Population Structures and Dynamics of Rhododendron Communities with Different Stages of Succession in Northwest Guizhou, China
Abstract
:1. Introduction
2. Results
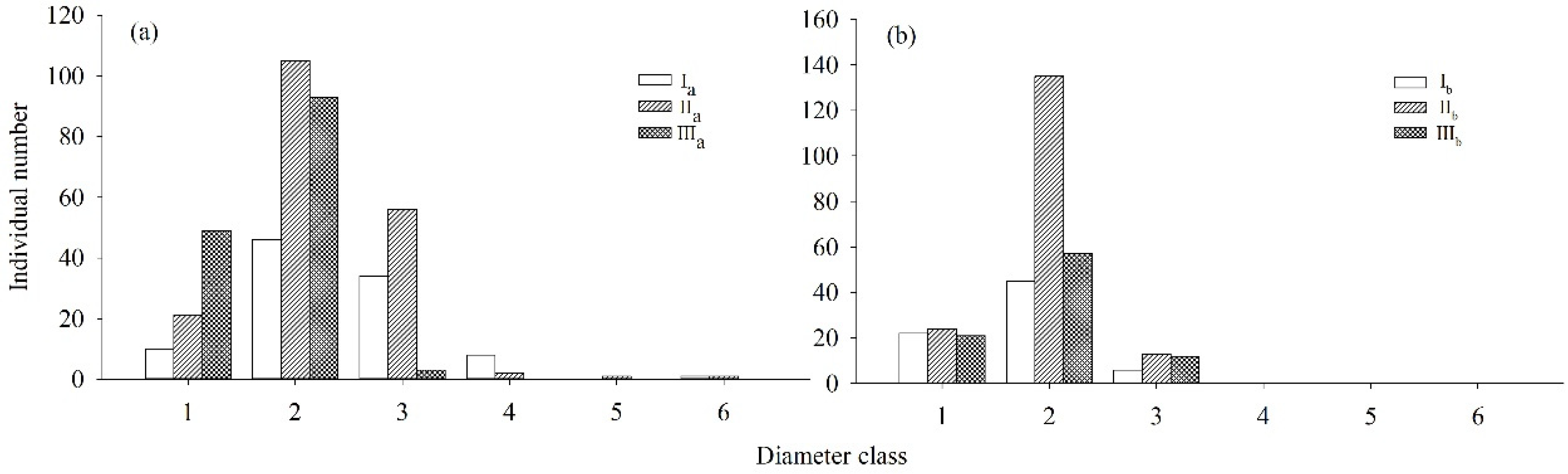
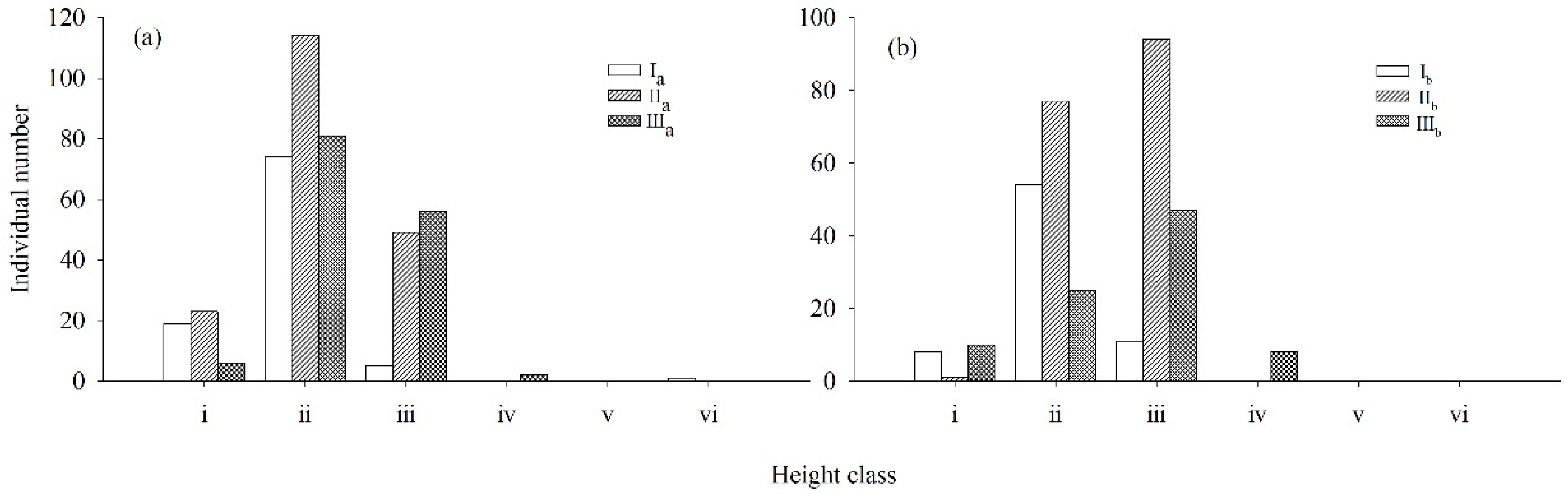
2.1. Quantification of Densities, Diameter Class/Height Structures, and Dynamics of Both Rhododendron Populations
2.2. Static Life Tables and Survival Rate Curves of Both Rhododendron Populations in Communities at Different Stages of Succession
2.3. Time Sequence Analysis of the Two Rhododendron Populations in Communities at Different Stages of Succession
3. Discussion
3.1. Population Structures and Types
3.2. Population Dynamics and Development
4. Materials and Methods
4.1. Overview of the Study Area
4.2. Sample Plot Setting and Survey Methods
4.3. Research Indicators and Data Analysis
4.3.1. Quantification of Population Dynamics
4.3.2. Static Life Tables and Survival Curves
4.3.3. Time Sequence Prediction
4.4. Data Processing and Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harper, J.L. Population Biology of Plant; Oxford University Press: London, UK, 1977; pp. 2–15. [Google Scholar]
- Galal, T.M. Size structure and dynamics of some woody perennials along elevation gradient in Wadi Gimal, Red Sea coast of Egypt. Flora 2011, 206, 638–645. [Google Scholar] [CrossRef]
- Li, W.; Zhang, G.F. Population structure and spatial pattern of the endemic and endangered subtropical tree Parrotia subaequalis (Hamamelidaceae). Flora 2015, 212, 10–18. [Google Scholar] [CrossRef]
- Holeksa, J.; Saniga, M.; Szwagrzyk, J.; Dziedzic, T.; Ferenc, S.; Wodka, M. Altitudinal variability of stand structure and regeneration in the subalpine spruce forests of the Pol’ana biosphere reserve, Central Slovakia. Eur. J. For. Res. 2007, 126, 303–313. [Google Scholar] [CrossRef]
- Druckenbrod, D.; Shugart, H.; Davies, I. Spatial pattern and process in forest stands within the Virginia piedmont. J. Veg. Sci. 2009, 16, 37–48. [Google Scholar] [CrossRef]
- Wu, J.X.; Zhang, X.M.; Deng, C.Z.; Liu, G.J. Structure and dynamic of Populus euphratica population along Tarim River. Pak. J. Bot. 2012, 44, 1651–1656. [Google Scholar]
- Omelko, A.; Ukhvatkina, O.; Zhmerenetsky, A.; Sibirina, L.; Petrenko, T.; Bobrovsky, M. From young to adult trees: How spatial patterns of plants with different life strategies change during age development in an old-growth Korean pine-broadleaved forest. For. Ecol. Manag. 2018, 411, 46–66. [Google Scholar] [CrossRef]
- Wen, L.; Song, T.S.; Du, H.M.; Wang, K.L. The succession characteristics and its driving mechanism of plant community in karst region, Southwest China. Acta Ecol. Sin. 2015, 35, 5822–5833. [Google Scholar] [CrossRef]
- Zeng, Q.C.; An, S.S.; Liu, Y. Soil bacterial community response to vegetation succession after fencing in the grassland of China. Sci. Total Environ. 2017, 609, 2–10. [Google Scholar] [CrossRef]
- Chamberlain, D.; Hyam, R.; Argent, G.; Fairweather, G.; Walter, K. The Genus Rhododendron: Its Classification and Synonymy; Royal Botanic Garden Edinburgh: Edinburgh, UK, 1996. [Google Scholar]
- Mackay, M.; Gardner, S. Geographic analysis of Red List Rhododendron (Ericaceae) taxa by country of origin identifies priorities for ex situ conservation. Blumea 2017, 62, 103–120. [Google Scholar] [CrossRef]
- Li, T.Q.; Liu, X.F.; Li, Z.G.; Ma, H.; Wan, Y.M.; Liu, X.X.; Fu, L.Y. Study on reproductive biology of Rhododendron longipedicellatum: A newly discovered and special threatened plant surviving in limestone habitat in southeast Yunnan China. Front. Plant Sci. 2018, 9, 33. [Google Scholar] [CrossRef]
- Ahmad, P.I.; Puni, L.; Pandey, R.; Pala, N.A.; Rather, M.M.; Rashid, M.; Malik, A.R. Influence of different treatments and techniques on rooting behavior of Rhododendron arboreum Sm. in Indian Himalayas. Acta Ecol. Sin. 2021, 41, 332–335. [Google Scholar] [CrossRef]
- Yu, F.Y.; Skidmore, A.K.; Wang, T.J.; Huang, J.H. Low-elevation endemic Rhododendrons in China are highly vulnerable to climate and land use change. Ecol. Indic. 2021, 126, 107699. [Google Scholar] [CrossRef]
- Min, T.L.; Fang, R.Z. On the origin and geographic distribution of genus Rhododendron L. Plant Divers. 1979, 1, 19–30. [Google Scholar]
- Berg, W. Rhododendron Exploration in Tibet; Digital Library and Archives of the Virginia Tech University Libraries: Blacksburg, VA, USA, 1987. [Google Scholar]
- Wang, Z.C.; Chen, X.; Ren, Z.M.; Hu, C.X.; Zhao, C.Y.; Bo, L.; Shen, S.K. Population structure and dynamic characteristies of an endangered and endemic species Rhododendron rex subsp. rex in Southwest, China. Seeds 2010, 29, 72–76. [Google Scholar]
- Chen, X.; Huang, J.Y.; Huang, C.L.; Chen, X. Study on the macromorphological characters of Rhododendron (Ericaceae) in Northwestern Guizhou. Seeds 2013, 32, 51–55. [Google Scholar]
- Rong, L.; Chen, X.; Wang, X.C. Leaf Anatomical Characters and Its Ecological Adaptation of 13 Species of Rhododendron in Baili Azalea Area. J. Anhui Agric. Sci. 2009, 37, 1084–1088. [Google Scholar] [CrossRef]
- Mayr, S.; Beikircher, B.; Obkircher, M.A.; Schmid, P. Hydraulic plasticity and limitations of alpine Rhododendron species. Oecologia 2010, 164, 321–330. [Google Scholar] [CrossRef]
- Jia, Z.Z.; Li, W.J.; Tian, A.; Wang, J.G. Ecological influence of tourism disturbance on the characteristics of different communities in Baili Rhododendron forest. Acta Ecol. Sin. 2021, 41, 4641–4649. [Google Scholar]
- Wang, Z.Y.; Wang, L.J.; Tian, X.L.; Zhang, Q.; Wang, J.G.; Shuai, H.G.; Chen, F.; Liu, M.; Jin, B.C.; Zhao, X.C. Characteristies and species diversity of different Rhododendron shrub communities in northwest Guizhou. Acta Ecol. Sin. 2023, 43, 693–701. [Google Scholar] [CrossRef]
- Masaki, T.; Ota, T.; Sugita, H.; Oohara, H.; Otani, T.; Nagaike, T.; Nakamura, S. Structure and dynamics of tree populations within unsuccessful conifer plantations near the Shirakami Mountains, a snowy region of Japan. For. Ecol. Manag. 2004, 194, 389–401. [Google Scholar] [CrossRef]
- Svensson, J.S.; Jeglum, J.K. Structure and dynamics of an undisturbed old-growth Norway spruce forest on the rising Bothnian coastline. For. Ecol. Manag. 2001, 151, 67–79. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhao, X.C.; Wang, L.J.; Wang, Z.Y.; Shuai, H.G.; Wang, Y.F.; Jin, B.C.; Chen, C. Effects of clearcutting on species composition and community renewal of Rhododendron shrubs in northwest Guizhou Province, China. Front. Ecol. Evol. 2023, 11, 1225466. [Google Scholar] [CrossRef]
- Mao, A.A.; Kaliamoorthy, S.; Ranyaphi, R.A.; Das, J.; Gupta, S.; Athili, J.; Yumnam, J.Y.; Chanu, L.I. In vitro micropropagation of three rare, endangered, and endemic Rhododendron species of Northeast India. In Vitro Cell. Dev. Biol.-Plant. 2011, 47, 674–681. [Google Scholar] [CrossRef]
- Yang, H.Q.; Liu, Y.H.; Liu, F.L.; Hu, C.X.; Zhao, C.Y.; Long, B.; Shen, S.K. population structure and dynamic characteristies of an endangered and endemic species Rhododendron rex subsp. rex in Southwest, China. Acta Bot. Boreali-Occident. Sin. 2020, 40, 2148–2156. [Google Scholar] [CrossRef]
- Lorenzo, P.; Palomera-Pérez, A.; Reigosa, M.J.; González, L. Allelopathic interference of invasive Acacia dealbata Link on the physiological parameters of native understory species. Plant Ecol. 2011, 212, 403–412. [Google Scholar] [CrossRef]
- Xu, W.B.; Zhao, M.; Waleta; Shi, Y.; Hu, T.H.; Yu, Y.W. Quantitative traits, age structure and self-thining rule of three shrubs on northest edge of Qinghai-Tibet plateau. Pratac. Sci. 2019, 36, 1207–1214. [Google Scholar]
- Bierzychudek, P. Plant Biodiversity and Population Dynamics. In Ecology and the Environment; Springer: New York, NY, USA, 2014; p. 2965. [Google Scholar] [CrossRef]
- Comita, L.S.; Hubbell, S.P. Local neighborhood and species’ shade tolerance influence survival in a diverse seedling bank. Ecololy 2009, 90, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Chen, X.A. Preliminary study on structure and regeneration of Rhododendron delavayi population in Baili Azalea Forest Park. Guizhou Sci. 2005, 23, 46–49. [Google Scholar] [CrossRef]
- Jiang, H.; He, B.; Luo, Q. A study of the relationship between the distribution of bryophytes and the environmental factors in Baili Azalea National Forest Park. Ecol. Sci. 2015, 34, 31–36. [Google Scholar] [CrossRef]
- Wu, J.X.; Zhang, X.M.; Deng, C.Z.; Liu, G.J.; Li, H. Characteristics and dynamics analysis of Populus euphratica populations in the middle reaches of Tarim River. J. Arid Land 2010, 2, 250–256. [Google Scholar] [CrossRef]
- Chen, X.D. A study on the method of quantitative analysis for plant population and community structural dynamics. Acta Ecol. Sin. 1998, 2, 104–107. [Google Scholar]
- Bisht, V.K.; Kuniyal, C.P.; Nautiyal, B.P.; Prasad, P. Spatial distribution and regeneration of Quercus semecarpifolia and Quercus floribunda in a subalpine forest of western Himalaya, India. Physiol. Mol. Biol. Plants 2013, 19, 443–448. [Google Scholar] [CrossRef]
- Long, T.; Wu, X.L.; Wang, Y.; Chen, J. The population status and threats of Taxus cuspidate, a plant species with extremely small populations in China. Glob. Ecol. Conserv. 2021, 26, e01495. [Google Scholar] [CrossRef]
- Hett, J.M.; Loucks, O.L. Age structure models of balsam fir and eastern hemlock. J. Ecol. 1976, 64, 1029–1044. [Google Scholar] [CrossRef]
- Xiao, Y.A.; He, P.; Li, X.H.; Deng, H.P. Study on numeric dynamics of natural populations of the endangered species Disanthus cercidifolius var. longipes. Chin. J. Plant Ecol. 2004, 28, 252–257. [Google Scholar] [CrossRef]




| Index (%) | Community | |||||
|---|---|---|---|---|---|---|
| Ia | IIa | IIIa | Ib | IIb | IIIb | |
| V1 | −78.26 | 80.00 | −47.31 | −51.11 | −82.22 | −63.16 |
| V2 | 26.01 | 46.67 | 96.77 | 86.67 | 90.37 | 78.95 |
| V3 | 76.47 | 96.43 | 100.00 | 100.00 | 100.00 | 100.00 |
| V4 | 100.00 | 50.00 | – | – | – | – |
| V5 | −100.00 | 0.00 | – | – | – | – |
| V6 | 100.00 | 100.00 | – | – | – | – |
| Vpi | 39.57 | 47.42 | 48.15 | 46.24 | 67.02 | 48.60 |
| V′pi | 6.59 | 7.90 | 2.67 | 1.28 | 0.86 | 0.76 |
| Species | Index | Community | Age Class | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| R. annae | ax (plant) | Ia | 10 | 46 | 34 | 8 | 0 | 1 |
| IIa | 21 | 105 | 56 | 2 | 1 | 1 | ||
| IIIa | 49 | 93 | 3 | 0 | 0 | 0 | ||
| a′x (plant) | Ia | 42 | 30 | 28 | 1 | 1 | 1 | |
| IIa | 89 | 61 | 33 | 2 | 1 | 1 | ||
| IIIa | 78 | 48 | 18 | 0 | 0 | 0 | ||
| lx (plant) | Ia | 1000 | 714 | 667 | 24 | 24 | 24 | |
| IIa | 1000 | 685 | 371 | 22 | 11 | 11 | ||
| IIIa | 1000 | 615 | 231 | – | – | – | ||
| lnlx | Ia | 6.91 | 6.57 | 6.5 | 3.17 | 3.17 | 3.17 | |
| IIa | 6.91 | 6.53 | 5.92 | 3.11 | 2.42 | 2.42 | ||
| IIIa | 6.91 | 6.42 | 5.44 | – | – | – | ||
| dx (plant) | Ia | 286 | 48 | 643 | 0 | 0 | 24 | |
| IIa | 315 | 315 | 348 | 11 | 0 | 11 | ||
| IIIa | 385 | 385 | 231 | – | – | – | ||
| qx (%) | Ia | 28.57 | 6.67 | 96.43 | 0 | 0 | 100 | |
| IIa | 31.46 | 45.9 | 93.94 | 50 | 0 | 100 | ||
| IIIa | 38.46 | 62.5 | 100 | – | – | – | ||
| Lx (plant) | Ia | 857 | 690 | 345 | 24 | 24 | 12 | |
| IIa | 843 | 528 | 197 | 17 | 11 | 6 | ||
| IIIa | 808 | 423 | 115 | – | – | – | ||
| Tx (plant) | Ia | 1952 | 1095 | 405 | 60 | 36 | 12 | |
| IIa | 1601 | 758 | 230 | 34 | 17 | 6 | ||
| IIIa | 1346 | 538 | 115 | – | – | – | ||
| ex (year) | Ia | 1.95 | 1.53 | 0.61 | 2.5 | 1.5 | 0.5 | |
| IIa | 1.6 | 1.11 | 0.62 | 1.5 | 1.5 | 0.5 | ||
| IIIa | 1.35 | 0.88 | 0.5 | – | – | – | ||
| Kx (%) | Ia | 0.34 | 0.07 | 3.33 | 0 | 0 | 3.17 | |
| IIa | 0.38 | 0.61 | 2.8 | 0.69 | 0 | 2.42 | ||
| IIIa | 0.49 | 0.98 | 5.44 | – | – | – | ||
| R. irroratum | ax (plant) | Ib | 53 | 61 | 11 | 1 | 0 | 0 |
| IIb | 24 | 135 | 13 | 0 | 0 | 0 | ||
| IIIb | 21 | 57 | 12 | 0 | 0 | 0 | ||
| a′x (plant) | Ib | 59 | 42 | 25 | 1 | 1 | 1 | |
| IIb | 98 | 57 | 16 | 0 | 0 | 0 | ||
| IIIb | 45 | 30 | 15 | 0 | 0 | 0 | ||
| lx (plant) | Ib | 1000 | 712 | 424 | 17 | 17 | 17 | |
| IIb | 1000 | 582 | 163 | – | – | – | ||
| IIIb | 1000 | 667 | 333 | – | – | – | ||
| lnlx | Ib | 6.91 | 6.57 | 6.05 | 2.83 | 2.83 | 2.83 | |
| IIb | 6.91 | 6.37 | 5.1 | – | – | – | ||
| IIIb | 6.91 | 6.5 | 5.81 | – | – | – | ||
| dx (plant) | Ib | 288 | 288 | 407 | 0 | 0 | 17 | |
| IIb | 418 | 418 | 163 | – | – | – | ||
| IIIb | 333 | 333 | 333 | – | – | – | ||
| qx (%) | Ib | 28.81 | 40.48 | 96 | 0 | 0 | 100 | |
| IIb | 41.84 | 71.93 | 100 | – | – | – | ||
| IIIb | 33.33 | 50 | 100 | – | – | – | ||
| Lx (plant) | Ib | 856 | 568 | 220 | 17 | 17 | 8 | |
| IIb | 791 | 372 | 82 | – | – | – | ||
| IIIb | 833 | 500 | 167 | – | – | – | ||
| Tx (plant) | Ib | 1686 | 831 | 263 | 42 | 25 | 8 | |
| IIb | 1245 | 454 | 82 | – | – | – | ||
| IIIb | 1500 | 667 | 167 | – | – | – | ||
| ex (year) | Ib | 1.69 | 1.17 | 0.62 | 2.5 | 1.5 | 0.5 | |
| IIb | 1.24 | 0.78 | 0.5 | – | – | – | ||
| IIIb | 1.5 | 1 | 0.5 | – | – | – | ||
| Kx (%) | Ib | 0.34 | 0.52 | 3.22 | 0 | 0 | 2.83 | |
| IIb | 0.54 | 1.27 | 5.1 | – | – | – | ||
| IIIb | 0.41 | 0.69 | 5.81 | – | – | – | ||
| Community | Exponential Function Model | R2 | Power Function Model | R2 |
|---|---|---|---|---|
| Ia | y = 9.948e−0.235x | 0.664 ** | y = 7.831x−0.438 | 0.501 ** |
| IIa | y = 10.733e−0.284x | 0.879 ** | y = 8.445x−0.640 | 0.720 ** |
| IIIa | y = 7.908e−0.120x | 0.953 ** | y = 7.040x−0.206 | 0.870 ** |
| Ib | y = 10.529e−0.276x | 0.722 ** | y = 7.980x−0.520 | 0.555 ** |
| IIb | y = 8.234e−0.152x | 0.933 ** | y = 7.096x−0.259 | 0.840 ** |
| IIIb | y = 7.600e−0.087x | 0.972 ** | y = 6.991x−0.150 | 0.902 ** |
| Species | Age class | Primary Data | M2(1) | M4(1) | M6(1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ia | IIa | IIIa | Ia | IIa | IIIa | Ia | IIa | IIIa | Ia | IIa | IIIa | ||
| R. annae | 1 | 10 | 21 | 49 | |||||||||
| 2 | 46 | 105 | 93 | 28 | 63 | 71 | |||||||
| 3 | 34 | 56 | 3 | 40 | 81 | 48 | |||||||
| 4 | 8 | 2 | 0 | 21 | 29 | 2 | 30 | 58 | 40 | ||||
| 5 | 0 | 1 | 0 | 4 | 2 | 0 | 22 | 37 | 17 | ||||
| 6 | 1 | 1 | 0 | 1 | 1 | 0 | 9 | 11 | 0 | 15 | 27 | 14 | |
| Total | 99 | 186 | 145 | 94 | 176 | 121 | 61 | 106 | 57 | 15 | 27 | 14 | |
| Species | Age class | Primary Data | M2(1) | M4(1) | M6(1) | ||||||||
| Ib | IIb | IIIb | Ib | IIb | IIIb | Ib | IIb | IIIb | Ib | IIb | IIIb | ||
| R. irroratum | 1 | 53 | 24 | 21 | |||||||||
| 2 | 61 | 135 | 57 | 57 | 80 | 39 | |||||||
| 3 | 11 | 13 | 12 | 36 | 74 | 35 | |||||||
| 4 | 1 | 0 | 0 | 6 | 7 | 6 | 33 | 54 | 27 | ||||
| 5 | 0 | 0 | 0 | 1 | 0 | 0 | 14 | 27 | 14 | ||||
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 12 | 21 | 10 | |
| Total | 126 | 172 | 90 | 100 | 161 | 80 | 49 | 83 | 41 | 12 | 21 | 10 | |
| Community Type | Mark | Altitude (m) | Longitude and Latitude | Rhododendron Important Value | Constructive Species |
|---|---|---|---|---|---|
| R. annae + Lyonia ovalifolia shrubs | Ia | 1807 | E 105°51′03.52″ N 27°24′5.53″ | 0.453 | Lyonia ovalifolia R. annae |
| R. annae shrubs | IIa | 1825 | E 105°51′58.12″ N 27°23′20.93″ | 0.595 | R. annae |
| R. annae + broad-leaved forest | IIIa | 1783 | E 105°51′52.23″ N 27°23′23.94″ | 0.485 | R. annae |
| R. irroratum + Lyonia ovalifolia shrubs | Ib | 1803 | E 105°52′58.88″ N 27°23′25.34″ | 0.417 | R. irroratum Lyonia ovalifolia |
| R. irroratum shrubs | IIb | 1804 | E 105°51′57.49″ N 27°23′24.64″ | 0.656 | R. irroratum |
| R. irroratum + broad-leaved forest | IIIb | 1811 | E 105°51′52.78″ N 27°23′22.93″ | 0.556 | R. irroratum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, J.; Wang, X.; Wang, L.; Wang, Y.; Wei, J.; Niu, Z.; Jian, L.; Jin, B.; Chen, C.; et al. Population Structures and Dynamics of Rhododendron Communities with Different Stages of Succession in Northwest Guizhou, China. Plants 2024, 13, 946. https://doi.org/10.3390/plants13070946
Zhang Y, Wang J, Wang X, Wang L, Wang Y, Wei J, Niu Z, Jian L, Jin B, Chen C, et al. Population Structures and Dynamics of Rhododendron Communities with Different Stages of Succession in Northwest Guizhou, China. Plants. 2024; 13(7):946. https://doi.org/10.3390/plants13070946
Chicago/Turabian StyleZhang, Yaoyao, Jianli Wang, Xiaojing Wang, Lingjun Wang, Yuefeng Wang, Junpeng Wei, Zijing Niu, Linye Jian, Baocheng Jin, Chao Chen, and et al. 2024. "Population Structures and Dynamics of Rhododendron Communities with Different Stages of Succession in Northwest Guizhou, China" Plants 13, no. 7: 946. https://doi.org/10.3390/plants13070946





