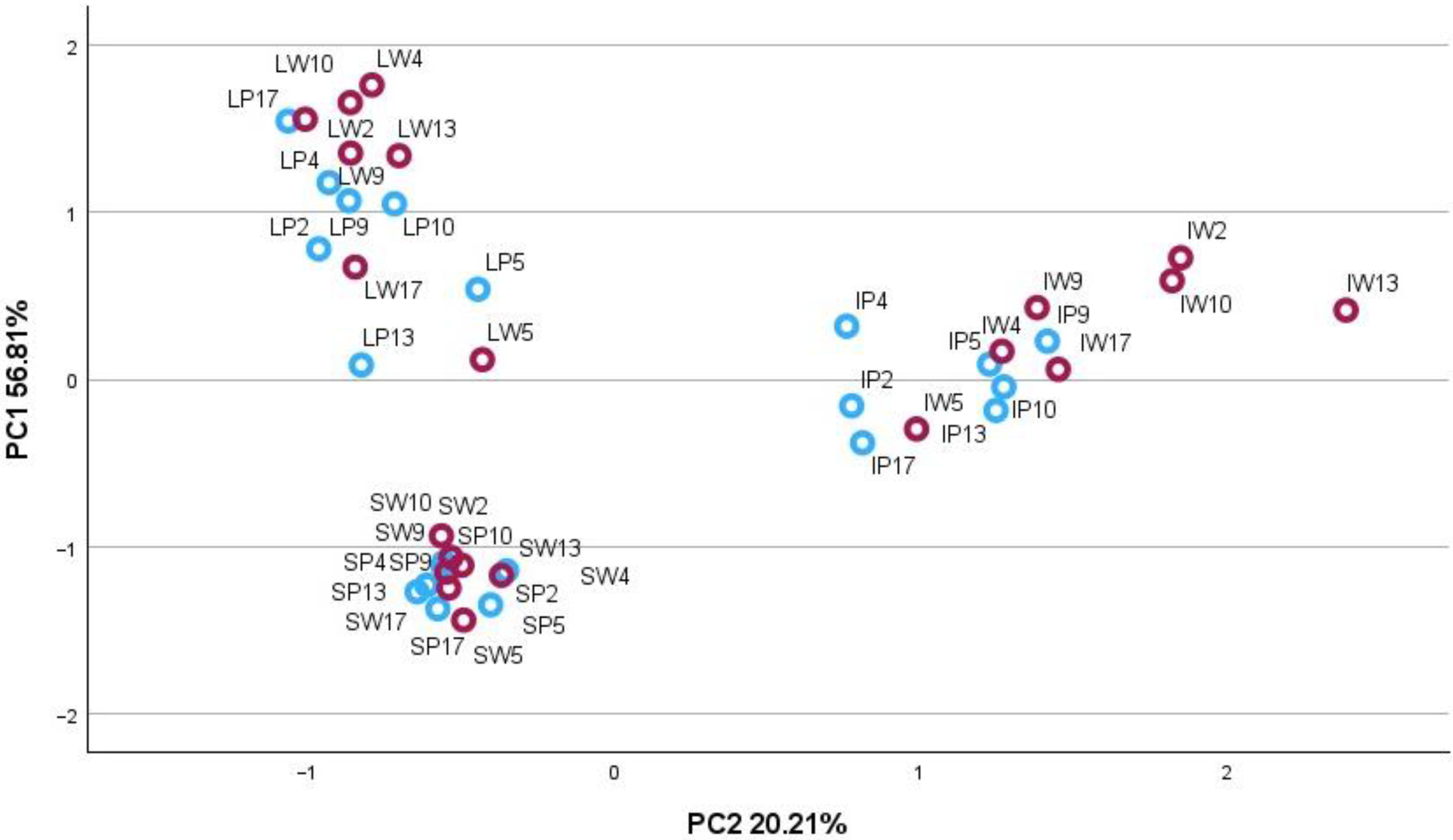
2.2. Principal Component Analysis
2.3. Assesment of Radical Scavenging and Reducing Activity of Achillea millefolium Extracts
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material
4.3. Preparation of Plant Extracts
4.4. HPLC-PDA Conditions
4.5. Antioxidant Activity Assays
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Battaglini, M.; Moles, N.; Ciofani, G. Natural Antioxidant Compounds as Potential Pharmaceutical Tools against Neurodegenerative Diseases. ACS Omega 2022, 7, 25974–25990. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.Y.; Lee, Y.C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-Derived Natural Products for Drug Discovery: Current Approaches and Prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Nayik, G.A. Immunity Boosting Medicinal Plants of the Western Himalayas; Springer: Singapore, 2023; pp. 1–614. [Google Scholar] [CrossRef]
- Richardson, I.B.K. Achillea L. In Flora Europaea; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: London, UK, 1976; Volume 4, pp. 159–165. [Google Scholar]
- Ehrendorfer, F.; Guo, Y.-P. Multidisciplinary Studies on Achillea Sensu Lato (Compositae-Anthemideae): New Data on Systematics and Phylogeography. Willdenowia 2006, 36, 69–87. [Google Scholar] [CrossRef]
- Ayoobi, F.; Shamsizadeh, A.; Fatemi, I.; Vakilian, A.; Allahtavakoli, M.; Hassanshahi, G.; Moghadam-Ahmadi, A. Bio-Effectiveness of the Main Flavonoids of Achillea Millefolium in the Pathophysiology of Neurodegenerative Disorders—A Review. Iran. J. Basic Med. Sci. 2017, 20, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Masoodi, M.H.; Rehman, M.U. Edible Plants in Health and Diseases: Volume II: Phytochemical and Pharmacological Properties; Springer Nature: Berlin, Germany, 2022; Volume 2, pp. 1–522. [Google Scholar]
- Villalva, M.; Jaime, L.; Villanueva-Bermejo, D.; Lara, B.; Fornari, T.; Reglero, G.; Santoyo, S. Supercritical Anti-Solvent Fractionation for Improving Antioxidant and Anti-Inflammatory Activities of an Achillea millefolium L. Extract. Food Res. Int. 2019, 115, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Dastgerdi, A.; Ezzatpanah, H.; Asgary, S.; Dokhani, S.; Rahimi, E. Phytochemical, Antioxidant and Antimicrobial Activity of the Essential Oil from Flowers and Leaves of Achillea millefolium Subsp. millefolium. J. Essent. Oil Bear. Plants 2017, 20, 395–409. [Google Scholar] [CrossRef]
- Farhadi, N.; Babaei, K.; Farsaraei, S.; Moghaddam, M.; Ghasemi Pirbalouti, A. Changes in Essential Oil Compositions, Total Phenol, Flavonoids and Antioxidant Capacity of Achillea millefolium at Different Growth Stages. Ind. Crops Prod. 2020, 152, 112570. [Google Scholar] [CrossRef]
- Syakri, S.; Ismail, I.; Amal, N.M.; Masjidi, N.A.; Tahir, K.A. Characterization and Anti-Aging Tests of Peel-Off Gel Masks Made from Ethanolic Extract of Yarrow (Achillea millefolium). Open Access Maced. J. Med. Sci. 2021, 9, 1156–1161. [Google Scholar] [CrossRef]
- Villalva, M.; Silvan, J.M.; Alarcón-Cavero, T.; Villanueva-Bermejo, D.; Jaime, L.; Santoyo, S.; Martinez-Rodriguez, A.J. Antioxidant, Anti-Inflammatory, and Antibacterial Properties of an Achillea millefolium L. Extract and Its Fractions Obtained by Supercritical Anti-Solvent Fractionation against Helicobacter Pylori. Antioxidants 2022, 11, 1849. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, I.; Ungureanu, C.; Avramescu, S.; Fierascu, R.C.; Ortan, A.; Soare, L.C.; Paunescu, A. In Vitro Antioxidant and Antifungal Properties of Achillea millefolium L. Rom. Biotechnol. Lett. 2015, 20, 10626–10636. [Google Scholar]
- Georgieva, L.; Gadjalova, A.; Mihaylova, D.; Pavlov, A. Achillea millefolium L.—Phytochemical Profile and in Vitro Antioxidant Activity. Int. Food Res. J. 2015, 22, 1347–1352. [Google Scholar]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem. Biodivers. 2021, 18, e2100345. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ul Hassan, M.; Rasool, T.; Iqbal, C.; Arshad, A.; Abrar, M.; Abrar, M.M.; Habib-ur-Rahman, M.; Noor, M.A.; Sher, A.; Fahad, S. Linking Plants Functioning to Adaptive Responses Under Heat Stress Conditions: A Mechanistic Review. J. Plant Growth Regul. 2022, 41, 2596–2613. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Quality from the Field: The Impact of Environmental Factors as Quality Determinants in Medicinal Plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Radušienė, J.; Karpavičienė, B.; Raudone, L.; Vilkickyte, G.; Çırak, C.; Seyis, F.; Yayla, F.; Marksa, M.; Rimkienė, L.; Ivanauskas, L. Trends in Phenolic Profiles of Achillea millefolium from Different Geographical Gradients. Plants 2023, 12, 746. [Google Scholar] [CrossRef]
- Mazandarani, M.; Behmanesh, B.; Rezaei, M.B.; Ghaeimi, E.O. Ecological Factors, Chemical Composition and Antibacterial Activity of the Essential Oil from Achillea millefolium L. in the North of Iran. Planta Med. 2007, 73, 179. [Google Scholar] [CrossRef]
- Aziz, A.; Badawy, E.M.; Zheljazkov, V.D.; Nicola, S.M.; Fouad, H. Yield and Chemical Composition of Essential Oil of Achillea millefolium L. as Affected by Harvest Time. Egypt J. Chem. 2019, 62, 533–540. [Google Scholar] [CrossRef]
- Radzhabov, G.K.; Aliev, A.M.; Musaev, A.M.; Islamova, F.I. Variability of the Constituent Composition of Achillea millefolium Essential Oils in the Wild Flora of Dagestan. Pharm. Chem. J. 2022, 56, 661–666. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Żwawiak, J.; Pawełczyk, A.; Olender, D.; Zaprutko, L. Structure and Activity of Pentacyclic Triterpenes Codrugs. A Review. Mini Rev. Med. Chem. 2021, 21, 1509–1526. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical Composition of the Essential Oils and Extracts of Achillea Species and Their Biological Activities: A Review. J. Ethnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef] [PubMed]
- Radusiene, J.; Gudaityte, O. Analysis of Phenotypic Variation in Cenopopulations of Achillea millefolium. Bot. Lith 2005, 11, 205–210. [Google Scholar]
- Ovile Mimi, C.; De-la-Cruz-Chacón, I.; Caixeta Sousa, M.; Aparecida Ribeiro Vieira, M.; Ortiz Mayo Marques, M.; Ferreira, G.; Silvia Fernandes Boaro, C. Chemophenetics as a Tool for Distinguishing Morphotypes of Annona emarginata (Schltdl.) H. Rainer. Chem. Biodivers. 2021, 18, e2100544. [Google Scholar] [CrossRef] [PubMed]
- Bimbiraite, K.; Ragazinskiene, O.; Maruska, A.; Kornysova, O. Comparison of the Chemical Composition of Four Yarrow (Achillea millefolium L.) Morphotypes. Biologija 2008, 54, 208–2012. [Google Scholar] [CrossRef]
- Garzoli, S.; Cicaloni, V.; Salvini, L.; Trespidi, G.; Iriti, M.; Vitalini, S. SPME-GC-MS Analysis of the Volatile Profile of Three Fresh Yarrow (Achillea millefolium L.) Morphotypes from Different Regions of Northern Italy. Separations 2023, 10, 51. [Google Scholar] [CrossRef]
- Marksa, M.; Zymone, K.; Ivanauskas, L.; Radušienė, J.; Pukalskas, A.; Raudone, L. Antioxidant Profiles of Leaves and Inflorescences of Native, Invasive and Hybrid Solidago Species. Ind. Crop. Prod. 2020, 145, 112123. [Google Scholar] [CrossRef]
- Pereira, C.; Barros, L.; Ferreira, I.C. Extraction, Identification, Fractionation and Isolation of Phenolic Compounds in Plants with Hepatoprotective Effects. J. Sci. Food Agric. 2016, 96, 1068–1084. [Google Scholar] [CrossRef] [PubMed]
- Raudone, L.; Radušiene, J.; Seyis, F.; Yayla, F.; Vilkickyte, G.; Marksa, M.; Ivanauskas, L.; Cırak, C. Distribution of Phenolic Compounds and Antioxidant Activity in Plant Parts and Populations of Seven Underutilized Wild Achillea Species. Plants 2022, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative Stress and Antioxidants—A Critical Review on In Vitro Antioxidant Assays. Antioxidants 2022, 11, 2388. [Google Scholar] [CrossRef] [PubMed]
- Mucha, P.; Skoczyńska, A.; Małecka, M.; Hikisz, P.; Budzisz, E. Overview of the Antioxidant and Anti-Inflammatory Activities of Selected Plant Compounds and Their Metal Ions Complexes. Molecules 2021, 26, 4886. [Google Scholar] [CrossRef] [PubMed]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Kukula-Koch, W. Achillea Species as Sources of Active Phytochemicals for Dermatological and Cosmetic Applications. Oxid. Med. Cell Longev. 2021, 2021, 6643827. [Google Scholar] [CrossRef] [PubMed]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview. In Plant Signaling Molecules: Role and Regulation under Stressful Environments; Elsevier: Amsterdam, The Netherlands, 2019; pp. 157–168. [Google Scholar] [CrossRef]
- Kundakovic, T.; Fokialakis, N.; Magiatis, P.; Kovacevic, N.; Chinou, I. Triterpenic Derivatives of Achillea Alexandri-Regis BORNM. & RUDSKI. Chem. Pharm. Bull. 2004, 52, 1462–1465. [Google Scholar] [CrossRef]
- Conforti, F.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Comparative Radical Scavenging and Antidiabetic Activities of Methanolic Extract and Fractions from Achillea ligustica ALL. Biol. Pharm. Bull. 2005, 28, 1791–1794. [Google Scholar] [CrossRef]
- Etehadpour, M.; Tavassolian, I. Ecological Factors Regulate Essential Oil Yield, Percent and Compositions of Endemic Yarrow (Achillea eriophora DC.) in Southeast Iran. Int. J. Hortic. Sci. Technol. 2019, 6, 201–215. [Google Scholar]
- Chandler, R.F.; Hooper, S.N.; Safe, L.M.; Hooper, D.L.; Jamieson, W.D.; Flinn, C.G. Herbal Remedies of the Maritime Indians: Sterols and Triterpenes of Achillea millefolium L. (Yarrow). J. Pharm. Sci. 1982, 71, 690–693. [Google Scholar] [CrossRef]
- Faustino, C.; Pinheiro, L.; Duarte, N. Triterpenes as Potential Drug Candidates for Rheumatoid Arthritis Treatment. Life 2023, 13, 1514. [Google Scholar] [CrossRef] [PubMed]
- Vilkickyte, G.; Petrikaite, V.; Marksa, M.; Ivanauskas, L.; Jakstas, V.; Raudone, L. Fractionation and Characterization of Triterpenoids from Vaccinium Vitis-idaea L. Cuticular Waxes and Their Potential as Anticancer Agents. Antioxidants 2023, 12, 465. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia 11. Yarrow. Millefolii herba. 2023. Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph.-eur.-11th-edition (accessed on 26 February 2024).
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A Neglected Panacea? A Review of Ethnobotany, Bioactivity, and Biomedical Research. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L. Optimization, Validation and Application of HPLC-PDA Methods for Quantification of Triterpenoids in Vaccinium Vitis-idaea L. Molecules 2021, 26, 1645. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying An Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) As A Measure Of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Cirak, C.; Radusiene, J.; Raudone, L.; Vilkickyte, G.; Seyis, F.; Marksa, M.; Ivanauskas, L.; Yayla, F. Phenolic Compounds and Antioxidant Activity of Achillea arabica Populations. South Afr. J. Bot. 2022, 147, 425–433. [Google Scholar] [CrossRef]
- Midway, S.; Robertson, M.; Flinn, S.; Kaller, M. Comparing multiple comparisons:practical guidance for choosing the best multiple comparisons test. PeerJ 2020, 8, e10387. [Google Scholar] [CrossRef]
- Arsham, H.; Lovric, M. Bartlett’s Test. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 87–88. [Google Scholar] [CrossRef]
- Kaiser, H.F. An Index of Factorial Simplicity. Psychometrika 1974, 39, 31–36. [Google Scholar] [CrossRef]




| Compound | IW 1 | IP | LW | LP | SW | SP |
|---|---|---|---|---|---|---|
| Oleanolic acid | 29.22 ± 14.64 2 | 80.03 ± 15.35 | 102.68 ± 44.53 | 139.25 ± 44.53 | 0 ± 0 | 0 ± 0 |
| ursolic | 0 ± 0 | 0 ± 0 | 16.78 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Maslinic acid | 18.02 ± 5.18 | 32.96 ± 9.66 | 32.18 ± 13.71 | 58.27 ± 13.71 | 14.31 ± 2.92 | 16.04 ± 6.26 |
| Corosolic acid | 46.1 ± 15.44 | 78.79 ± 12.14 | 86.81 ± 3 | 91.1 ± 3 | 5.36 ± 0.55 | 1.39 ± 0.86 |
| Betulinic acid | 72.38 ± 18.52 | 197.27 ± 66.68 | 440.54 ± 78.94 | 519.01 ± 51.77 | 0 ± 0 | 0 ± 0 |
| Betulin | 2327.85 ± 726.91 | 1114.17 ± 257.8 | 956.93 ± 110.63 | 992.32 ± 153.83 | 1016.3 ± 5.5 | 888.95 ± 0 |
| Uvaol | 24.87 ± 14.45 | 23.38 ± 6.68 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Betulinic acid methyl ester | 226.15 ± 77.43 | 239.78 ± 75.7 | 230.81 ± 63.13 | 225.74 ± 63.13 | 23.84 ± 6.72 | 19.12 ± 2.69 |
| beta-Amyrin | 571.63 ± 56.64 | 388.28 ± 97.07 | 153.61 ± 33.32 | 115.71 ± 33.32 | 15.15 ± 3.9 | 16.31 ± 6.8 |
| beta-Sitosterol | 283.77 ± 68.47 | 211.71 ± 53.62 | 166.02 ± 50.38 | 165.21 ± 50.38 | 118.36 ± 16.33 | 125.77 ± 31.3 |
| Alfa-amyrin | 153.83 ± 37.42 | 84.95 ± 10.24 | 297.29 ± 33.4 | 250.44 ± 33.4 | 19.41 ± 11.61 | 9.08 ± 6.67 |
| Nicotiflorin | 0 ± 0 | 0 ± 0 | 237.18 ± 33.8 | 138.88 ± 33.8 | 0 ± 0 | 0 ± 0 |
| Isovitexin | 14.03 ± 4.05 | 25.18 ± 12.2 | 27.15 ± 12.46 | 24.92 ± 8.46 | 0 ± 0 | 0 ± 0 |
| Hesperidin | 491.5 ± 152.82 | 670.9 ± 188.85 | 1104.06 ± 35.51 | 1380.83 ± 50.58 | 238.54 ± 113.48 | 285.37 ± 121.65 |
| Luteolin-7-O-glucuronide | 2176.98 ± 444.59 | 1540.17 ± 310.57 | 5068.57 ± 345.75 | 3459.83 ± 345.75 | 611.39 ± 302.49 | 292.64 ± 199.77 |
| Neochlorogenic acid | 402.67 ± 53.65 | 482.4 ± 61.5 | 1184.96 ± 99.82 | 1190.55 ± 99.82 | 263.4 ± 47.24 | 268.3 ± 52.92 |
| Chlorogenic acid | 7969.08 ± 226.96 | 7848.88 ± 346.64 | 28,123.17 ± 1013.01 | 28,934.28 ± 1864.98 | 4583.7 ± 579.72 | 4076.34 ± 333.15 |
| 4-O-caffeoylquinic acid | 1313.7 ± 229.52 | 212.6 ± 69.91 | 405.28 ± 393.89 | 1058.9 ± 393.89 | 685.87 ± 46.00 | 96 ± 51.24 |
| 3.4-O-dicaffeoylquinic acid | 2545.78 ± 540.08 | 2546.82 ± 340.77 | 3749.7 ± 784.63 | 3338.91 ± 784.63 | 651.83 ± 188.26 | 714.49 ± 184.64 |
| 3.5-O-dicaffeoylquinic acid | 9014.24 ± 732.64 | 8502.93 ± 762.67 | 16,064.82 ± 984.88 | 14,974.35 ± 984.88 | 1486.04 ± 245.46 | 1344.03 ± 503.54 |
| 1.5-O-dicaffeoylquinic acid | 2595.3 ± 213.95 | 2414.05 ± 100.41 | 4957.9 ± 807.78 | 5765.53 ± 807.78 | 1070.17 ± 448.67 | 932.41 ± 481.21 |
| 4.5-O-dicaffeoylquinic acid | 1005.02 ± 239.56 | 1310.5 ± 188.35 | 2075.26 ± 702.21 | 2402.77 ± 702.21 | 590.36 ± 230.26 | 605.63 ± 217.05 |
| Caffeic acid | 17.46 ± 7.65 | 8.48 ± 3.94 | 64.14 ± 37.48 | 34.86 ± 17.48 | 33.93 ± 15.52 | 12.54 ± 9.12 |
| Quercitrin | 174.5 ± 23.63 | 106.46 ± 19.88 | 306.19 ± 134.67 | 229.69 ± 134.67 | 110.86 ± 56.22 | 42.19 ± 19.23 |
| Rutin | 176.2 ± 84.9 | 304.7 ± 42.42 | 2634.46 ± 862.93 | 2965.2 ± 1397.95 | 734.99 ± 414.21 | 1095.84 ± 440.83 |
| Quercetin | 27.61 ± 3 | 27.8 ± 5 | 18.76 ± 8.01 | 21.17 ± 8.01 | 19.83 ± 2.74 | 19.46 ± 3.05 |
| Isoquercitrin | 0 ± 0 | 0 ± 0 | 86.01 ± 9.83 | 137.9 ± 27.87 | 5.3 ± 0.25 | 42.76 ± 19.14 |
| Luteolin | 110.98 ± 3.13 | 2685.86 ± 384.43 | 144.96 ± 27.41 | 128.29 ± 27.41 | 137.77 ± 7.54 | 133.14 ± 5.5 |
| Luteolin-7-O-glucoside | 2792.46 ± 446.2 | 2016.26 ± 376.03 | 1032.26 ± 344.69 | 749.36 ± 344.69 | 89.37 ± 34.81 | 63.85 ± 28.59 |
| Luteolin-7-O-rutinoside | 756.05 ± 156.91 | 680.43 ± 99.55 | 1296.21 ± 655.24 | 780.66 ± 655.24 | 232.89 ± 97.13 | 50.41 ± 34.16 |
| Apigenin | 559.24 ± 65.3 | 413.08 ± 69.63 | 11.03 ± 2.15 | 2.49 ± 0.15 | 0 ± 0 | 0 ± 0 |
| Apigenin-O-7-glucoside | 3535.22 ± 462.02 | 2724.33 ± 338.68 | 258.94 ± 102.82 | 184.97 ± 102.82 | 27.1 ± 11.6 | 22.21 ± 9.07 |
| Santin | 249.57 ± 25.88 | 220.59 ± 11.77 | 248.04 ± 84.09 | 257.24 ± 84.09 | 207.03 ± 0.93 | 147.97 ± 93.59 |
| Cynarin | 0 ± 0 | 0 ± 0 | 74.99 ± 34.59 | 30.2 ± 34.59 | 28.49 ± 23.71 | 2.87 ± 6.75 |
| Pop. No | Administrative Location | Latitude (°N) | Longitude (°E) | Elevation (m.a.s.l.) | Habitat |
|---|---|---|---|---|---|
| 1 | Einororys, Alytus distr. | 54.44614 | 24.38971 | 120 | Mesophytic grassland |
| 2 | Geruliai, Alytus distr. | 54.53120 | 24.27056 | 130 | Mesophytic grassland |
| 3 | Užugriovis, Vilnius distr. | 54.82782 | 25.24644 | 161 | Dry grassland |
| 4 | Bernatonys, Vilnius distr. | 54.90934 | 25.32271 | 160 | Mesophytic grassland |
| 5 | Vorėnai, Molėtai distr. | 55.35779 | 25.61012 | 158 | Mesophytic grassland |
| 6 | Juodžionys, Biržai distr. | 56.24353 | 24.87915 | 60 | Mesophytic grassland |
| 7 | Dubingiai, Molėtai distr. | 55.05911 | 25.43509 | 175 | Pine forest, roadside |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raudone, L.; Vilkickyte, G.; Marksa, M.; Radusiene, J. Comparative Phytoprofiling of Achillea millefolium Morphotypes: Assessing Antioxidant Activity, Phenolic and Triterpenic Compounds Variation across Different Plant Parts. Plants 2024, 13, 1043. https://doi.org/10.3390/plants13071043
Raudone L, Vilkickyte G, Marksa M, Radusiene J. Comparative Phytoprofiling of Achillea millefolium Morphotypes: Assessing Antioxidant Activity, Phenolic and Triterpenic Compounds Variation across Different Plant Parts. Plants. 2024; 13(7):1043. https://doi.org/10.3390/plants13071043
Chicago/Turabian StyleRaudone, Lina, Gabriele Vilkickyte, Mindaugas Marksa, and Jolita Radusiene. 2024. "Comparative Phytoprofiling of Achillea millefolium Morphotypes: Assessing Antioxidant Activity, Phenolic and Triterpenic Compounds Variation across Different Plant Parts" Plants 13, no. 7: 1043. https://doi.org/10.3390/plants13071043











