Comprehensive Analysis of Autophagy-Related Genes in Rice Immunity against Magnaporthe oryzae
Abstract
:1. Introduction
2. Results
2.1. Identification of 37 OsATGs in the Rice Genome
2.2. Physicochemical Properties and Subcellular Localization of OsATGs
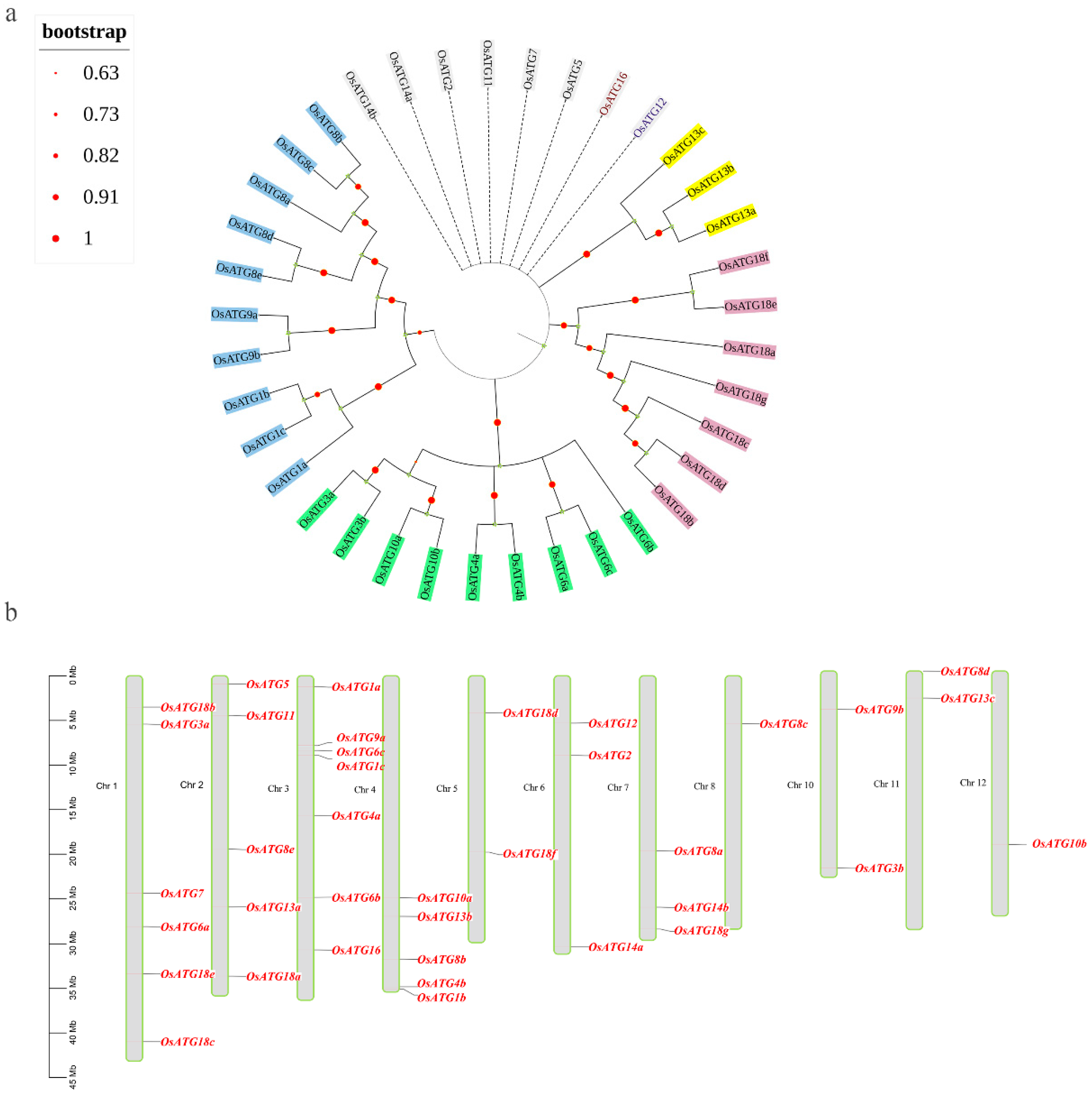
2.3. Phylogenetic Relationship and Chromosomal Localization of OsATGs
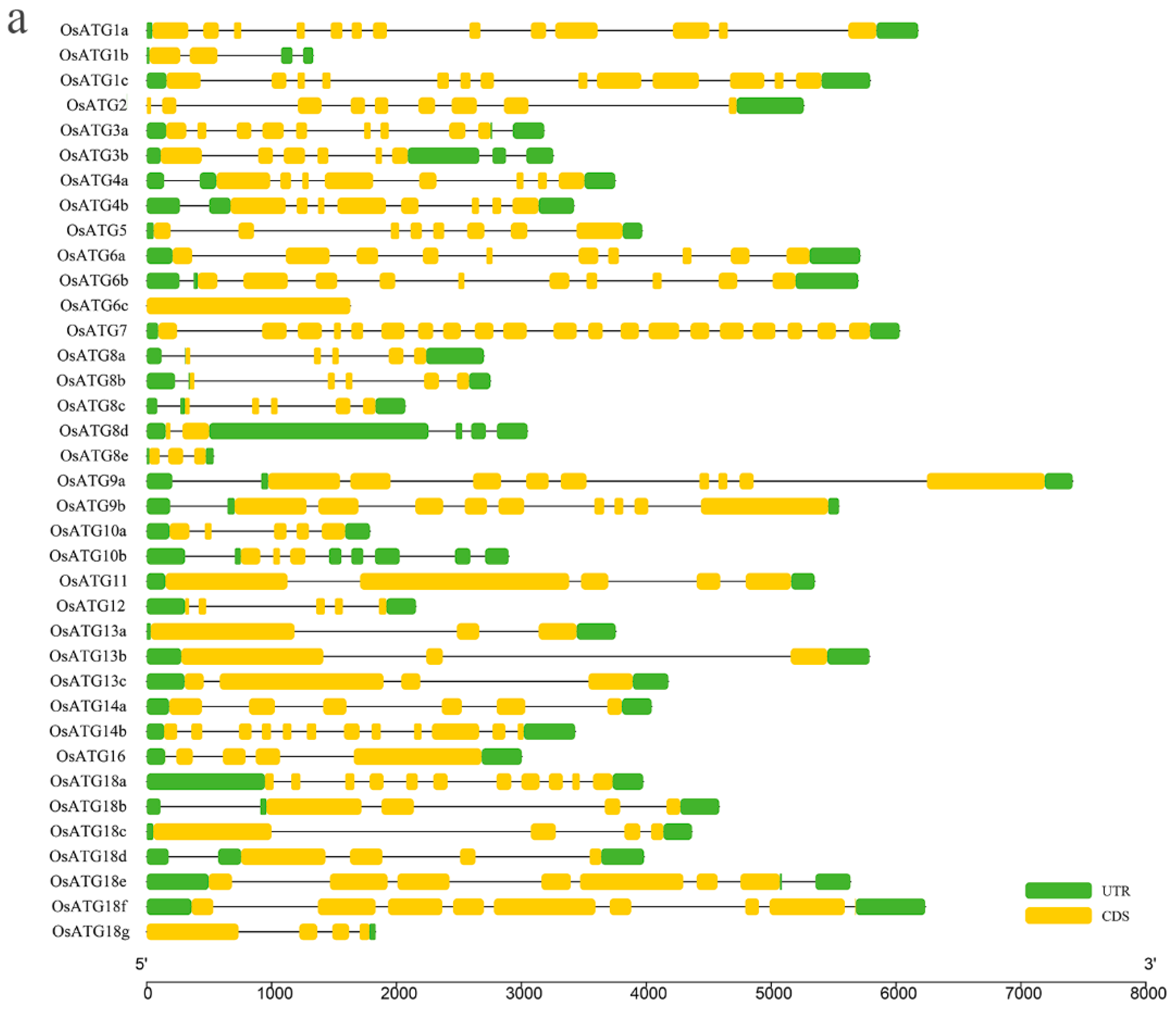
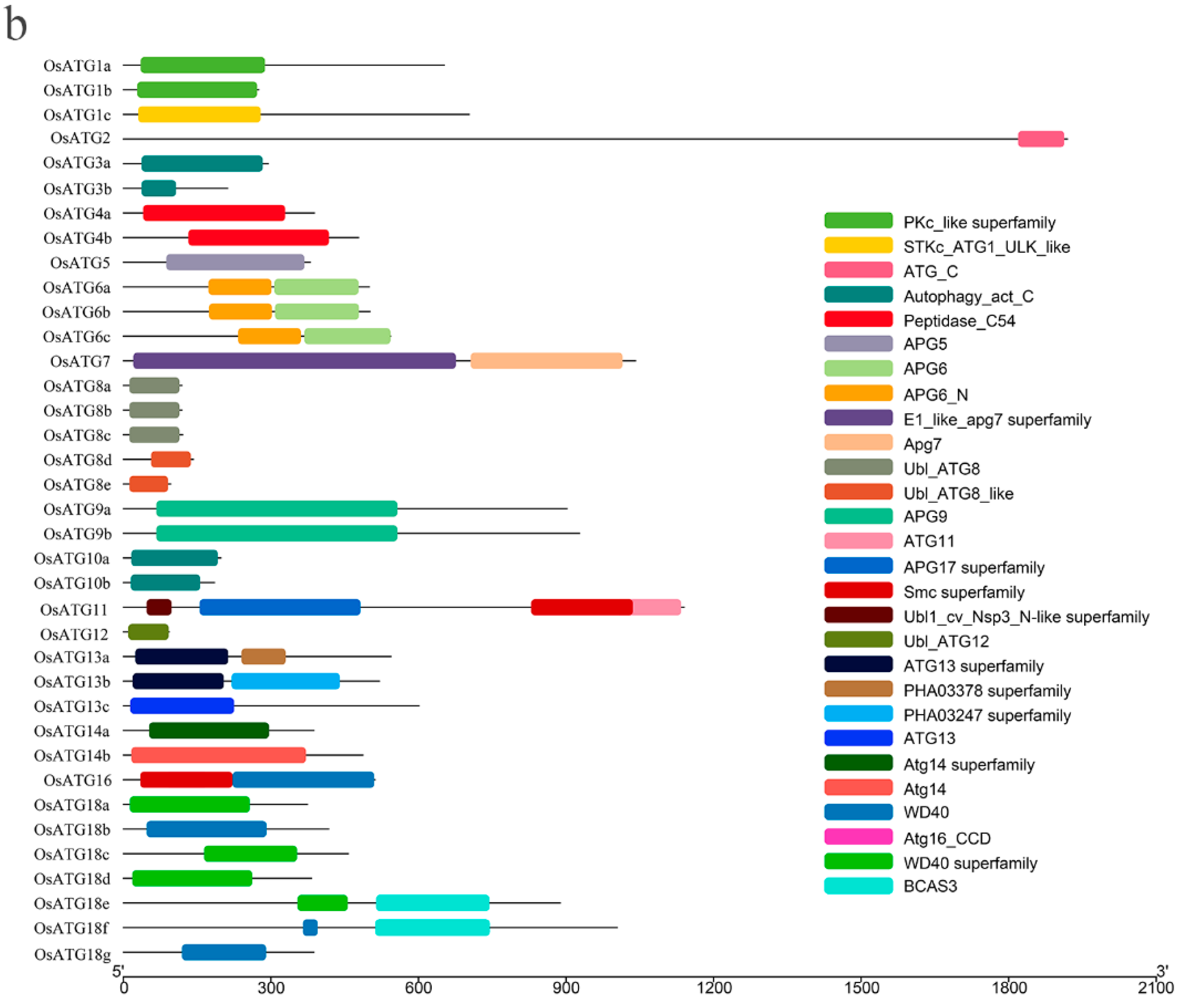
2.4. Gene Structure and Function Domain of OsATGs
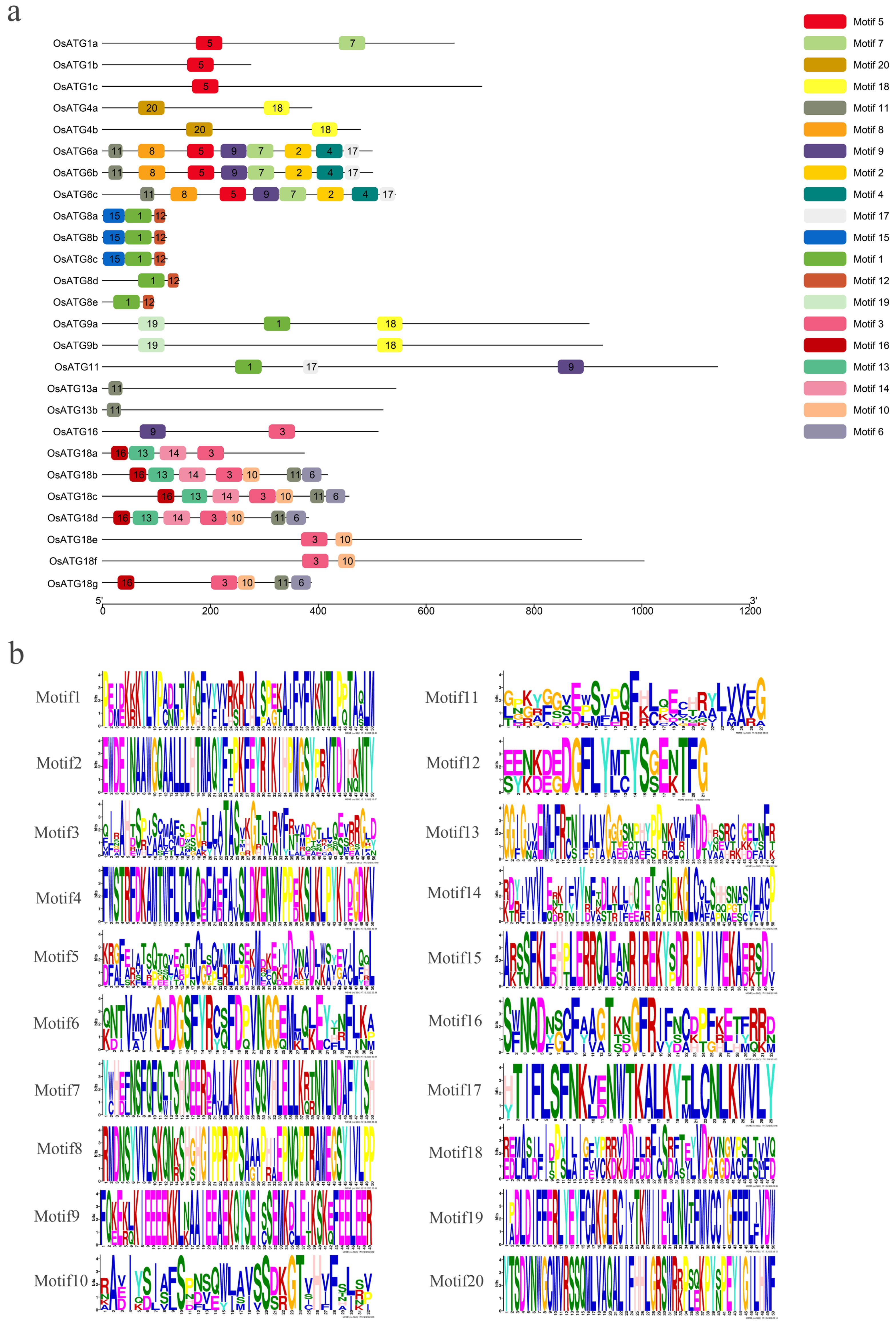
2.5. Protein Motif of OsATGs
2.6. Genetic cis-Regulatory Elements of OsATGs
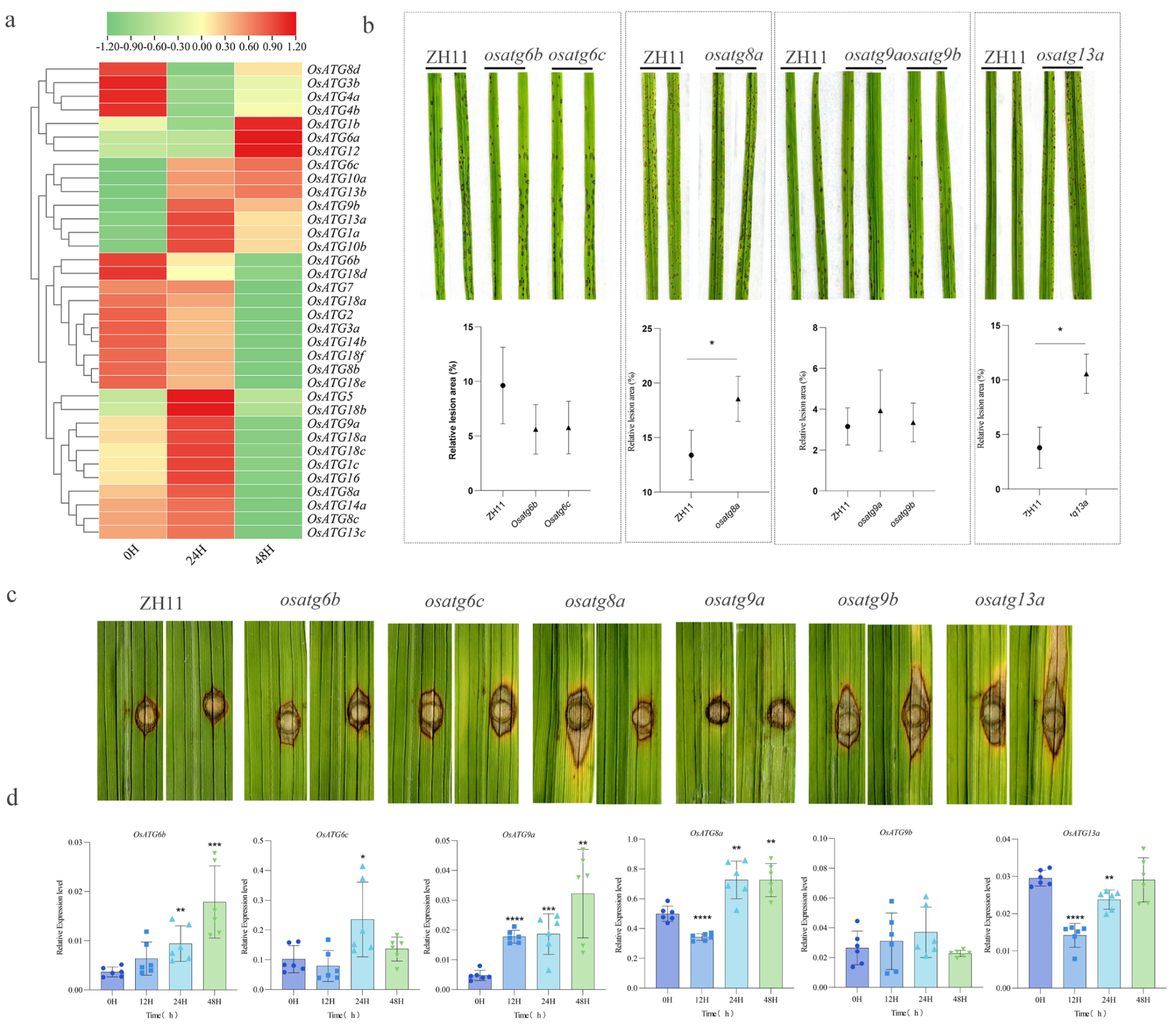
2.7. Resistance to Rice Blast Fungus of OsATGs
2.8. Response to Hormone Treatments of OsATGs
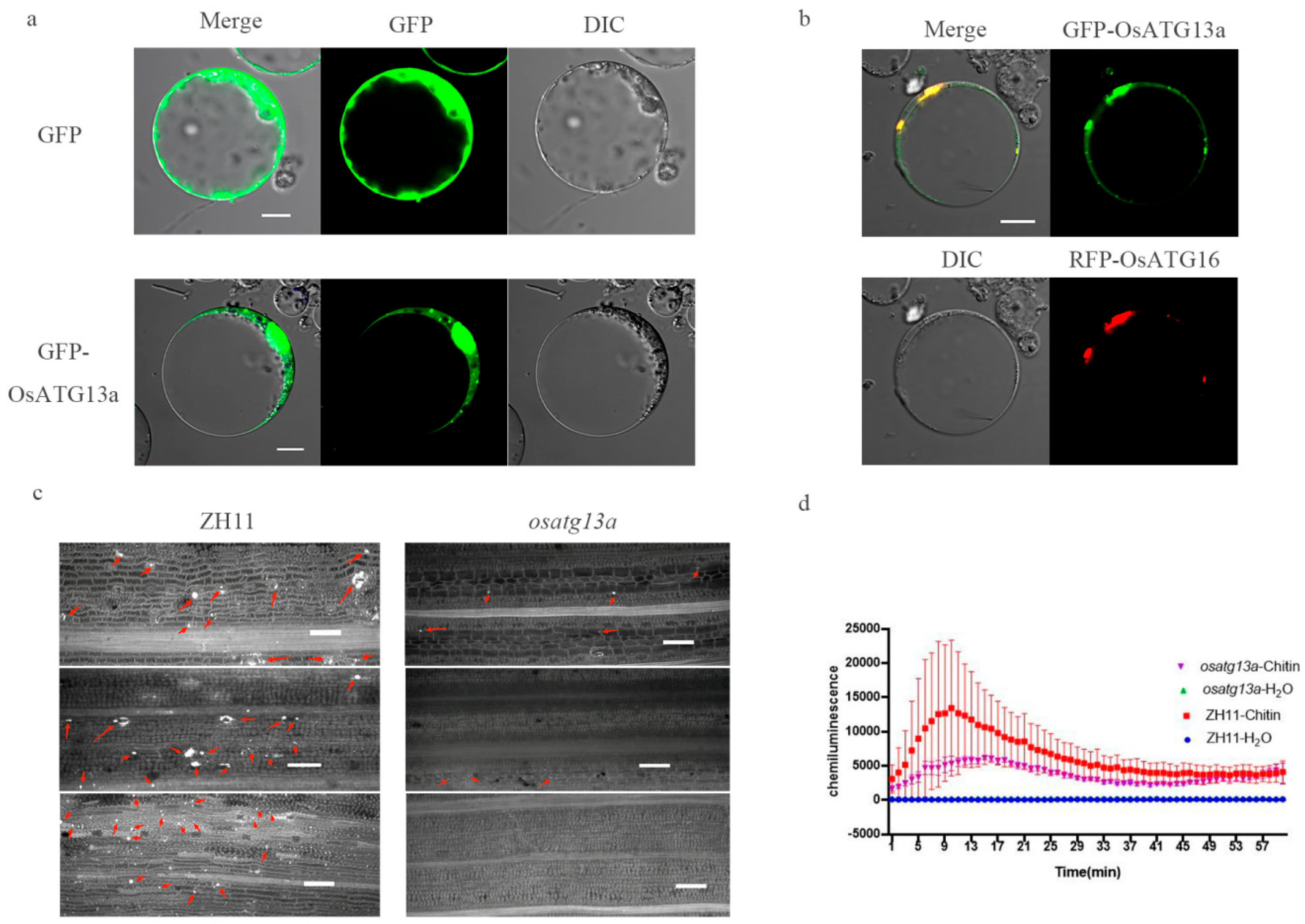
2.9. Subcellular Localization and Role of Immune Reaction of OsATG13a
2.10. Possible Interaction Proteins of OsATG13a
3. Materials and Methods
3.1. Identification of OsATGs in Rice
3.2. Bioinformatics Characterization Analysis
3.3. Analysis of Gene Expression Patterns
3.4. Plants and Materials
3.5. Disease Resistance Analysis
3.6. Pathogen Infection and Hormone Induction Treatment
3.7. Subcellular Localization and Immune Response Analysis
3.8. Analysis of Protein Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Wang, T.; Han, J.; Li, M.; Zhao, Y.; Su, T.; Ma, C. Plant Autophagy: An Intricate Process Controlled by Various Signaling Pathways. Front. Plant Sci. 2021, 12, 754982. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Levine, B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell 2014, 157, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Marion, J.; Le Bars, R.; Besse, L.; Batoko, H.; Satiat-Jeunemaitre, B. Multiscale and Multimodal Approaches to Study Autophagy in Model Plants. Cells 2018, 7, 5. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Cregg, J.M.; Dunn, W.A., Jr.; Emr, S.D.; Sakai, Y.; Sandoval, I.V.; Sibirny, A.; Subramani, S.; Thumm, M.; Veenhuis, M.; et al. A unified nomenclature for yeast autophagy-related genes. Dev. Cell 2013, 5, 539–545. [Google Scholar] [CrossRef]
- Takeshige, K.; Baba, M.; Tsuboi, S.; Noda, T.; Ohsumi, Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 1992, 119, 301–311. [Google Scholar] [CrossRef]
- Wen, X.; Klionsky, D.J. An overview of macroautophagy in yeast. J. Mol. Biol. 2016, 428, 1681–1699. [Google Scholar] [CrossRef]
- Xia, K.; Liu, T.; Ouyang, J.; Wang, R.; Fan, T.; Zhang, M. Genome-wide identification, classification, and expression analysis of autophagy-associated gene homologues in rice (Oryza sativa L.). DNA Res. Int. J. Rapid Publ. Rep. Genes Genomes 2011, 18, 363–377. [Google Scholar] [CrossRef]
- Li, F.; Chung, T.; Pennington, J.G.; Federico, M.L.; Kaeppler, H.F.; Kaeppler, S.M.; Otegui, M.S.; Vierstra, R.D. Autophagic recycling plays a central role in maize nitrogen remobilization. Plant Cell 2015, 27, 1389–1408. [Google Scholar] [CrossRef]
- Huang, W.; Ma, D.N.; Liu, H.L.; Luo, J.; Wang, P.; Wang, M.L.; Guo, F.; Wang, Y.; Zhao, H.; Ni, D.J. Genome-Wide Identification of CsATGs in Tea Plant and the Involvement of CsATG8e in Nitrogen Utilization. Int. J. Mol. Sci. 2020, 21, 7043. [Google Scholar] [CrossRef]
- Luo, K.; Li, J.; Lu, M.; An, H.; Wu, X. Genome-Wide Identification and Expression Analysis of Rosa roxburghii Autophagy-Related Genes in Response to Top-Rot Disease. Biomolecules 2023, 13, 556. [Google Scholar] [CrossRef] [PubMed]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef] [PubMed]
- Avila-Ospina, L.; Marmagne, A.; Soulay, F.; Masclaux-Daubresse, C. Identification of Barley (Hordeum vulgare L.) Autophagy Genes and Their Expression Levels during Leaf Senescence, Chronic Nitrogen Limitation and in Response to Dark Exposure. Agronomy 2016, 6, 15. [Google Scholar] [CrossRef]
- Islam, M.M.; Ishibashi, Y.; Nakagawa, A.C.; Tomita, Y.; Iwaya-Inoue, M.; Arima, S.; Zheng, S.H. Nitrogen redistribution and its relationship with the expression of GmATG8c during seed filling in soybean. J. Plant Physiol. 2016, 192, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Jones, M.L. Silencing ATG6 and PI3K accelerates petal senescence and reduces flower number and shoot biomass in petunia. Plant Sci. Int. J. Exp. Plant Biol. 2021, 302, 110713. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Wang, C.; Zhao, Y.; Yang, Y.; Shan, W.; Kuang, J.; Lu, W.; Fan, Z.; Su, X.; Lin, H.; et al. Molecular characterization of leaf senescence-associated autophagy genes in postharvest Chinese flowering cabbage and identifying their transcriptional activator BrMYB108. Postharvest Biol. Technol. 2022, 185, 111785. [Google Scholar] [CrossRef]
- Huang, L.; Yu, L.J.; Zhang, X.; Fan, B.; Wang, F.Z.; Dai, Y.S.; Qi, H.; Zhou, Y.; Xie, L.J.; Xiao, S. Autophagy regulates glucose-mediated root meristem activity by modulating ROS production in Arabidopsis. Autophagy 2019, 15, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Sera, Y.; Hanamata, S.; Sakamoto, S.; Ono, S.; Kaneko, K.; Mitsui, Y.; Koyano, T.; Fujita, N.; Sasou, A.; Masumura, T.; et al. Essential roles of autophagy in metabolic regulation in endosperm development during rice seed maturation. Sci. Rep. 2019, 9, 18544. [Google Scholar] [CrossRef]
- Signorelli, S.; Tarkowski, Ł.P.; Van den Ende, W.; Bassham, D.C. Linking Autophagy to Abiotic and Biotic Stress Responses. Trends Plant Sci. 2019, 24, 413–430. [Google Scholar] [CrossRef]
- Avin-Wittenberg, T. Autophagy and its role in plant abiotic stress management. Plant Cell Environ. 2019, 42, 1045–1053. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, Y.; Bassham, D.C. Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 2009, 5, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, P.; Jia, X.; Huo, L.; Che, R.; Ma, F. Improvement of drought tolerance by overexpressing MdATG18a is mediated by modified antioxidant system and activated autophagy in transgenic apple. Plant Biotechnol. J. 2018, 16, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Rana, R.M.; Dong, S.; Ali, Z.; Huang, J.; Zhang, H.S. Regulation of ATG6/Beclin-1 homologs by abiotic stresses and hormones in rice (Oryza sativa L.). Genet. Mol. Res. GMR 2012, 11, 3676–3687. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zeng, Z.; Liu, C.; Yuan, W.; Hou, N.; Bian, H.; Zhu, M.; Han, N. A barley homolog of yeast ATG6 is involved in multiple abiotic stress responses and stress resistance regulation. Plant Physiol. Biochem. 2017, 115, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.Y.; Wang, Y.J.; Jiao, J.L.; Wang, H.Z. Silencing of ATG2 and ATG7 promotes programmed cell death in wheat via inhibition of autophagy under salt stress. Ecotoxicol. Environ. Saf. 2021, 225, 112761. [Google Scholar] [CrossRef]
- Shin, J.H.; Yoshimoto, K.; Ohsumi, Y.; Jeon, J.S.; An, G. OsATG10b, an autophagosome component, is needed for cell survival against oxidative stresses in rice. Mol. Cells 2009, 27, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Guo, Z.; Jia, X.; Sun, X.; Wang, P.; Gong, X.; Ma, F. Increased autophagic activity in roots caused by overexpression of the autophagy-related gene MdATG10 in apple enhances salt tolerance. Plant Sci. Int. J. Exp. Plant Biol. 2020, 294, 110444. [Google Scholar] [CrossRef]
- Lenz, H.D.; Haller, E.; Melzer, E.; Kober, K.; Wurster, K.; Stahl, M.; Bassham, D.C.; Vierstra, R.D.; Parker, J.E.; Bautor, J.; et al. Autophagy differentially controls plant basal immunity to biotrophic and necrotrophic pathogens. Plant J. Cell Mol. Biol. 2011, 66, 818–830. [Google Scholar] [CrossRef]
- Lai, Z.; Wang, F.; Zheng, Z.; Fan, B.; Chen, Z. A critical role of autophagy in plant resistance to necrotrophic fungal pathogens. Plant J. Cell Mol. Biol. 2011, 66, 953–968. [Google Scholar] [CrossRef]
- Li, F.; Zhang, M.; Zhang, C.; Zhou, X. Nuclear autophagy degrades a geminivirus nuclear protein to restrict viral infection in solanaceous plants. New Phytol. 2020, 225, 1746–1761. [Google Scholar] [CrossRef]
- Jiang, L.; Lu, Y.; Zheng, X.; Yang, X.; Chen, Y.; Zhang, T.; Zhao, X.; Wang, S.; Zhao, X.; Song, X.; et al. The plant protein NbP3IP directs degradation of Rice stripe virus p3 silencing suppressor protein to limit virus infection through interaction with the autophagy-related protein NbATG8. New Phytol. 2021, 229, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, B.; Moreau, K.; Jahreiss, L.; Puri, C.; Rubinsztein, D.C. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat. Cell Biol. 2010, 12, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liu, J. RLKdb: A comprehensive curated receptor-like kinase family database. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gao, C.; Sun, P.; Wang, W.; Tang, D. Arabidopsis E3 ligase KEG associates with and ubiquitinates MKK4 and MKK5 to regulate plant immunity. J. Integr. Plant Biol. 2021, 63, 327–339. [Google Scholar] [CrossRef]
- Xin, Y.; Meng, S.; Ma, B.; He, W.; He, N. Mulberry genes MnANR and MnLAR confer transgenic plants with resistance to Botrytis cinerea. Plant Sci. Int. J. Exp. Plant Biol. 2020, 296, 110473. [Google Scholar] [CrossRef] [PubMed]
- Muñiz García, M.N.; Grossi, C.; Ulloa, R.M.; Capiati, D.A. The protein phosphatase 2A catalytic subunit StPP2Ac2b enhances susceptibility to Phytophthora infestans and senescence in potato. PLoS ONE 2022, 17, e0275844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dong, S.; Wang, M.; Wang, W.; Song, W.; Dou, X.; Zheng, X.; Zhang, Z. The role of vacuolar processing enzyme (VPE) from Nicotiana benthamiana in the elicitor-triggered hypersensitive response and stomatal closure. J. Exp. Bot. 2010, 61, 3799–3812. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wang, G.; Bai, R.; Zhao, H.; Ge, Z.; Shi, H. The self-association of cytoplasmic malate dehydrogenase 1 promotes malate biosynthesis and confers disease resistance in cassava. Plant Physiol. Biochem. 2023, 201, 107814. [Google Scholar] [CrossRef]
- Duvaud, S.; Gabella, C.; Lisacek, F.; Stockinger, H.; Ioannidis, V.; Durinx, C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021, 49, W216–W227. [Google Scholar] [CrossRef]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Profiti, G.; Casadio, R. BUSCA: An integrative web server to predict subcellular localization of proteins. Nucleic Acids Res. 2018, 46, W459–W466. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Fang, H.; Zhang, C.; Zhang, F.; Hao, Z.; You, X.; Shi, X.; Park, C.H.; Hua, K.; et al. Two VOZ transcription factors link an E3 ligase and an NLR immune receptor to modulate immunity in rice. Mol. Plant 2021, 14, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Yang, F.; Niu, Y.; Lin, Y.; Chen, Z.; Li, G.; Luo, Q.; Wang, F.; Wang, M. Loss function of SL (Sekiguchi lesion) in the rice cultivar Minghui 86 leads to enhanced resistance to (hemi)biotrophic pathogens. BMC Plant Biol. 2020, 20, 507. [Google Scholar] [CrossRef]
- Wilson, R.A. Magnaporthe oryzae . Trends Microbiol. 2021, 29, 663–664. [Google Scholar] [CrossRef]
- Pennisi, E. Armed and dangerous. Science 2010, 327, 804–805. [Google Scholar]
- Kumar, A.A.; Mishra, P.; Kumari, K.; Panigrahi, K.C. Environmental stress influencing plant development and flowering. Front. Biosci. (Sch. Ed.) 2012, 4, 1315–1324. [Google Scholar]
- Su, J.; Spears, B.J.; Kim, S.H.; Gassmann, W. Constant vigilance: Plant functions guarded by resistance proteins. Plant J. Cell Mol. Biol. 2018, 93, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Dong, J.; Wang, T. Autophagy in Plant Abiotic Stress Management. Int. J. Mol. Sci. 2021, 22, 4075. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Hashimi, S.M.; Liu, J.Z. Emerging Roles of the Selective Autophagy in Plant Immunity and Stress Tolerance. Int. J. Mol. Sci. 2020, 21, 6321. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, J.Q.; Chen, Z. The perplexing role of autophagy in plant innate immune responses. Mol. Plant Pathol. 2014, 15, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar]
- Zhou, J.M.; Zhang, Y. Plant Immunity: Danger Perception and Signaling. Cell 2022, 181, 978–989. [Google Scholar] [CrossRef]
- Gupta, A.; Bhardwaj, M.; Tran, L.P. Jasmonic Acid at the Crossroads of Plant Immunity and Pseudomonas syringae Virulence. Int. J. Mol. Sci. 2020, 21, 7482. [Google Scholar] [CrossRef]
- Naidoo, S.; Visser, E.A.; Zwart, L.; Toit, Y.D.; Bhadauria, V.; Shuey, L.S. Dual RNA-Sequencing to Elucidate the Plant-Pathogen Duel. Curr. Issues Mol. Biol. 2018, 27, 127–142. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, W.; Hu, W.; Liu, G.; Wu, C.; Liu, W.; Zeng, H.; He, C.; Shi, H. Genome-wide analysis of autophagy-related genes in banana highlights MaATG8s in cell death and autophagy in immune response to Fusarium wilt. Plant Cell Rep. 2017, 36, 1237–1250. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wang, P.; He, C.; Shi, H. MeWRKY20 and its interacting and activating autophagy-related protein 8 (MeATG8) regulate plant disease resistance in cassava. Biochem. Biophys. Res. Commun. 2017, 494, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Ismayil, A.; Liu, Y. Autophagy in Plant-Virus Interactions. Annu. Rev. Virol. 2020, 7, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ge, W.; Chang, H.; Xin, X.; Ji, R. Discovery of BrATG6 and its potential role in Brassica rapa L. resistance to infection by Plasmodiophora brassicae. Gene 2021, 791, 145711. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Sun, H.; Zhang, W.; Pei, D.; He, Y.; Wang, H. Wheat homologs of yeast ATG6 function in autophagy and are implicated in powdery mildew immunity. BMC Plant Biol. 2015, 15, 95. [Google Scholar] [CrossRef] [PubMed]
- Dagdas, Y.F.; Pandey, P.; Tumtas, Y.; Sanguankiattichai, N.; Belhaj, K.; Duggan, C.; Leary, A.Y.; Segretin, M.E.; Contreras, M.P.; Savage, Z.; et al. Host autophagy machinery is diverted to the pathogen interface to mediate focal defense responses against the Irish potato famine pathogen. eLife 2018, 7, e37476. [Google Scholar] [CrossRef] [PubMed]
- Mamun, M.A.; Tang, C.; Sun, Y.; Islam, M.N.; Liu, P.; Wang, X.; Kang, Z. Wheat Gene TaATG8j Contributes to Stripe Rust Resistance. Int. J. Mol. Sci. 2018, 19, 1666. [Google Scholar] [CrossRef]



| Gene Name | Amino Acid | Molecular Weight | Theoretical pI | Instability Index | Aliphatic Index | GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| OsATG1a | 652 | 72,903.85 | 6.21 | 40.47 | 92.33 | −0.299 | nucleus |
| OsATG1b | 275 | 30,840.6 | 8.49 | 55.13 | 102.36 | −0.054 | nucleus |
| OsATG1c | 703 | 77,457.54 | 6.88 | 56.48 | 84.22 | −0.403 | endomembrane system |
| OsATG2 | 1919 | 212,230.11 | 5.07 | 44.47 | 85.83 | −0.265 | endomembrane system |
| OsATG3a | 294 | 33,057.95 | 4.52 | 47.56 | 76.22 | −0.469 | endomembrane system |
| OsATG3b | 212 | 23,369.21 | 4.58 | 42.9 | 72.59 | −0.478 | nucleus |
| OsATG4a | 388 | 42,519.74 | 4.64 | 37.54 | 82.22 | −0.168 | nucleus |
| OsATG4b | 478 | 52,525.64 | 4.66 | 52.67 | 78.33 | −0.252 | nucleus |
| OsATG5 | 380 | 42,348.98 | 5 | 55.69 | 83.11 | −0.377 | organelle membrane |
| OsATG6a | 500 | 55,898.42 | 5.96 | 39.39 | 79.26 | −0.362 | nucleus |
| OsATG6b | 501 | 56,254.86 | 5.86 | 42.41 | 75.75 | −0.418 | nucleus |
| OsATG6c | 543 | 61,148.31 | 5.95 | 42.94 | 80.29 | −0.414 | organelle membrane |
| OsATG7 | 1041 | 113,579.91 | 5.78 | 36.32 | 92.1 | −0.110 | nucleus |
| OsATG8a | 119 | 13,663.71 | 8.78 | 40.57 | 81.09 | −0.469 | nucleus |
| OsATG8b | 119 | 13,732.86 | 8.78 | 40.36 | 89.33 | −0.403 | nucleus |
| OsATG8c | 120 | 13,888.07 | 8.8 | 38.82 | 88.58 | −0.364 | nucleus |
| OsATG8d | 142 | 16,008.41 | 6.89 | 63.38 | 73.45 | −0.297 | nucleus |
| OsATG8e | 96 | 10,864.61 | 6.89 | 43.08 | 83.12 | 0.015 | nucleus |
| OsATG9a | 902 | 103,663.59 | 6.15 | 52.01 | 86.65 | −0.231 | nucleus |
| OsATG9b | 927 | 106,325.6 | 6.96 | 51.01 | 82.84 | −0.272 | nucleus |
| OsATG10a | 198 | 22,303.36 | 5.81 | 42.93 | 81.16 | −0.305 | nucleus |
| OsATG10b | 185 | 20,232.67 | 4.64 | 45.96 | 87.41 | −0.185 | nucleus |
| OsATG11 | 1140 | 126,809.03 | 5.77 | 41.66 | 82.09 | −0.490 | nucleus |
| OsATG12 | 93 | 10,461.01 | 9.05 | 37.9 | 92.26 | −0.101 | nucleus |
| OsATG13a | 544 | 57,756.13 | 9.19 | 67.32 | 67.9 | −0.341 | nucleus |
| OsATG13b | 520 | 55,863.62 | 8.46 | 70.96 | 65 | −0.499 | nucleus |
| OsATG13c | 601 | 65,461.48 | 7.29 | 62.02 | 75.49 | −0.485 | nucleus |
| OsATG14a | 387 | 42,764.6 | 8.48 | 59.28 | 87.31 | −0.400 | nucleus |
| OsATG14b | 487 | 54,156.64 | 9.71 | 55.13 | 76.32 | −0.514 | nucleus |
| OsATG16 | 511 | 56,463.94 | 6.82 | 46.52 | 83.07 | −0.396 | nucleus |
| OsATG18a | 374 | 40,875.54 | 8.87 | 45.23 | 87.38 | −0.140 | endomembrane system |
| OsATG18b | 417 | 46,186.52 | 8.33 | 37.42 | 78.08 | −0.196 | endomembrane system |
| OsATG18c | 457 | 48,721.81 | 6.12 | 49.43 | 75.36 | −0.296 | endomembrane system |
| OsATG18d | 382 | 42,553.34 | 6.51 | 32.37 | 76.07 | −0.170 | plasma membrane |
| OsATG18e | 888 | 95,991.19 | 6.57 | 46.55 | 78.5 | −0.315 | endomembrane system |
| OsATG18f | 1004 | 108,586.38 | 5.67 | 46.9 | 77.19 | −0.275 | nucleus |
| OsATG18g | 387 | 42,221.48 | 8 | 49.56 | 69.77 | −0.311 | nucleus |
| Name in Study | Gene ID | Function Prediction | Reference about Immunity |
|---|---|---|---|
| OsRLK170576 | XP_015639675.1 | LRR-like protein kinase (RLK ID: OsatE170576) | Yin and Liu 2023 [33] |
| OsKEG1 | XP_015639441.1 | E3 ubiquitin-protein ligase KEG | Gao et al., 2021 [34] |
| OsANR1 | XP_015634145.1 | Anthocyanidin reductase ANR | Xin et al., 2020 [35] |
| OsPP2A-4 | XP_015632306.1 | Serine/threonine-protein phosphatase PP2A-4 | Muñiz García et al., 2022 [36] |
| OsVPE1 | XP_015619322.1 | Vacuolar-processing enzyme VPE | Zhang et al., 2010 [37] |
| OsMDH1 | XP_015634484.1 | Malate dehydrogenase MDH | Zhou et al., 2023 [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Pei, M.; Liu, S.; Wang, X.; Gong, S.; Chen, J.; Zhang, Y.; Wang, Z.; Lu, G.; Li, Y. Comprehensive Analysis of Autophagy-Related Genes in Rice Immunity against Magnaporthe oryzae. Plants 2024, 13, 927. https://doi.org/10.3390/plants13070927
Xie X, Pei M, Liu S, Wang X, Gong S, Chen J, Zhang Y, Wang Z, Lu G, Li Y. Comprehensive Analysis of Autophagy-Related Genes in Rice Immunity against Magnaporthe oryzae. Plants. 2024; 13(7):927. https://doi.org/10.3390/plants13070927
Chicago/Turabian StyleXie, Xuze, Mengtian Pei, Shan Liu, Xinxiao Wang, Shanshan Gong, Jing Chen, Ye Zhang, Zonghua Wang, Guodong Lu, and Ya Li. 2024. "Comprehensive Analysis of Autophagy-Related Genes in Rice Immunity against Magnaporthe oryzae" Plants 13, no. 7: 927. https://doi.org/10.3390/plants13070927







