Artemisia spp. Essential Oils: From Their Ethnobotanical Use to Unraveling the Microbiota Modulation Potential
Abstract
1. Introduction
2. Results
2.1. Qualitative Analysis
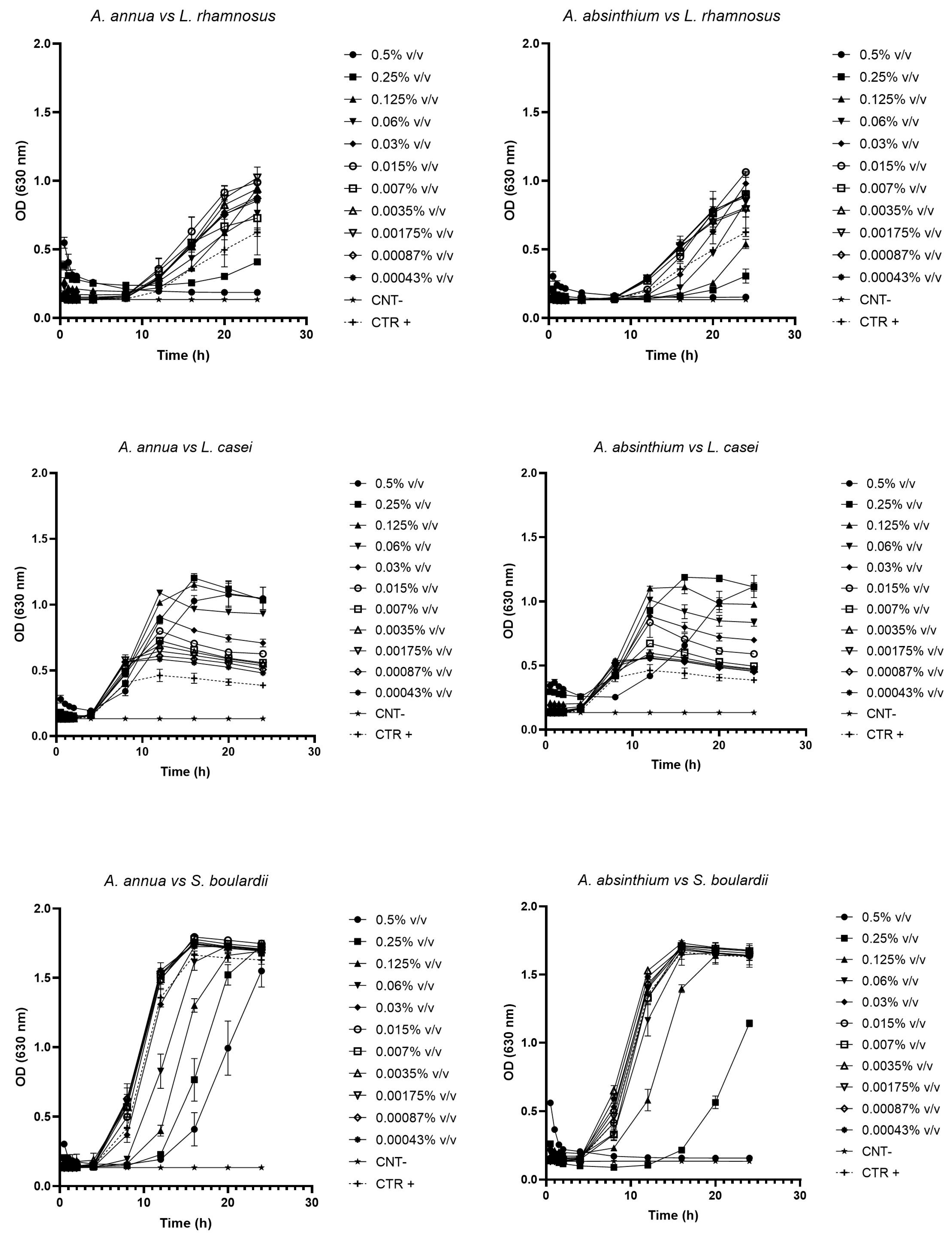
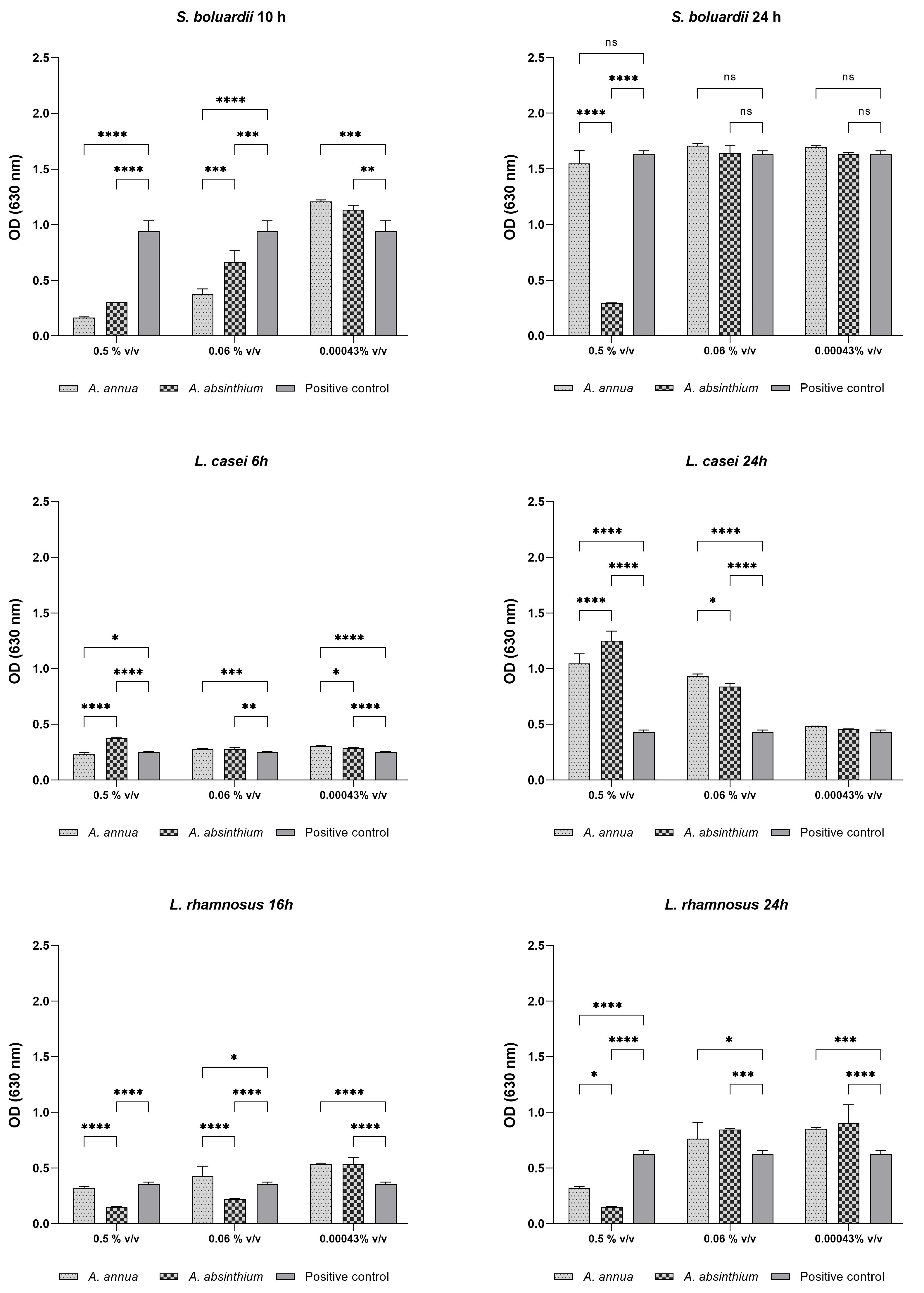
2.2. Growth Curve Testing
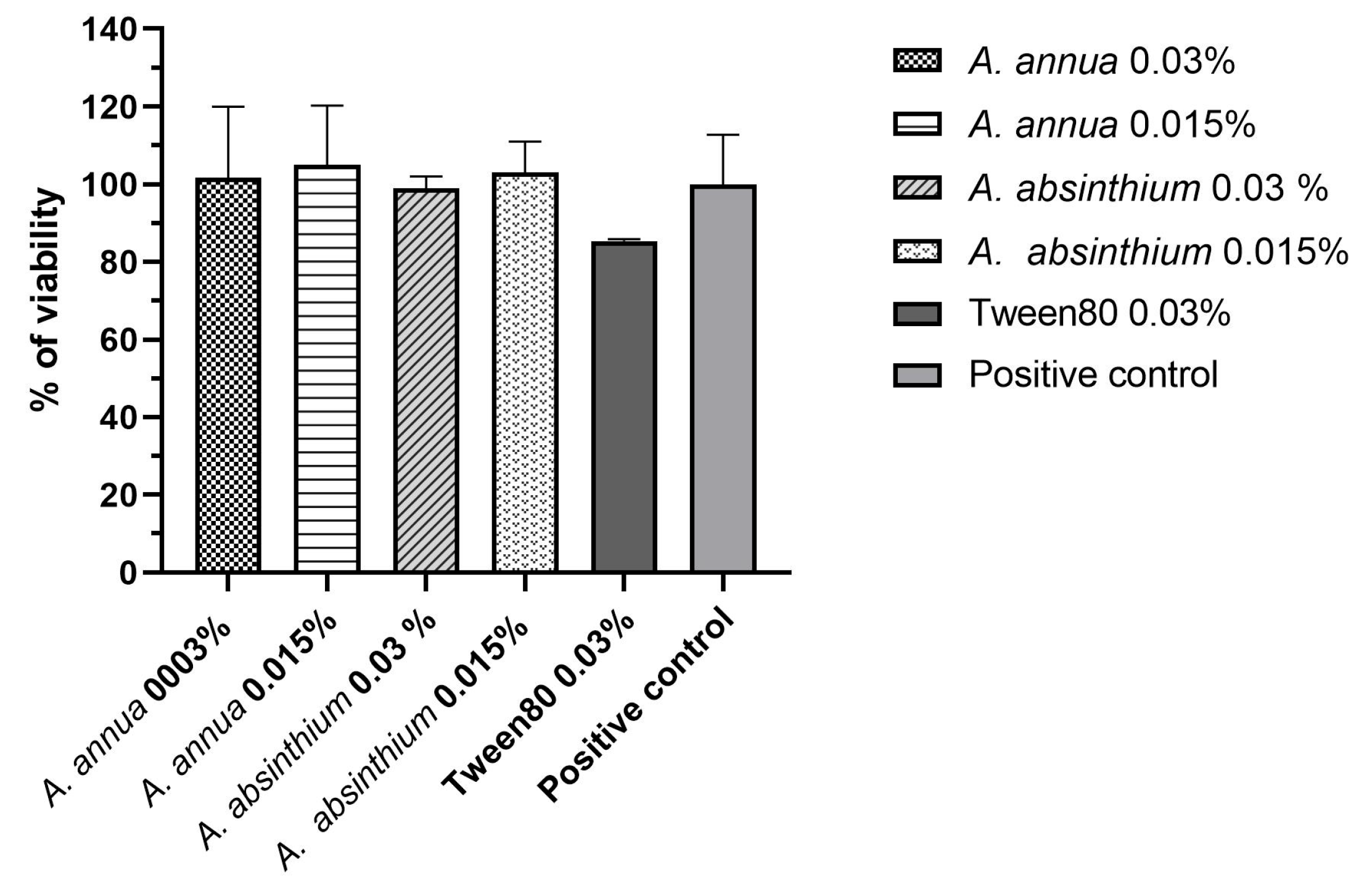
2.3. Cell Viability
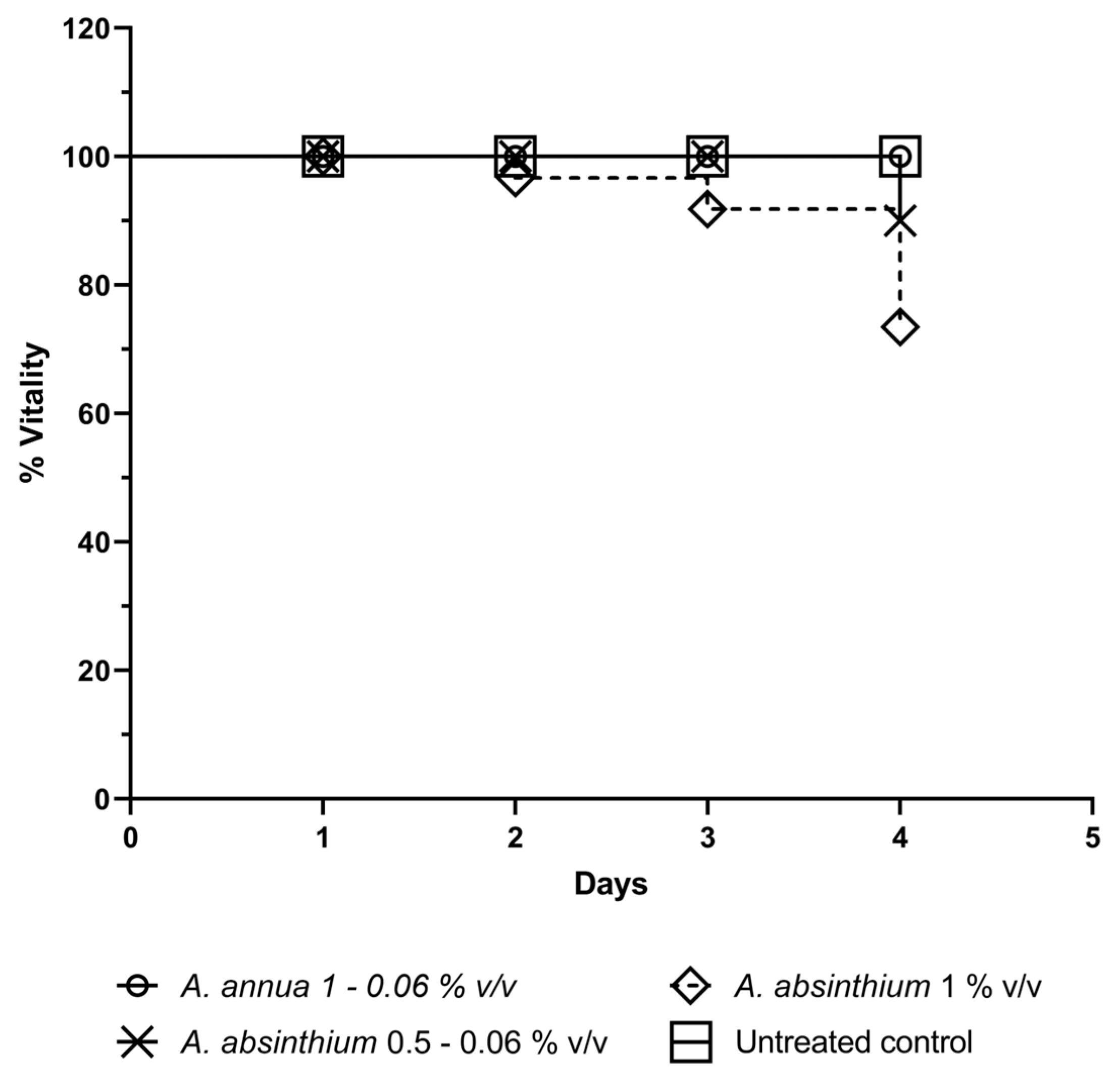
2.4. In Vivo Toxicity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction of Essential Oils
4.3. Analysis of Essential Oils
4.4. Bacterial Strains, Culture Medium and Chemicals
4.5. Prebiotic Activity of EOs
4.6. Growth Curve Testing
4.7. Cell Culture
4.8. Cell Viability
4.9. In Vivo Toxicity Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia Species with High Biological Values as a Potential Source of Medicinal and Cosmetic Raw Materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef] [PubMed]
- Hildegard von Bingen Association. Available online: https://www.santa-ildegarda-di-bingen.it/it/s-ildegarda/article-le-sue-opere/ (accessed on 15 February 2024).
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.A.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.-Importance in the History of Medicine, the Latest Advances in Phytochemistry and Therapeutical, Cosmetological and Culinary Uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef]
- Panesar, P.S.; Joshi, V.K.; Panesar, R.; Abrol, G.S. Vermouth: Technology of Production and Quality Characteristics. Adv. Food Nutr. Res. 2011, 63, 251–283. [Google Scholar] [CrossRef]
- Vogt, D.D. Absinthium: A Nineteenth-Century Drug of Abuse. J. Ethnopharmacol. 1981, 4, 337–342. [Google Scholar] [CrossRef]
- Albert-Puleo, M. Van Gogh’s Vision: Thujone Intoxication. JAMA 1981, 246, 42. [Google Scholar] [CrossRef]
- Holstege, C.P.; Baylor, M.R.; Rusyniak, D.E. Absinthe: Return of the Green Fairy. Semin. Neurol. 2002, 22, 89–93. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Walch, S.G.; Padosch, S.A.; Kröner, L.U. Absinthe—A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Soon, L.; Ng, P.Q.; Chellian, J.; Madheswaran, T.; Panneerselvam, J.; Gupta, G.; Nammi, S.; Hansbro, N.; Hsu, A.; Dureja, H.; et al. Therapeutic Potential of Artemisia Vulgaris: An Insight into Underlying Immunological Mechanisms. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Zouari, S.; Ayadi, I.; Fakhfakh, N.; Jdir, H.; Aloui, L.; Kossentini, M.; Rebai, A.; Zouari, N. Essential Oil Variation in Wild Populations of Artemisia Saharae (Asteraceae) from Tunisia: Chemical Composition, Antibacterial and Antioxidant Properties. Bot. Stud. 2014, 55, 76. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Poulev, A.; O’Neal, J.; Wnorowski, G.; Malek, D.E.; Jäger, R.; Raskin, I. Toxicological Evaluation of the Ethanolic Extract of Artemisia Dracunculus L. for Use as a Dietary Supplement and in Functional Foods. Food Chem. Toxicol. 2004, 42, 585–598. [Google Scholar] [CrossRef]
- Zhigzhitzhapova, S.V.; Dylenova, E.P.; Gulyaev, S.M.; Randalova, T.E.; Taraskin, V.V.; Tykheev, Z.A.; Radnaeva, L.D. Composition and Antioxidant Activity of the Essential Oil of Artemisia Annua L. Nat. Prod. Res. 2020, 34, 2668–2671. [Google Scholar] [CrossRef] [PubMed]
- Chebbac, K.; Ghneim, H.K.; El Moussaoui, A.; Bourhia, M.; El Barnossi, A.; Ouaritini, Z.B.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; Giesy, J.P.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia Aragonensis Lam. against Drug-Resistant Microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef] [PubMed]
- Chebbac, K.; Benziane Ouaritini, Z.; El Moussaoui, A.; Chalkha, M.; Lafraxo, S.; Bin Jardan, Y.A.; Nafidi, H.A.; Bourhia, M.; Guemmouh, R. Antimicrobial and Antioxidant Properties of Chemically Analyzed Essential Oil of Artemisia Annua L. (Asteraceae) Native to Mediterranean Area. Life 2023, 13, 807. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.A.; Khan, N.M.; Ahmad, S.; Nasruddin; Aziz, R.; Ullah, I.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A. Phytochemical Profiling, Antioxidant, Antimicrobial and Cholinesterase Inhibitory Effects of Essential Oils Isolated from the Leaves of Artemisia Scoparia and Artemisia absinthium. Pharmaceuticals 2022, 15, 1221. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Díaz, R.A.; Ibáñez-Escribano, A.; Burillo, J.; de las Heras, L.; del Prado, G.; Agulló-Ortuño, M.T.; Julio, L.F.; González-Coloma, A. Trypanocidal, Trichomonacidal and Cytotoxic Components of Cultivated Artemisia Absinthium Linnaeus (Asteraceae) Essential Oil. Mem. Inst. Oswaldo Cruz 2015, 110, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Naß, J.; Efferth, T. The Activity of Artemisia Spp. and Their Constituents against Trypanosomiasis. Phytomedicine 2018, 47, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pang, B.; Yan, X.; Shang, X.; Hu, X.; Shi, J. Prebiotic Properties of Different Polysaccharide Fractions from Artemisia Sphaerocephala Krasch Seeds Evaluated by Simulated Digestion and in Vitro Fermentation by Human Fecal Microbiota. Int. J. Biol. Macromol. 2020, 162, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Davila, M.M.; Papada, E. The Role of Plant-Derived Natural Products in the Management of Inflammatory Bowel Disease-What Is the Clinical Evidence So Far? Life 2023, 13, 1703. [Google Scholar] [CrossRef]
- Ksibi, N.; Saada, M.; Yeddes, W.; Limam, H.; Tammar, S.; Wannes, W.A.; Msaada, K. Phytochemical Profile, Antioxidant and Antibacterial Activities of Artemisia Absinthium L. Collected in Tunisian Regions. J. Mex Chem. Soc. 2022, 66, 312–329. [Google Scholar] [CrossRef]
- Riahi, L.; Ghazghazi, H.; Ayari, B.; Aouadhi, C.; Klay, I.; Chograni, H.; Zoghlami, N. Effect of Environmental Conditions on Chemical Polymorphism and Biological Activities among Artemisia Absinthium L. Essential Oil Provenances Grown in Tunisia. Ind. Crops Prod. 2015, 66, 96–102. [Google Scholar] [CrossRef]
- Bachrouch, O.; Ferjani, N.; Haouel, S.; Jemâa, J.M.B. Main Compounds and Insecticidal Activities of Two Tunisian Artemisia Essential Oils towards Two Important Beetle Parasites. Crops Ind. Prod. 2015, 65, 127–133. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Magdy Beshbishy, A.; Murata, T.; Zaragoza-Bastida, A.; et al. Bioactive Compounds, Pharmacological Actions, and Pharmacokinetics of Wormwood (Artemisia Absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Jelodar, N.B.; Modarresi, M.; Bagheri, N.; Jamali, A. Increase of Chamazulene and α-Bisabolol Contents of the Essential Oil of German Chamomile (Matricaria chamomilla L.) Using Salicylic Acid Treatments under Normal and Heat Stress Conditions. Foods 2016, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Michelakis, E.C.; Evergetis, E.; Koulocheri, S.D.; Haroutounian, S.A. Exploitation of Artemisia Arborescens as a Renewable Source of Chamazulene: Seasonal Variation and Distillation Conditions. Nat. Prod. Commun. 2016, 11, 1513–1516. [Google Scholar] [CrossRef]
- Baldino, L.; Reverchon, E.; Della Porta, G. An Optimized Process for SC-CO2 Extraction of Antimalarial Compounds from Artemisia Annua L. J. Supercrit. Fluids 2017, 128, 89–93. [Google Scholar] [CrossRef]
- Donato, R.; Santomauro, F.; Bilia, A.R.; Flamini, G.; Sacco, C. Antibacterial Activity of Tuscan Artemisia Annua Essential Oil and Its Major Components against Some Foodborne Pathogens. LWT-Food Sci. Technol. 2015, 64, 1251–1254. [Google Scholar] [CrossRef]
- Bilia, A.R.; Santomauro, F.; Sacco, C.; Bergonzi, M.C.; Donato, R. Essential Oil of Artemisia Annua L.: An Extraordinary Component with Numerous Antimicrobial Properties. Evid. Based Complement. Altern. Med. 2014, 2014, 159819. [Google Scholar] [CrossRef] [PubMed]
- Das, S. Artemisia Annua (Qinghao): A Pharmacological Review. Nternational J. Pharm. Sci. Res. 2012, 3, 4573–4577. [Google Scholar]
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and Thujone-Containing Herbal Medicinal and Botanical Products: Toxicological Assessment. Regul. Toxicol. Pharmacol. 2013, 65, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Somade, O.T. Camphor Toxicity: A Review of Recent Findings. In Proceedings of the National Academy of Sciences India Section B—Biological Sciences; Springer: Berlin/Heidelberg; Germany, 2022. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; Garciá-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Carpena, M.; Otero, P.; Gullón, P.; Prieto, M.A.; Simal-Gandara, J. Culinary and Nutritional Value of Edible Wild Plants from Northern Spain Rich in Phenolic Compounds with Potential Health Benefits. Food Funct. 2020, 11, 8493–8515. [Google Scholar] [CrossRef]
- Ianni, S. Ippocrasso. In Storia Di Un Vino Speziato; Ed Borè Srl.: Lecce, Italy, 2022. [Google Scholar]
- Zhou, X.; Qiao, K.; Wu, H.; Zhang, Y. The Impact of Food Additives on the Abundance and Composition of Gut Microbiota. Molecules 2023, 28, 631. [Google Scholar] [CrossRef] [PubMed]
- Bottari, B.; Levante, A.; Neviani, E.; Gatti, M. How the Fewest Become the Greatest. L. Casei’s Impact on Long Ripened Cheeses. Front. Microbiol. 2018, 9, 415614. [Google Scholar] [CrossRef] [PubMed]
- Cosme, F.; Inês, A.; Vilela, A. Consumer’s Acceptability and Health Consciousness of Probiotic and Prebiotic of Non-Dairy Products. Food Res. 2021, 151, 110842. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency; Committee on Herbal Medicinal Products (HMPC). Assessment Report on Artemisia Absinthium L., Herba. 2017. Available online: https://www.ema.europa.eu/en/medicines/herbal/absinthii-herba (accessed on 15 February 2024).
- Council of Europe European Pharmacopoeia; Strasbourg, France. 2020. Available online: https://www.edqm.eu/en/the-european-pharmacopoeia-commission (accessed on 15 February 2024).
- Jennings, W.G.; Shibamoto, T. Qualitative Analysis of Flavor and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: Cambridge, MA, USA, 1980; ISBN 9780123842503. [Google Scholar]
- Davies, N.W. Gas Chromatographic Retention Indices of Monoterpenes and Sesquiterpenes on Methyl Silicone and Carbowax 20M Phases. J. Chromatogr. 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 1998. [Google Scholar]
- Goodner, K.I. Practical Retention Index Models of OV-101, DB-1, DB-5, and DB-Wax for Flavour and Fragrance Compounds. LWT-Food Sci. Technol. 2008, 41, 951–958. [Google Scholar] [CrossRef]
- McLafferty, F.W. The Wiley Registry of Mass Spectral Data, with Nist Spectral Data, 7th. ed.; John Wiley & Sons: New York, NY, USA, 1998. [Google Scholar]
| N | Compound Name | AAb-EO | AA-EO | KI a | KI b | Identification c |
|---|---|---|---|---|---|---|
| % | % | |||||
| 1 | 1-Ethyl-2-methylcyclopentane | - | 0.1 | 761 | - | 1,2 |
| 2 | trans-7-Methyl-3-octene | - | 0.1 | 797 | - | 1,2 |
| 3 | 1-Nonene | - | 0.4 | 823 | - | 1,2 |
| 4 | Tricyclene | 0.1 | - | 843 | 1047 | 1,2 |
| 5 | α-Thujene | 0.1 | 0.2 | 850 | - | 1,2,3 |
| 6 | α-Pinene | 0.8 | 5.4 | 854 | 1036 | 1,2,3 |
| 7 | Camphene | 1.4 | 0.2 | 866 | 1075 | 1,2,3 |
| 8 | β-Pinene | 0.6 | 14.4 | 889 | 1120 | 1,2,3 |
| 9 | Myrcene | 0.2 | 0.2 | 909 | 1145 | 1,2 |
| 10 | α-Phellandrene | 0.4 | 0.1 | 917 | 1177 | 1,2,3 |
| 11 | α-Terpinene | 1.5 | 1.1 | 929 | 1170 | 1,2,3 |
| 12 | p-Cymene | 2.2 | 1.3 | 937 | 1250 | 1,2 |
| 13 | Limonene | 0.3 | - | 940 | 1180 | 1,2,3 |
| 14 | Eucalyptol | 0.6 | 4.4 | 941 | 1210 | 1,2,3 |
| 15 | cis-Ocimene | 0.2 | 0.1 | 954 | 1225 | 1,2 |
| 16 | γ-Terpinene | 2.0 | 2.3 | 970 | 1221 | 1,2,3 |
| 17 | cis-Sabinene hydrate | 1.6 | - | 977 | - | 1,2 |
| 18 | Terpinolene | 0.7 | 0.5 | 996 | 1289 | 1,2,3 |
| 19 | α-Pinene oxide | 0.1 | - | 1003 | 1384 | 1,2 |
| 20 | cis-Thujone | 1.4 | - | 1008 | 1430 | 1,2 |
| 21 | Linalool | - | 0.2 | 1009 | 1506 | 1,2,3 |
| 22 | 1,3,8-p-Menthatriene | 0.2 | - | 1013 | - | 1,2 |
| 23 | trans-Thujone | 19.6 | - | 1021 | 1442 | 1,2 |
| 24 | cis-p-Menth-2-en-1-ol | 0.4 | - | 1028 | - | 1,2 |
| 25 | trans-Chrysanthenol | - | 0.1 | 1030 | - | 1,2 |
| 26 | allo-Ocimene | - | 0.3 | 1035 | 1382 | 1,2 |
| 27 | Camphor | 12.2 | 0.6 | 1049 | 1491 | 1,2,3 |
| 28 | Isopulegol | 0.1 | - | 1050 | 1533 | 1,2 |
| 29 | cis-Chrysanthenol | 0.3 | 4.3 | 1052 | - | 1,2 |
| 30 | Eucarvone | 0.1 | 1061 | - | 1,2 | |
| 31 | Pinocarvone | 0.1 | - | 1062 | - | 1,2 |
| 32 | Borneol | 0.7 | - | 1067 | 1715 | 1,2 |
| 33 | neoiso-Isopulegol | 0.1 | - | 1073 | - | 1,2 |
| 34 | Terpinen-4-ol | 1.7 | 1.3 | 1079 | 1636 | 1,2 |
| 35 | trans-Isocitral | 0.1 | - | 1084 | - | 1,2 |
| 36 | α-Terpineol | 0.3 | 0.8 | 1092 | 1662 | 1,2,3 |
| 37 | cis-Piperitol | 0.1 | - | 1095 | - | 1,2 |
| 38 | trans-4-Caranone | 0.3 | - | 1100 | - | 1,2 |
| 39 | trans-Piperitol | 0.2 | - | 1102 | 1690 | 1,2 |
| 40 | 2,6-Dimethyl-2-vinyl-5-heptenoic acid | 0.1 | - | 1118 | - | 1,2 |
| 41 | 1-(3-methylbutyl)-Cyclopentene | 0.1 | - | 1128 | - | 1,2 |
| 42 | Cumin aldehyde | 0.1 | - | 1133 | 1802 | 1,2 |
| 43 | Carvotanacetone | 0.5 | - | 1138 | 1697 | 1,2 |
| 44 | trans-Chrysanthenyl acetate | - | 6.3 | 1156 | - | 1,2 |
| 45 | Perilla aldehyde | 0.1 | - | 1165 | 1785 | 1,2 |
| 46 | Bornyl acetate | - | 0.2 | 1176 | 1575 | 1,2 |
| 47 | Carvacrol | 3.9 | 0.4 | 1195 | 2219 | 1,2,3 |
| 48 | δ-Elemene | 0.2 | 1.1 | 1218 | 1479 | 1,2 |
| 49 | trans-Carvyl acetate | - | 0.1 | 1225 | - | 1,2 |
| 50 | α-Longipinene | - | 0.5 | 1228 | - | 1,2 |
| 51 | α-Cubebene | 0.1 | - | 1230 | 1445 | 1,2 |
| 52 | Ionene | 0.1 | - | 1233 | - | 1,2 |
| 53 | Silphiperfol-5,7(14)-diene | - | 0.1 | 1236 | - | 1,2 |
| 54 | α-Ylangene | - | 1.0 | 1251 | 1492 | 1,2 |
| 55 | α-Copaene | 0.6 | 0.3 | 1254 | 1477 | 1,2 |
| 56 | β-Bourbonene | 0.6 | 0.2 | 1262 | 1498 | 1,2 |
| 57 | β-Cubebene | 0.1 | - | 1269 | 1525 | 1,2 |
| 58 | β-Elemene | 0.4 | 1.6 | 1272 | - | 1,2 |
| 59 | γ,4-dimethyl-Benzenebutanal | 0.2 | - | 1284 | - | 1,2 |
| 60 | Longifolene | 2.8 | - | 1295 | 1574 | 1,2 |
| 61 | α-Cedrene | 0.1 | - | 1297 | - | 1,2 |
| 62 | β-Cedrene | 0.4 | 0.2 | 1298 | 1587 | 1,2 |
| 63 | β-Ylangene | - | 3.9 | 1299 | 1492 | 1,2 |
| 64 | β-Copaene | 0.1 | 5.0 | 1313 | - | 1,2 |
| 65 | β-Gurjunene | - | 1.0 | 1320 | 1655 | 1,2 |
| 66 | Aromandendrene | 0.4 | 0.3 | 1321 | 1631 | 1,2 |
| 67 | cis-Cadina-1(6),4-diene | 0.1 | - | 1332 | - | 1,2 |
| 68 | Propanoic acid, 2-methyl-, 1,7,7-trimethylbicyclo[2.2.1]hept-2-yl ester, exo- | 0.2 | - | 1337 | - | 1,2 |
| 69 | α-Himachalene | - | 0.4 | 1340 | - | 1,2 |
| 70 | β-Chamigrene | 0.9 | - | 1345 | 1724 | 1,2 |
| 71 | allo-Aromadendrene | - | 2.8 | 1347 | 1660 | 1,2 |
| 72 | Germacrene D | 0.8 | 0.3 | 1349 | 1712 | 1,2 |
| 73 | cis-Muurola-4(14),5-diene | - | 5.8 | 1352 | - | 1,2 |
| 74 | β-Selinene | 0.6 | - | 1353 | 1725 | 1,2 |
| 75 | γ-Gurjunene | - | 3.8 | 1355 | - | 1,2 |
| 76 | β-Ionone | 0.1 | - | 1358 | 1907 | 1,2 |
| 77 | γ-Muurolene | - | 1.2 | 1366 | 1725 | 1,2 |
| 78 | ar-Curcumene | - | 1.7 | 1370 | 1786 | 1,2 |
| 79 | α-Farnesene | - | 1.6 | 1383 | 1752 | 1,2 |
| 80 | Cadina-3,9-diene | - | 1.1 | 1393 | - | 1,2 |
| 81 | Elemol | 0.5 | - | 1413 | 2076 | 1,2 |
| 82 | Viridiflorene | - | 2.6 | 1415 | - | 1,2 |
| 83 | α-Cedrene epoxide | 0.1 | - | 1417 | - | 1,2 |
| 84 | Bicyclogermacrene | - | 2.3 | 1432 | 1756 | 1,2 |
| 85 | Spathulenol | 0.4 | 0.8 | 1439 | - | 1,2 |
| 86 | Caryophyllene oxide | 2.0 | - | 1442 | 2008 | 1,2 |
| 87 | trans-β-Guaiene | - | 0.6 | 1451 | - | 1,2 |
| 88 | Salvial-4(14)-en-1-one | 0.5 | - | 1452 | 2037 | 1,2 |
| 89 | Aristolene epoxide | - | - | 1453 | - | 1,2 |
| 90 | trans-γ-Bisabolene | - | 0.2 | 1465 | - | 1,2 |
| 91 | Humulene epoxide II | 0.2 | - | 1466 | - | 1,2 |
| 92 | Junenol | 0.6 | - | 1474 | - | 1,2 |
| 93 | Guaiol | - | 0.7 | 1488 | 2094 | 1,2 |
| 94 | 3,6-Dihydrochamazulene | 7.4 | - | 1489 | - | 1,2 |
| 95 | γ-Eudesmol | - | 3.2 | 1492 | 2178 | 1,2 |
| 96 | Ylangenol | 0.3 | - | 1494 | - | 1,2 |
| 97 | α-Acorenol | - | 3.6 | 1498 | - | 1,2 |
| 98 | 1-epi-Cubenol | 0.1 | - | 1500 | - | 1,2 |
| 99 | β-Eudesmol | 0.7 | 0.7 | 1500 | 2215 | 1,2 |
| 100 | α-Cadinol | - | 1.0 | 1522 | 2224 | 1,2 |
| 101 | Cedr-8(15)-en-10-ol | 0.3 | - | 1523 | - | 1,2 |
| 102 | Aromadendrene oxide | - | 0.5 | 1535 | - | 1,2 |
| 103 | Germacra-4(15),5,10(14)-trien-1-α-ol | 0.3 | - | 1537 | - | 1,2 |
| 104 | α-Bisabolol | - | 0.5 | 1538 | 2232 | 1,2 |
| 105 | Chamazulene | 11.7 | - | 1581 | - | 1,2,3 |
| 106 | 6,10,14-trimethyl-2-Pentadecanone | 0.2 | - | 1691 | 2131 | 1,2 |
| 107 | Geranyl-α-terpinene | 3.9 | - | 1834 | - | 1,2 |
| 108 | Geranyl-p-cymene | 3.5 | - | 1837 | - | 1,2 |
| 109 | Campesterol acetate | - | 0.1 | 2697 | - | 1,2 |
| 110 | Olean-12-en-3β-ol acetate | - | 0.1 | 2685 | - | 1,2 |
| 111 | 3β-Stigmasta-5,22-dien-3-ol acetate | - | 0.2 | 2709 | - | 1,2 |
| 112 | Stigmastan-3,5,22-trien | - | 0.2 | 2745 | - | 1,2 |
| 113 | β-Sitosterol acetate | - | 0.9 | 2766 | - | 1,2 |
| 114 | 5β-Cholestan-3-one, cyclic ethylene acetal | - | 0.2 | 2987 | - | 1,2 |
| Total | 96.9 | 97.8 | ||||
| Monoterpenes hydrocarbons | 10.7 | 26.1 | ||||
| Oxygenated monoterpenes | 44.6 | 18.8 | ||||
| Sesquiterpenes hydrocarbons | 34.8 | 39.6 | ||||
| Oxygenated sesquiterpenes | 6.0 | 11.0 | ||||
| Others | 0.8 | 2.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polito, F.; Di Mercurio, M.; Rizzo, S.; Di Vito, M.; Sanguinetti, M.; Urbani, A.; Bugli, F.; De Feo, V. Artemisia spp. Essential Oils: From Their Ethnobotanical Use to Unraveling the Microbiota Modulation Potential. Plants 2024, 13, 967. https://doi.org/10.3390/plants13070967
Polito F, Di Mercurio M, Rizzo S, Di Vito M, Sanguinetti M, Urbani A, Bugli F, De Feo V. Artemisia spp. Essential Oils: From Their Ethnobotanical Use to Unraveling the Microbiota Modulation Potential. Plants. 2024; 13(7):967. https://doi.org/10.3390/plants13070967
Chicago/Turabian StylePolito, Flavio, Mattia Di Mercurio, Silvia Rizzo, Maura Di Vito, Maurizio Sanguinetti, Andrea Urbani, Francesca Bugli, and Vincenzo De Feo. 2024. "Artemisia spp. Essential Oils: From Their Ethnobotanical Use to Unraveling the Microbiota Modulation Potential" Plants 13, no. 7: 967. https://doi.org/10.3390/plants13070967
APA StylePolito, F., Di Mercurio, M., Rizzo, S., Di Vito, M., Sanguinetti, M., Urbani, A., Bugli, F., & De Feo, V. (2024). Artemisia spp. Essential Oils: From Their Ethnobotanical Use to Unraveling the Microbiota Modulation Potential. Plants, 13(7), 967. https://doi.org/10.3390/plants13070967








