Abstract
Mixed cultivation with legumes may alleviate the nitrogen (N) limitation of monoculture Eucalyptus. However, how leaf functional traits respond to N in mixed cultivation with legumes and how they affect tree growth are unclear. Thus, this study investigated the response of leaf functional traits of Eucalyptus urophylla × Eucalyptus grandis (E. urophylla × E. grandis) and Dalbergia odorifera (D. odorifera) to mixed culture and N application, as well as the regulatory pathways of key traits on seedling growth. In this study, a pot-controlled experiment was set up, and seedling growth indicators, leaf physiology, morphological parameters, and N content were collected and analyzed after 180 days of N application treatment. The results indicated that mixed culture improved the N absorption and photosynthetic rate of E. urophylla × E. grandis, further promoting seedling growth but inhibiting the photosynthetic process of D. odorifera, reducing its growth and biomass. Redundancy analysis and path analysis revealed that leaf nitrogen content, pigment content, and photosynthesis-related physiological indicators were the traits most directly related to seedling growth and biomass accumulation, with the net photosynthetic rate explaining 50.9% and 55.8% of the variation in growth indicators for E. urophylla × E. grandis and D. odorifera, respectively. Additionally, leaf morphological traits are related to the trade-off strategy exhibited by E. urophylla × E. grandis and D. odorifera based on N competition. This study demonstrated that physiological traits related to photosynthesis are reliable predictors of N nutrition and tree growth in mixed stands, while leaf morphological traits reflect the resource trade-off strategies of different tree species.
1. Introduction
As part of the plant economic spectrum, leaf functional traits are defined as the morphological/structural, chemical, and physiological characteristics of leaves that play a key role in the whole life cycle of plants [1,2]. These traits essentially reflect a plant’s trade-off strategy between the cost of leaf construction and photosynthetic output; therefore, these traits reflect the plant’s economics [3]. Regarding leaf construction, leaf area (LA), leaf thickness (LT), leaf volume (LV), leaf fresh mass (LFM), and leaf dry mass (LDM) are the basic leaf traits that directly reflect leaf construction strategies [4,5,6]. For example, a larger LA represents a larger area for light capture but also means more demand for resources such as water and nutrients [4]. LFM, LDM, and leaf mass fraction (LMF) were assessed at the leaf level and individual level for plant investment in leaf tissues and organs, respectively [7,8]. Moreover, the demand for light drives an increase in the light capture area per unit biomass (i.e., specific leaf area, SLA), which contributes to leaf photosynthesis and improved N use efficiency [9,10,11]. Leaf tissue density (LTD) and leaf dry matter content (LDMC) are used to estimate the compactness level and extent of leaf tissue growth, which may be related to plant responses to soil nutrient resources [6,12]. Regarding output, the net photosynthetic rate (Pn) is a decisive indicator of photosynthetic efficiency in leaves and is influenced by stomatal conductance (gs), transpiration rate (Tr), and intercellular carbon dioxide concentration (Ci) [13,14], while chlorophyll content (Chl) determines the flux size of photons received in leaves [15]. In addition, Rubisco carboxylation efficiency (CE) reflects the carboxylation efficiency of the key rate-limiting enzyme of photosynthesis [16], and leaf water-use efficiency (WUE) reflects the water use by the leaves and is closely related to the photosynthetic process [17]. On this basis, the plant fast–slow economic spectrum theory proposes that differences in plant traits exhibited along a resource gradient are key to the successful survival of different species [1]. That is, fast-growing species adapt to resource-rich environments and build simpler leaves using fewer resources with looser structures but higher N contents and photosynthetic rates, while slow-growing species build more complex leaves using more resources with denser structures but lower N contents to adapt to barren conditions [18]. However, additional research is needed to supplement our knowledge of leaf functional traits in response to N.
N is an essential, massive element that limits plant growth and development and plays an important role in leaf construction and production. Studies have shown that the chloroplast N content can reach 75% of the total leaf N content (LNC), and 30–40% of this N is allocated to ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), whose main role is to assimilate CO2 and is a rate-limiting enzyme for photosynthesis [19,20]. Evans and Clarke (2019) allocated leaf N according to function, indicating that the N associated with photosynthesis accounts for 54% of the total leaf N content, while the remaining N is used for the construction of the nucleus, cell wall, mitochondria, cytoplasm, and other parts of the plant [21]. Recent studies have shown that N addition increases the total N content and SLA and decreases the LDMC in the leaves of two herbaceous species, resulting in a preference for a “quick investment-return” survival strategy [22]. For herbaceous plants, N addition has an asymmetric effect on changes in leaf functional traits and biomass [23]. Recent studies of woody plants have shown that changes in the functional traits of tree leaves (e.g., SLA and LDMC) are correlated with soil N dynamics [24,25,26]. Therefore, changes in the functional traits of plant leaves may be good predictors of environmental N availability.
Eucalyptus species are timber species that are widely planted worldwide, and their advantages of rapid growth and high productivity compensate for the shortage of quality timber and slow the deforestation of natural forests [27]. However, the current multigenerational succession management model used for pure Eucalyptus stands places greater demands and challenges on soil fertility as well as the environment [28,29], and land degradation limits the growth of Eucalyptus [29,30]. In particular, the availability of N in the soil is one of the main factors limiting the productivity of agroforestry [31]. N fertilization can increase productivity to a certain extent. However, excessive N application can accelerate environmental pollution [32,33]. Recent studies have indicated that intercropping Eucalyptus plants with leguminous tree species can improve plant nitrogen use efficiency and the potential for ecological service functions [34,35,36]. In mixed systems where nitrogen-fixing plants are present or under fertilization conditions, plant leaves are the direct beneficiaries of improved N use, and the response of leaf functional traits to improved N conditions will directly determine plant “gain” [37,38]. However, the adaptive changes in the functional leaf traits of these two plant species in mixed systems and their impact on plant growth are unknown.
Based on this information, this study used controlled pot experiments of Eucalyptus urophylla × eucalyptus grandis (E. urophylla × E. grandis) and Dalbergia odorifera (D. odorifera) to investigate the effects of mixed cultivation, N addition, and co-treatments on leaf functional traits as well as further on the growth and biomass of seedlings. In this study, leaf morphological/structural, physiological, and chemical traits related to plant growth and development were collected according to previous methods (Table S1) [39,40,41,42,43,44,45,46]. The objectives of this research were (1) to understand the adaptive mechanisms of leaf traits in E. urophylla × E. grandis and D. odorifera in response to mixed cultivation and N application, (2) to test the hypothesis that mixed plantation and N application and their cotreatments promote the growth and development of E. urophylla × E. grandis and D. odorifera, and (3) to elucidate the correlation between key leaf traits and the growth of E. urophylla × E. grandis and D. odorifera, as well as their respective regulatory pathways.
2. Results
2.1. Responses of Leaf Physiological Traits to Mixed Planting and N Application
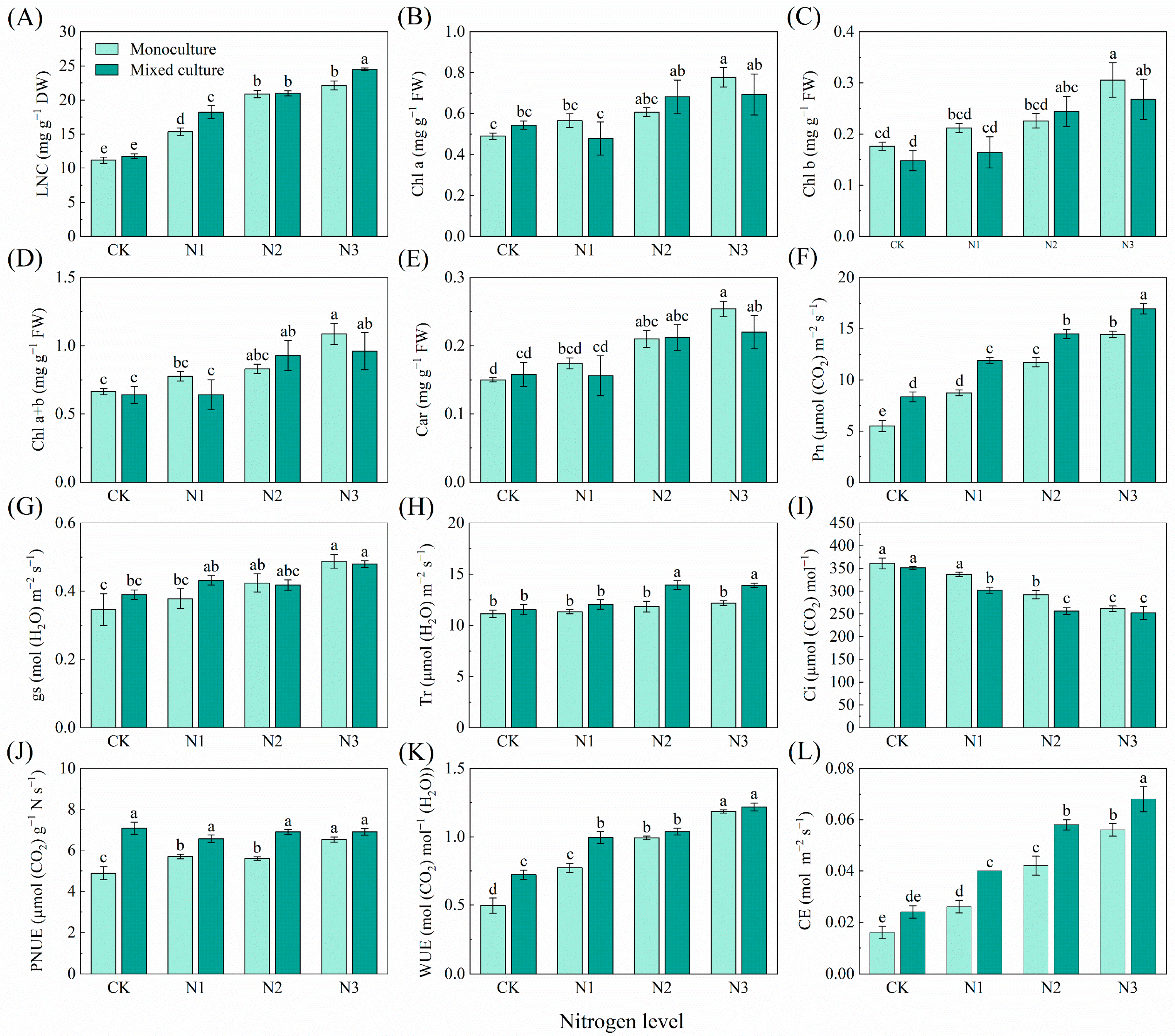
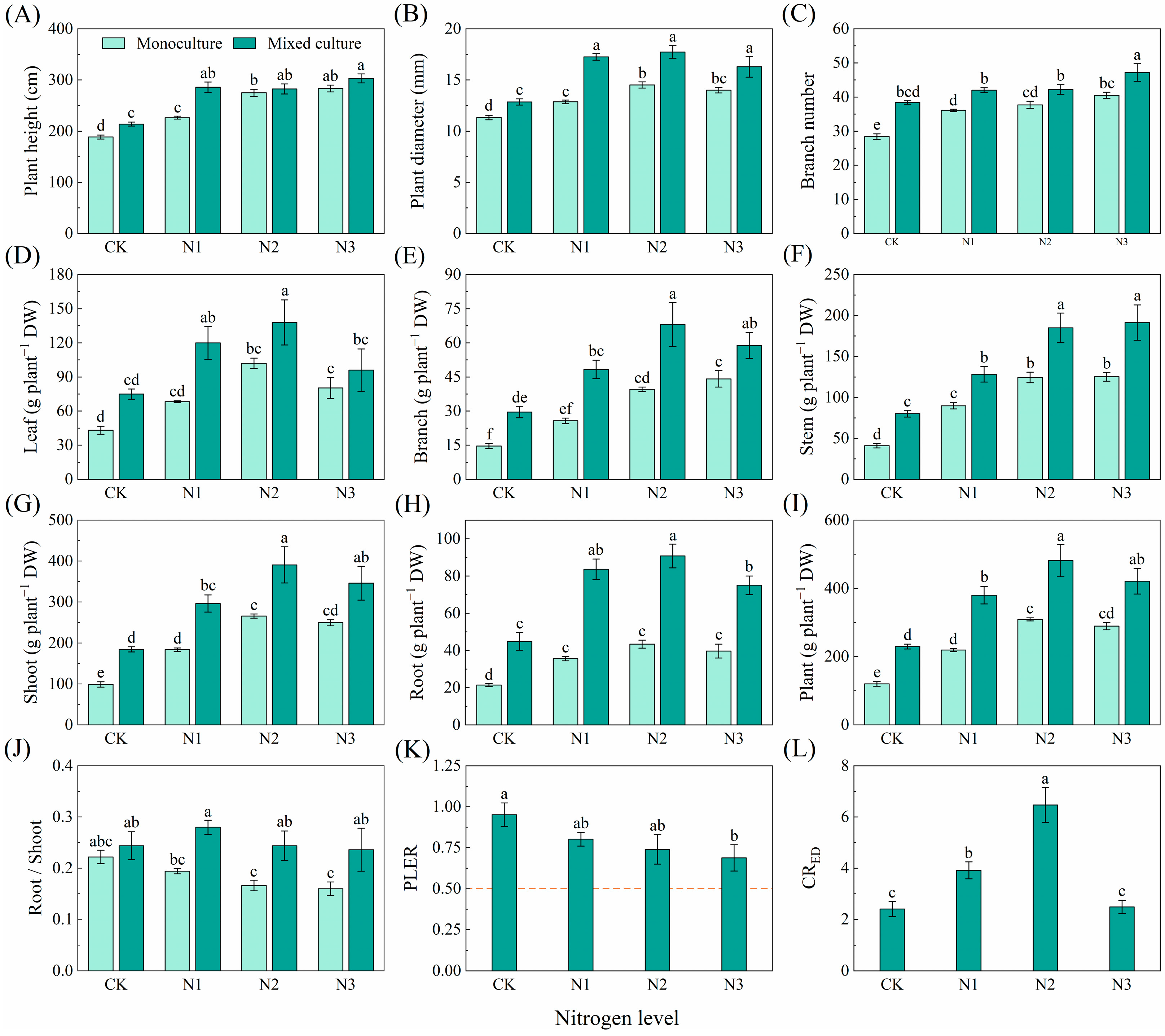
With increasing N application, there was a trend toward significantly increasing LNC, Chl a, Chl b, Chl a+b, Car, Pn, gs, photosynthetic N use sufficiency (PNUE), WUE, and CE, and decreasing Ci in the monoculture E. urophylla × E. grandis. Only mixed culture had no obvious effect on the LNC, Chl a, Chl b, Chl a+b, Car, gs, Tr, Ci, or CE of E. urophylla × E. grandis (Figure 1). Cotreatments of mixed cultivation and N addition significantly increased the LNC, Pn, gs, PNUE, WUE, and CE of E. urophylla × E. grandis (Figure 1A,F,G,J–L). However, the Chl a, Chl b, Chl a+b, Car, and Tr of E. urophylla × E. grandis increased significantly only under the higher N addition treatments (N2 (6 g urea pot−1) and N3 (12 g urea pot−1)) in the mixed plantation (Figure 1B–E,H). Moreover, cotreatment with mixed culture and N fertilization significantly reduced the Ci of the leaves of E. urophylla × E. grandis (Figure 1I).
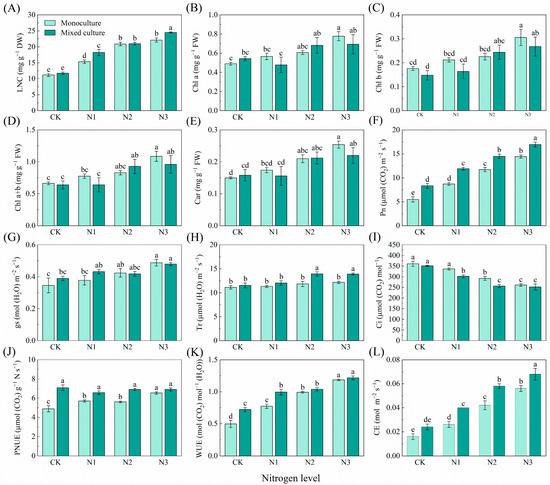
Figure 1.
Effects of mixed planting and N addition on the physiological traits of E. urophylla × E. grandis seedlings. The differences in (A) leaf nitrogen content (LNC), (B) chlorophyll a (Chl a), (C) chlorophyll b (Chl b), (D) total chlorophyll content (Chl a+b), (E) carotenoid (Car), (F) net photosynthetic rate (Pn), (G) stomatal conductance (gs), (H) transpiration rate (Tr), (I) intercellular CO2 concentration (Ci), (J) photosynthetic nitrogen use sufficiency (PNUE), (K) water-use efficiency (WUE), and (L) carboxylation efficiency of Rubisco (CE) in E. urophylla × E. grandis were compared via one-way ANOVA. Significant differences (p < 0.05; n = 5) between treatments are indicated by different lowercase letters.
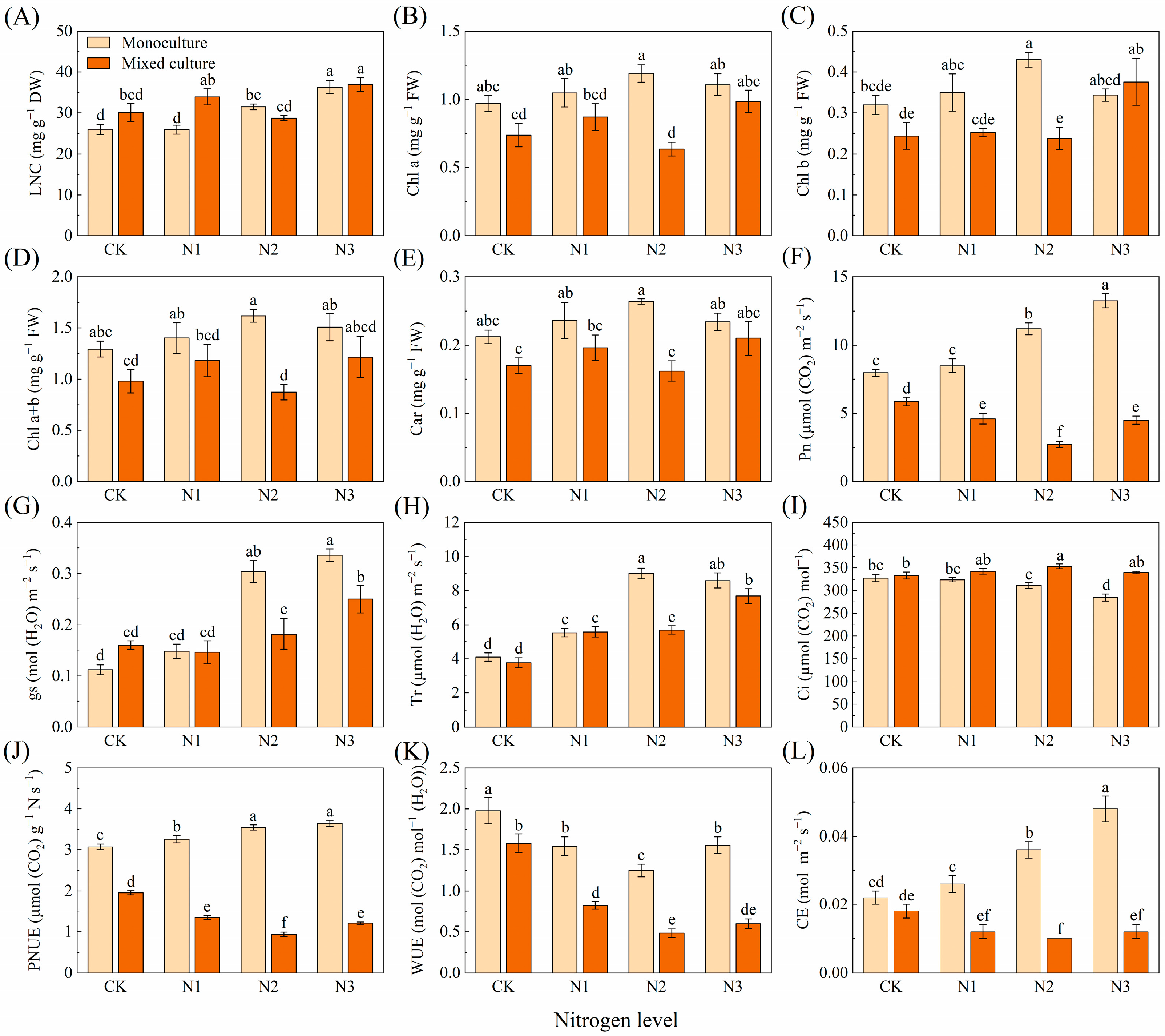
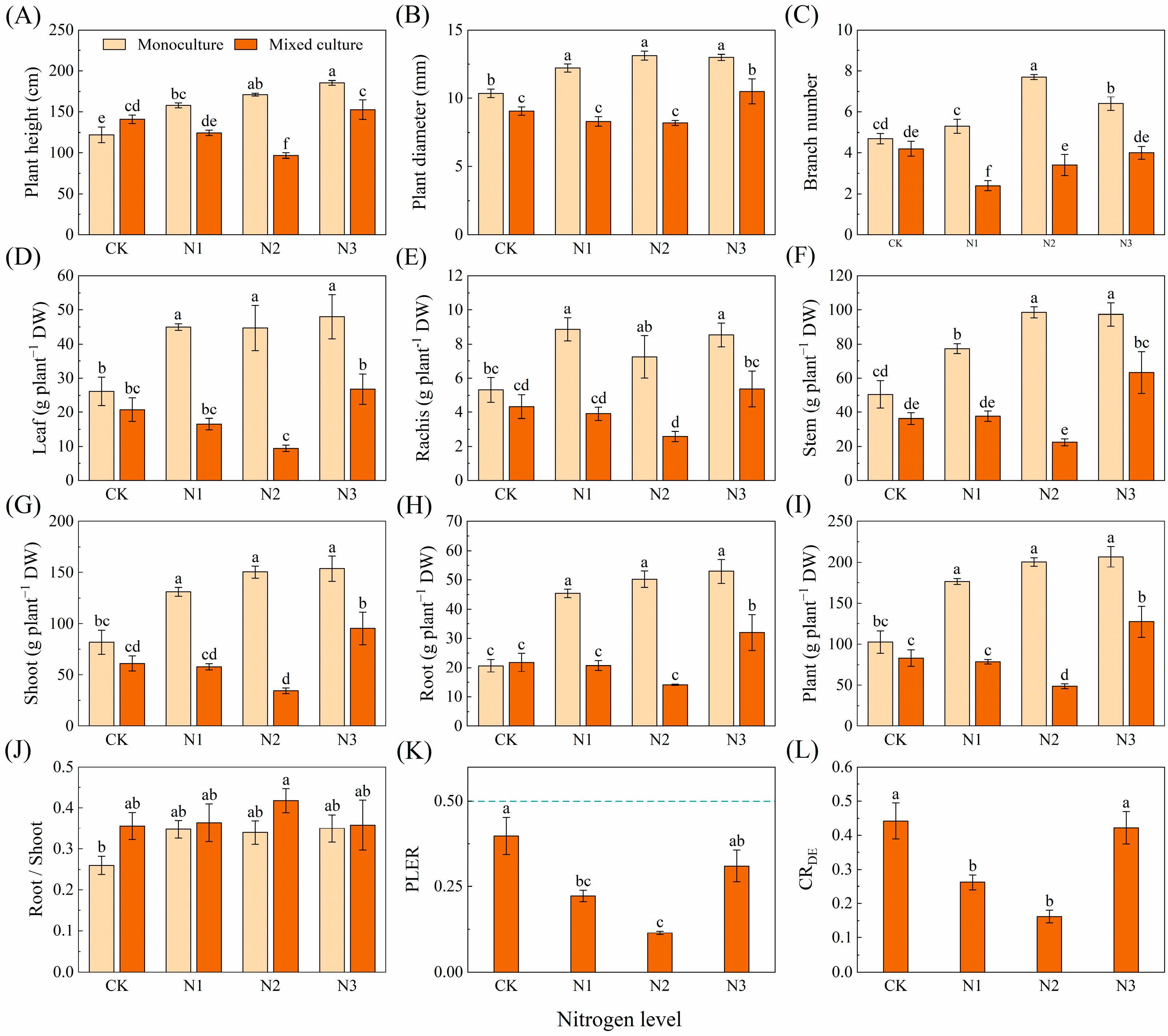
For D. odorifera, the mixed plantation treatment significantly reduced the leaf Pn, PNUE, and WUE (Figure 2F,J,K). The high N addition treatments (N2 and N3) significantly increased the LNC, Chl b, Pn, gs, Tr, PNUE, and CE but decreased the Ci and WUE (Figure 2A,C,F–L). The cotreatment of mixed planting and different N applications significantly increased LNC (N1 (3 g urea pot−1) and N3), gs (N2 and N3), Tr (N1, N2, and N3), and Ci (N2) and reduced the Chl a (N2), Chl a+b (N2), Pn (N1, N2, and N3), PNUE (N1, N2, and N3), WUE (N1, N2 and N3), and CE (N1, N2, and N3) of D. odorifera (Figure 2).
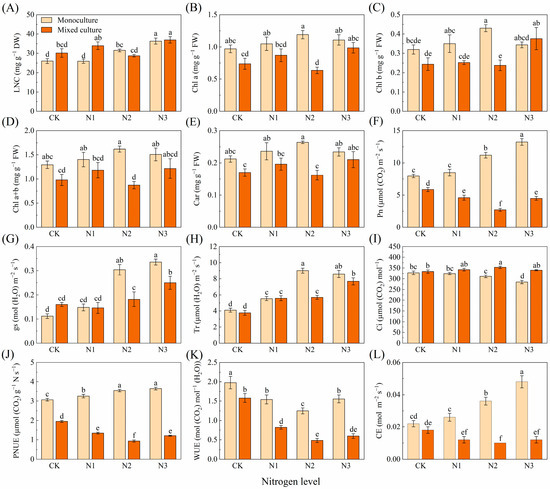
Figure 2.
Effects of mixed planting and N addition on the physiological traits of D. odorifera seedlings. The differences in (A) leaf nitrogen content (LNC), (B) chlorophyll a (Chl a), (C) chlorophyll b (Chl b), (D) total chlorophyll content (Chl a+b), (E) carotenoid (Car), (F) net photosynthetic rate (Pn), (G) stomatal conductance (gs), (H) transpiration rate (Tr), (I) intercellular CO2 concentration (Ci), (J) photosynthetic nitrogen use sufficiency (PNUE), (K) water-use efficiency (WUE), and (L) carboxylation efficiency of Rubisco (CE) in D. odorifera were compared via one-way ANOVA. Significant differences (p < 0.05; n = 5) between treatments are indicated by different lowercase letters.
2.2. Responses of Leaf Morphological Traits to Mixed Planting and N Application
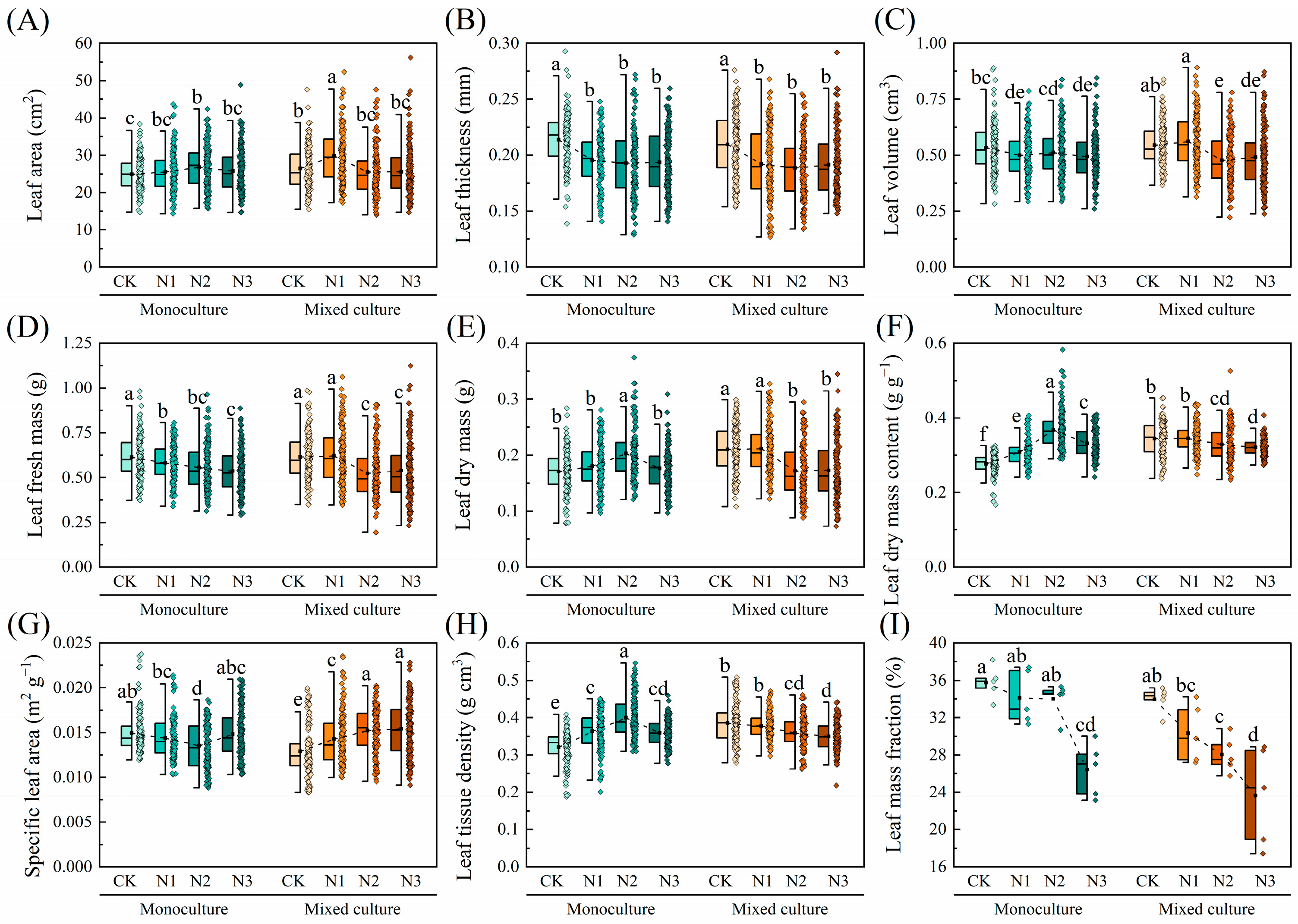
Compared to monoculture, mixed cultivation significantly increased the LA, LDM, LDMC, and LTD of E. urophylla × E. grandis, while notably reducing the SLA (p < 0.05). On the other hand, N addition increased the LA, LDM, LDMC, and LTD of E. urophylla × E. grandis, while reducing its LT, LV, LFM, SLA, and LMF (p < 0.05). Under the combined treatment of low N addition (CK and N1) and mixed cultivation, the LA, LV, LFM, LDM, LDMC, and LTD of E. urophylla × E. grandis were all higher than those under the combined treatment of low N addition (CK and N1) and monoculture. Conversely, the aforementioned traits under the combined treatment of high N addition (N2 and N3) and mixed cultivation were unchanged or reduced compared to the monoculture treatment. In addition, compared to monoculture, low N addition treatments (CK and N1) reduced the SLA of E. urophylla × E. grandis leaves under mixed cultivation, while high N treatments (N2 and N3) increased the SLA. Notably, the LMF of E. urophylla × E. grandis under both monoculture and mixed cultivation showed a decreasing trend with increasing N application (Figure 3).
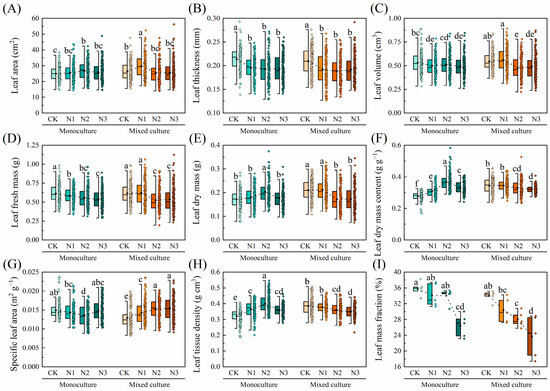
Figure 3.
Effects of mixed cultivation and N addition on leaf morphological traits of E. urophylla × E. grandis. (A) leaf area (LA), (B) leaf thickness (LT), (C) leaf volume (LV), (D) leaf fresh mass (LFM), (E) leaf dry mass (LDM), (F) leaf dry mass content (LDMC), (G) specific leaf area (SLA), (H) leaf tissue density (LTD), and (I) leaf mass fraction (LMF). Dashed lines indicate trends in the mean values of leaf traits with different N application treatments. Different lowercase letters indicate significant differences between different treatment combinations (p < 0.05; n = 50).
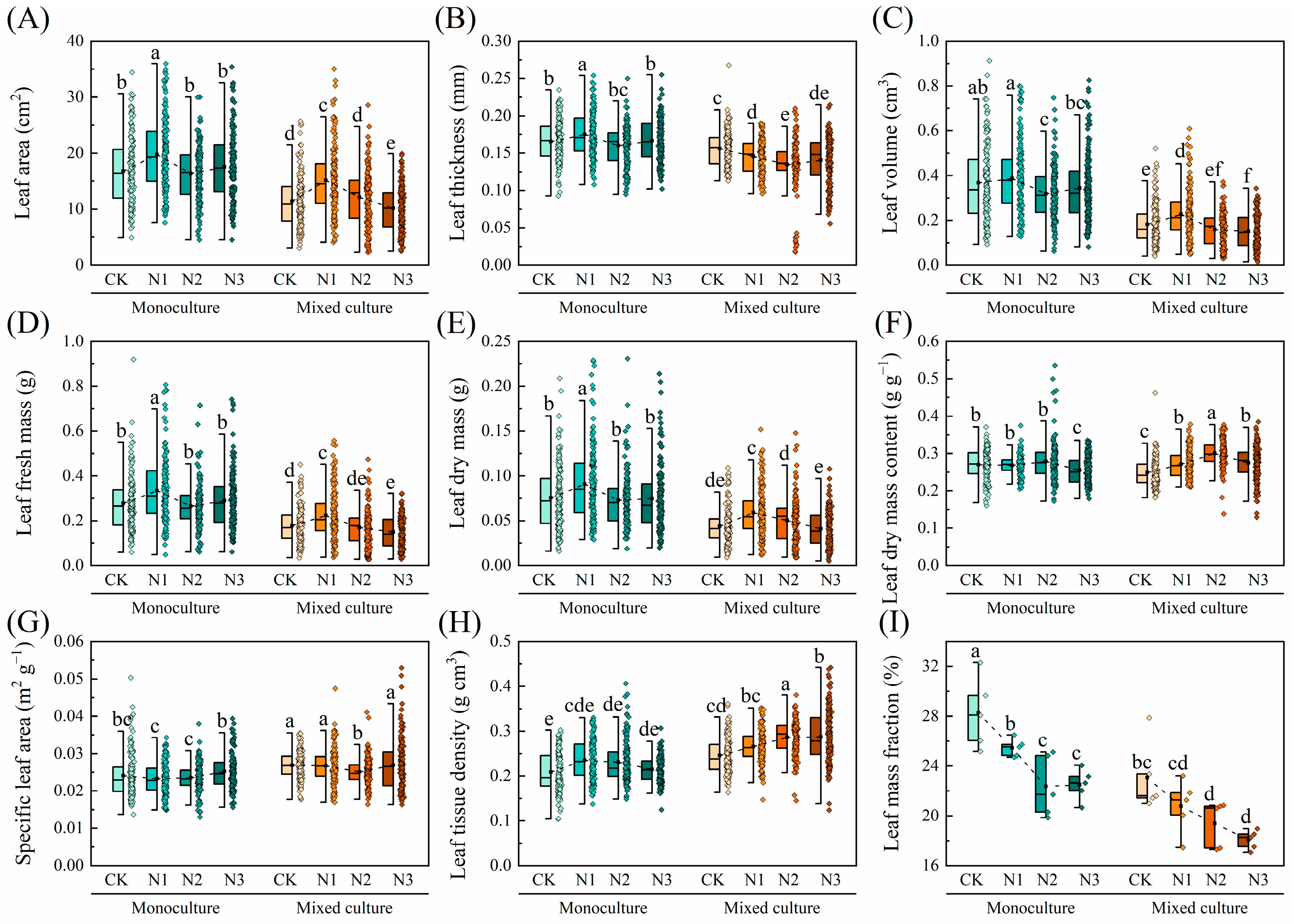
Compared to monoculture, mixed cultivation treatment significantly reduced the LA, LT, LV, LFM, LDM, LDMC, and LMF of D. odorifera under all nitrogen application treatments but significantly increased the SLA and LTD (p < 0.05). The overall leaf morphological traits of D. odorifera were insensitive to N addition, and only the LMF decreased with increasing N application rate (Figure 4).
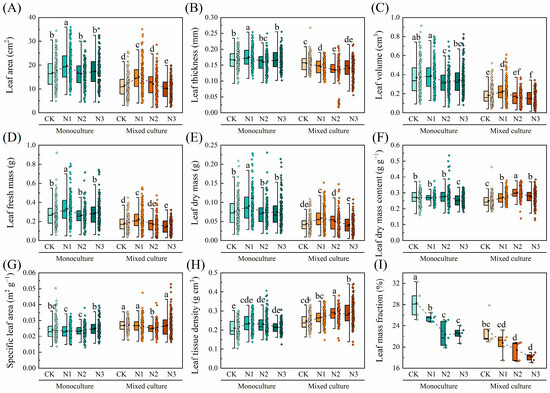
Figure 4.
Effects of mixed cultivation and N addition on the leaf morphological traits of D. odorifera. (A) leaf area (LA), (B) leaf thickness (LT), (C) leaf volume (LV), (D) leaf fresh mass (LFM), (E) leaf dry mass (LDM), (F) leaf dry mass content (LDMC), (G) specific leaf area (SLA), (H) leaf tissue density (LTD), and (I) leaf mass fraction (LMF). Dashed lines indicate trends in the mean values of leaf traits with different N application treatments. Different lowercase letters indicate significant differences between different treatment combinations (p < 0.05; n = 50).
2.3. Response of Tree Species Growth to Mixed Plantation and N Addition
Compared to monoculture, the mixed plantation significantly promoted plant height, diameter, branch number, and biomass of leaf, branch, stem, root, shoot, and total plant of E. urophylla × E. grandis (Figure 5). Different N application treatments significantly increased the plant height, diameter, branch number, and biomass of leaf, branch, stem, root, shoot, and whole plant but reduced the root/shoot of E. urophylla × E. grandis under monoculture treatment (Figure 5A–J). Under the cotreatment of mixed cultivation and N application, plant height, diameter, branch number, and the biomass of leaf, branch, stem, root, shoot, and total plant of E. urophylla × E. grandis were significantly greater than those under the other treatments (Figure 5A–I). The PLER of E. urophylla × E. grandis was greater than 0.5, but N application reduced the PLER (Figure 5K). The N1 and N2 treatments significantly increased the CRED of E. urophylla × E. grandis (Figure 5L).
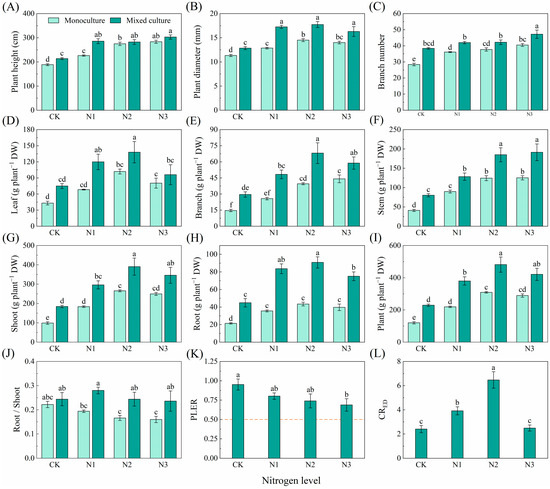
Figure 5.
Effects of mixed planting and N addition on the growth of E. urophylla × E. grandis. The differences in (A) plant height, (B) diameter, (C) branch number, biomass of (D) leaf, (E) branch, (F) stem, (G) shoot (gs), (H) root, (I) whole plant, (J) root/shoot, (K) PLER, and (L) CRED were compared via one-way ANOVA. The partial land equivalent ratio (PLER) was calculated based on plant aboveground parts (K), and the competitive ratio (CRED) was calculated based on the total biomass (L). The shoot biomass of monoculture and mixed cultivation seedlings are equal at the dotted line in subfigure (K). Different lowercase letters indicate significant differences in the data between different treatments (p < 0.05; n = 5).
N application obviously increased the height, diameter, branch number, and biomass of the leaves, rachises, stems, shoots, roots, and whole plants of D. odorifera in the monoculture treatment (p < 0.05). Mixed cultivation did not significantly affect the height or branch number or the biomass of the leaf, leaf rachis, stem, shoot, root, or whole plant of D. odorifera but did significantly reduce the diameter (Figure 6A–I). Under different nitrogen application treatments, mixed cultivation significantly (p < 0.05) reduced height, ground diameter, branch number, and biomass of leaf, rachis, stem, shoot, root, and plant of D. odorifera compared to monoculture (Figure 6A–I). The PLER and CRDE of D. odorifera were lower than 0.5 and 1, respectively, and the N1 and N2 treatments significantly reduced the PLER and CRDE (Figure 6K,L).
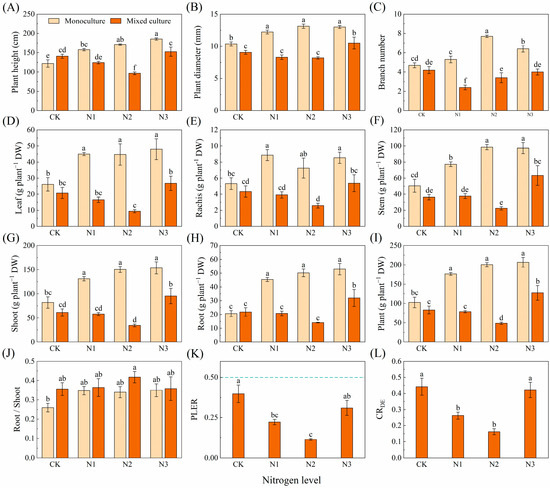
Figure 6.
Effects of mixed planting and N addition on the growth of D. odorifera. The differences in (A) plant height, (B) diameter, (C) branch number, biomass of (D) leaf, (E) branch, (F) stem, (G) shoot (gs), (H) root and (I) whole plant, (J) root/shoot, (K) PLER, and (L) CRED were compared via one-way ANOVA. The partial land equivalent ratio (PLER) was calculated based on plant aboveground parts (K), and competitive ratios (CRDE) were calculated based on the total biomass (L). The shoot biomass of monoculture and mixed cultivation seedlings are equal at the dotted line in subfigure (K). Different lowercase letters indicate significant differences in the data between different treatments (p < 0.05; n = 5).
2.4. Relationships between Leaf Functional Traits and Their Contributions to Plant Growth
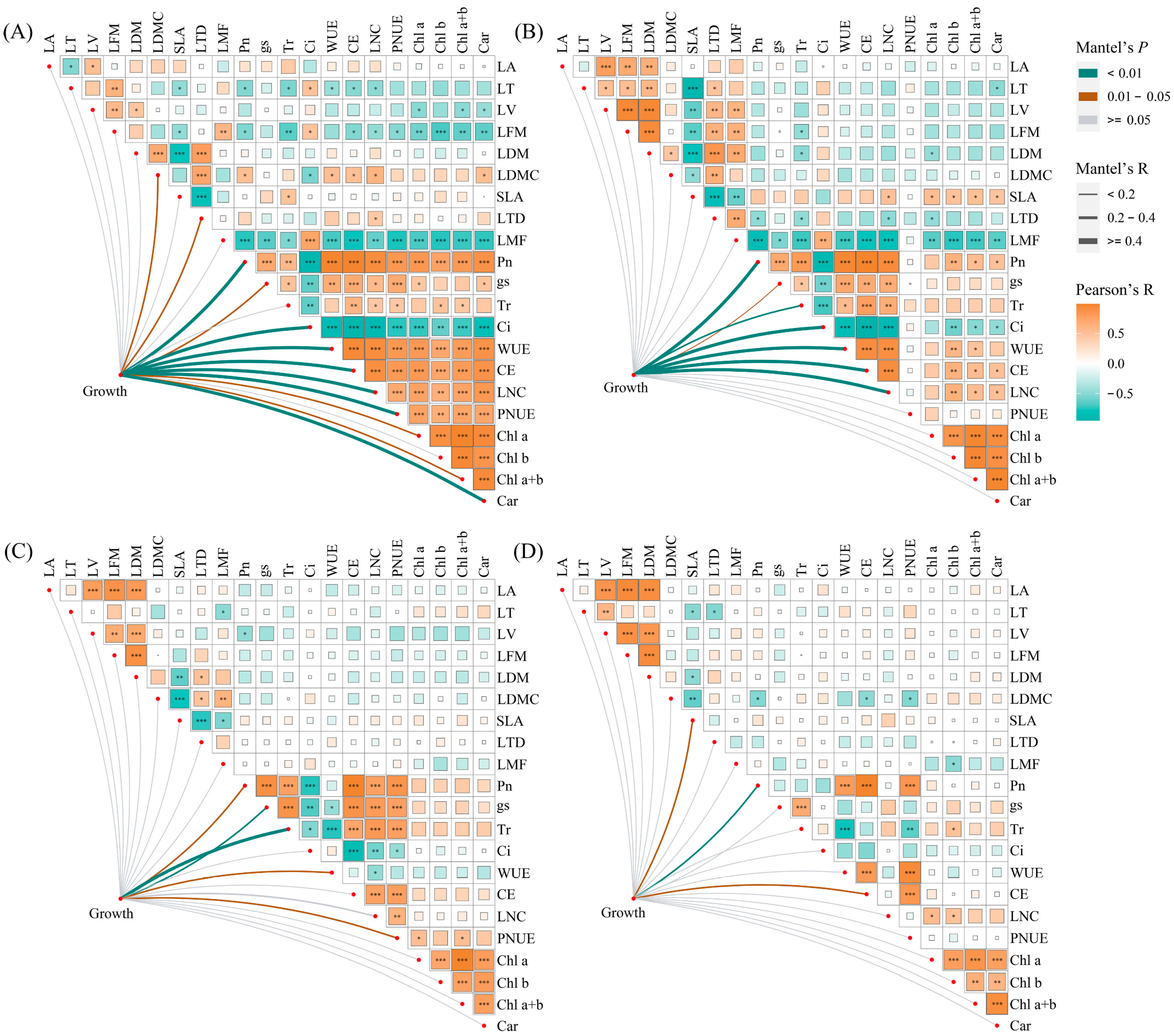
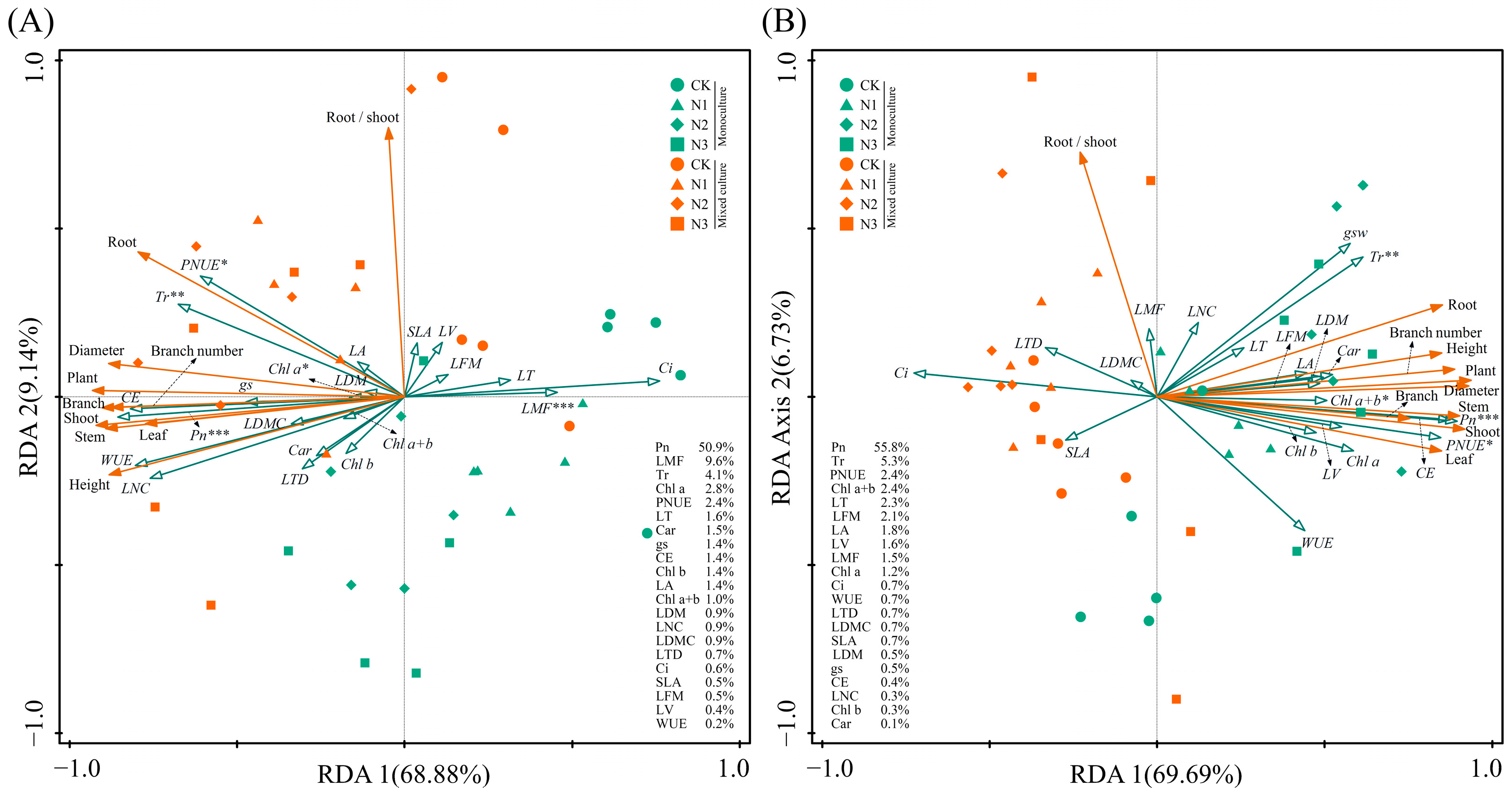
Under monoculture treatment, there was a significant positive correlation between Pn, gs, Tr, WUE, CE, LNC, PNUE, Chl a, Chl b, Chl a+b, and Car of E. urophylla × E. grandis (0.395 < R < 0.990, p < 0.05). Yet, they were all significantly negatively correlated with Ci (−0.948 < R < −0.623, p < 0.01). Regarding leaf morphological traits, the LA of E. urophylla × E. grandis showed a significant positive correlation with LV (R = 0.533, p = 0.015) and a negative correlation with LT (R = −0.527, p = 0.017). The SLA was significantly negatively correlated with LT, LFM, LDM, and LTD (−0.798 < R < −0.471, p < 0.05). And LDMC was significantly positively correlated with LDM and LTD (R = 0.689 and 0.731, p = 0.001 and 0.000). Pn, Tr, CE, and LNC showed significant negative correlations with LT, LFM, and LMF (−0.739 < R < −0.467, p < 0.05), but significant positive correlations with LDMC (0.512 < R < 0.611, p < 0.05). Additionally, Chl a, Chl b, Chl a+b, and Car exhibited significant negative correlations with LV, LFM, and LMF (−0.748 < R < −0.443, p < 0.05) (Figure 7A). Under mixed cultivation conditions, significant positive correlations are observed among Pn, gs, Tr, WUE, CE, and LNC (0.451 < R < 0.965, p < 0.05). Additionally, leaf pigments display highly significant positive correlations (0.847 < R < 0.958, p < 0.01). Furthermore, significant positive relationships are also evident between Pn, WUE, CE, LNC, and the pigments Chl b, Chl a+b, and Car (0. 473 < R < 0.623, p < 0.05). Regarding leaf morphological traits, significant positive correlations were found among LT, LV, LFM, LDM, LTD, and LMF of E. urophylla × E. grandis (0. 466 < R < 0.935, p < 0.05), while all exhibited significant negative correlations with SLA (−0.887 < R < −0.603, p < 0.01). Moreover, there was a general negative correlation between the morphological and physiological traits of E. urophylla × E. grandis, except for SLA and Ci (Figure 7B). Mantel’s test results indicate that the growth indices of monoculture E. urophylla × E. grandis are highly significantly correlated with Pn, Ci, WUE, CE, LNC, PNUE, and Car (R > 0.4, p < 0.01), and are significantly correlated with gs, Chl a, Chl a+b, LDMC, and LTD (0.2 < R < 0.4, p < 0.05) (Figure 7A). For mixed-cultivated E. urophylla × E. grandis, leaf Pn, Ci, WUE, CE, and LNC all show highly significant correlations with growth indices (R > 0.4, p < 0.01), as does Tr (0.2 < R < 0.4, p < 0.01). The gs also exhibits a significant correlation with growth indices (0.2 < R < 0.4, p < 0.05) (Figure 7B). The results of the RDA indicated that Pn, LMF, Tr, Chl a, and PNUE significantly influenced the growth of E. urophylla × E. grandis, explaining 50.9%, 9.6%, 4.1%, 2.8%, and 2.4%, respectively, of the variation in growth indices (Figure 8A).
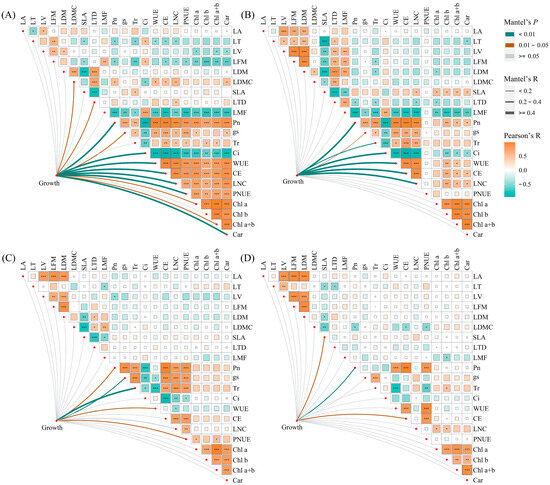
Figure 7.
Correlations between leaf functional traits and Mantel test results for the relationships between leaf functional traits and growth. (A) E. urophylla × E. grandis in monoculture; (B) E. urophylla × E. grandis in mixed culture; (C) D. odorifera in monoculture; and (D) D. odorifera in mixed culture. The growth variables included plant height, ground diameter, branch number, leaf, branch, stem, root, shoot, whole plant biomass, and root/shoot. * Significant at p < 0.05; ** Significant at p < 0.01; *** Significant at p < 0.001.
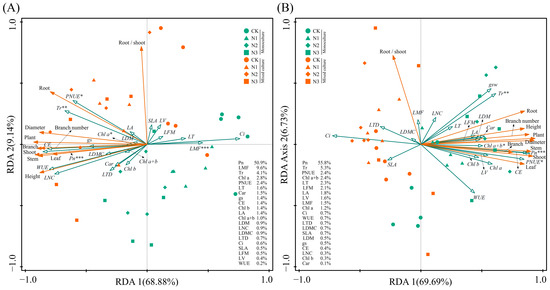
Figure 8.
Redundancy analysis showing the effects of leaf functional traits on the growth indices of E. urophylla × E. grandis (A) and D. odorifera (B). *, **, and *** indicate significant differences at the 0.05, 0.01, and 0.001 levels, respectively.
In the monoculture of D. odorifera, there were significantly positive correlations between Pn, gs, Tr, CE, LNC, and PNUE (0.612 < R < 0.980, p < 0.01), but all were negatively correlated with Ci and WUE (−0.849 < R < −0.446, p < 0.05). The SLA had strong negative correlations with LFM, LDM, LDMC, and LTD (−0.759 < R < −0.541, p < 0.05). Furthermore, the correlation between leaf physiological and morphological traits is relatively weak and not statistically significant (−0.4 < R < 0.4, p > 0.05) (Figure 7A). Under mixed cultivation treatment, leaf Pn, WUE, CE, and PNUE of D. odorifera are all highly significantly positively correlated (0.828 < R < 0.990, p < 0.001). The LNC and the contents of Chl a and Chl b exhibit significant correlations (R = 0.546 and 0.495, p < 0.05). Significant positive correlations are observed among the LA, LV, LFM, and LDM of D. odorifera (0.831 < R < 0.944, p < 0.001). Correlations between the physiological and morphological traits of D. odorifera leaves from mixed culture were generally weak, but there were strong associations between LDMC and leaf Pn, CE, and PNUE (−0.525 < R < −0.498, p < 0.05) (Figure 7D). In addition, Mantel’s test showed significant associations between D. odorifera growth indicators and Pn, gs, Tr, WUE, and PNUE in the monoculture treatment (R > 0.2, p < 0.05), while these leaf traits were only SLA, Pn, and CE in the mixed cultivation treatment (0.2 < R < 0.4, p < 0.05) (Figure 7C,D). According to the RDA results, the growth of D. odorifera was significantly affected by Pn, Tr, PNUE, and Chl a+b, which explained 55.8%, 5.3%, 2.4%, and 2.4%, respectively, of the variation in the growth indices (Figure 8B).
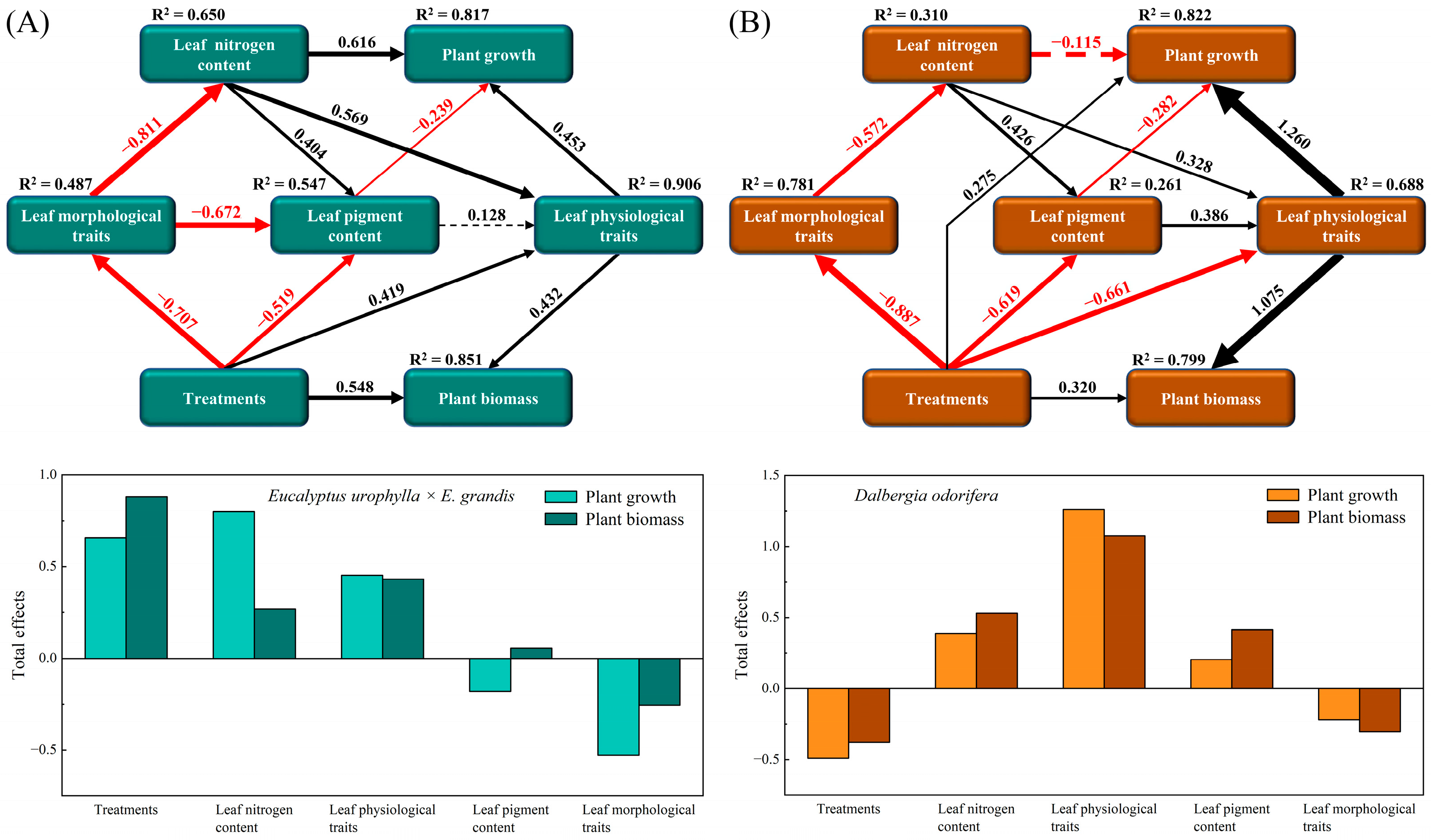
2.5. Potential Pathways of Leaf Trait Regulation of Plant Growth
According to the PLS-SEM results, the growth indices and biomass of E. urophylla × E. grandis and D. odorifera are jointly regulated by multiple leaf traits (Figure 9). Specifically, the growth (height, diameter, and branch number) of E. urophylla × E. grandis was directly regulated by LNC (0.616), leaf physiological traits (0.453), and leaf pigment content (−0.239), while its biomass (leaf, branch, stem, and root) was directly positively influenced by treatment (0.548) and leaf physiological traits (0.432). Overall, the growth of E. urophylla × E. grandis was positively regulated by treatment, LNC, and leaf physiological traits but negatively regulated by leaf morphological traits (Figure 9A). Treatment (0.275) and leaf physiological indicators (1.260) had direct positive impacts on the growth (height, diameter, and branch number) of D. odorifera, while leaf pigment content (−0.282) directly negatively regulated its growth. Moreover, the biomass (leaf, branch, stem, and root) of D. odorifera was directly positively regulated by treatment (0.320) and leaf physiological traits (1.075). Overall, LNC, leaf physiological traits, and leaf pigment content had positive regulatory effects on D. odorifera, while treatment and leaf morphological traits had overall negative impacts on its growth (Figure 9B).
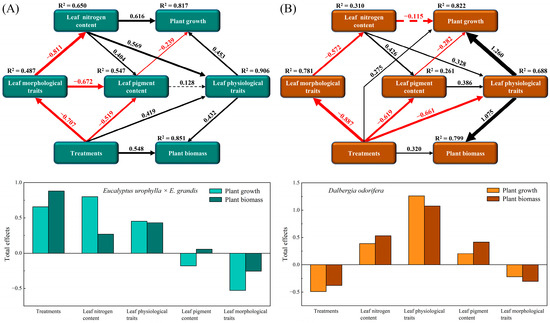
Figure 9.
Cascading relationships between plant growth and leaf functional traits. Partial least squares path modeling was employed to disentangle the major pathways by which different treatments influenced leaf nitrogen content; leaf morphological traits (LA, LT, LV, LFM, LDM, LDMC, SLA, LTD, and LMF); leaf physiological traits (Pn, gs, Tr, WUE, CE, and PNUE); leaf pigment contents (Chl a, Chl b, and Car); plant growth (height, diameter, and branch number); and plant biomass (leaf, branch, stem, and root) in E. urophylla × E. grandis (A) and D. odorifera (B). The solid black and red arrows represent positive and negative significant relationships, respectively, while the dashed black and red arrows represent positive and negative nonsignificant relationships, respectively. The numbers on the paths indicate the correlation coefficients, and the thickness of the arrows indicates the strength of the correlation.
3. Discussion
3.1. Adaptation of Leaf Traits of E. urophylla × E. grandis and D. odorifera to Mixed Plantation and N Addition Treatments
In general, variation in the functional traits of plant leaves is considered an adaptive change in response to the external environment [47,48]. In the present study, the variation in leaf morphological, physiological, and nutritional traits of E. urophylla × E. grandis and D. odorifera in response to mixed and N treatments was investigated (Figure 1, Figure 2, Figure 3 and Figure 4). The results suggest that the two species have different adaptation strategies.
N addition has been shown to increase the LNC based on mass [49,50]. The results of the present study showed that mixed culture alone did not significantly alter the LNC of E. urophylla × E. grandis, whereas N application alone significantly increased the LNC of E. urophylla × E. grandis (Figure 1A), indicating that soil N availability is the main driver of LNC. In mixed systems, plant acquisition of N depends on the competitive ability of the species [51,52]. Obviously, the interspecific competition in mixed cultivation tilted the balance of resource acquisition compared to the relatively equal intraspecific competition of the same plant species in monoculture. A higher root biomass allows for a greater soil N uptake capacity in E. urophylla × E. grandis [53], even robbing part of the N in D. odorifera to achieve interspecific N transfer [54,55]. Regarding leaf physiological traits, the Pn in E. urophylla × E. grandis under monoculture increased with N application, and this change was accompanied by an increase in gs and CE and a decrease in Ci (Figure 1). This result is consistent with most N addition experiments [56,57], indicating that N application alleviated the N limitation of photosynthesis. Correlation analysis revealed that N application increased the LNC and thus significantly increased leaf photosynthesis (Figure 7). An important reason is that the increase in leaf N promotes chlorophyll synthesis (Figure 8A) and improves light use efficiency [58,59]. Second, the increase in Pn benefited from the increase in CE (Figure 5), which improved the CO2 conversion efficiency [44,46]. In addition, N addition may indirectly improve photosynthetic efficiency by improving leaf stomatal permeability (gs), WUE, and photoprotective capacity (by Car) [60,61,62]. In the present study, the mixed treatment and the interaction with N application increased the photosynthesis of E. urophylla × E. grandis leaves to different degrees while significantly decreasing the photosynthetic rate of D. odorifera (Figure 1 and Figure 2). A reason for this finding is that the significant reduction in nonstomatal factors such as WUE and CE in D. odorifera leaves grown in mixed cultivation limits the photosynthetic response process [63]. Moreover, the significant decrease in chlorophyll and carotenoid contents in D. odorifera plants grown in a mixed cultivation system reduced the light capture and photoprotective capacity of the leaves [43]. The combined effects of mixed culture and N application increased the water and nutrient competitive ability of E. urophylla × E. grandis and decreased that of D. odorifera, which resulted in the overall improvement and decline of the leaf photosynthetic physiological functions of E. urophylla × E. grandis and D. odorifera, respectively [64,65].
Leaf morphological traits are important for the regulation of environmental adaptation in plants. Different plants can achieve similar resource acquisition capacities in both above- and below-ground parts in two ways: one way is to obtain more leaf biomass but less root biomass at lower SLAs, and the other way is to gain less leaf biomass but more root biomass at higher SLAs [66,67]. The results indicate that mixed cultivation of E. urophylla × E. grandis and D. odorifera tends to favor the second method of resource acquisition compared to monoculture. This means that mixed cultivation promotes the allocation of more root biomass for nutrient uptake by E. urophylla × E. grandis and D. odorifera (Figure 5 and Figure 6J) while reducing the material used for leaf construction, thereby increasing the light capture area per unit dry mass (Figure 3 and Figure 4). Takigahira and Yamawo (2019) suggested that this strategy may be linked to interspecific competition between E. urophylla × E. grandis and D. odorifera in mixed cultivation [68]. Moreover, some studies have shown that LMF increases with nutrient availability but decreases with light [69]. In this study, a noteworthy phenomenon was the decrease in the LMF of E. urophylla × E. grandis and D. odorifera with increasing N application under both monoculture and mixed cultivation, which was contrary to the results of Zou et al. [66], who found that N application increased the LMF of Machilus pauhoi seedlings. The results of this study suggest that the LMF under mixed cultivation decreased with increasing N application. This difference may be due to the improved leaf light conditions in the mixed system, which depend on the increased light harvesting capacity resulting from increased height [70]. Generally, it is believed that a high SLA and a low LDMC represent rapid nutrient acquisition and promote plant growth in fertile soils, while a low SLA and a high LDMC are commonly found in plants in poor environments [22,71]. It is clear that SLA and LDMC had opposite responses to N gradients in E. urophylla × E. grandis and D. odorifera, although this trend was weaker for D. odorifera (Figure 3 and Figure 4). This finding suggested that E. urophylla × E. grandis and D. odorifera may have employed different resource acquisition strategies under monoculture. N addition decreased the SLA of monocultured E. urophylla × E. grandis and increased LDMC, which may be due to N causing an imbalance of N and P in the trees, leading to a phosphorus limitation [24]; another possibility is that intraspecific N competition in E. urophylla × E. grandis resulted in a conservative resource acquisition strategy, which better explains how the N3 treatment alleviated the N limitation in this study [68]. Furthermore, mixed culture changed the response trends of SLA and LDMC to N gradients in E. urophylla × E. grandis and D. odorifera (Figure 3 and Figure 4). These findings suggested that mixed cultivation and N application enhance the resource availability of E. urophylla × E. grandis and reduce the resource accessibility of D. odorifera. This may be due to N intensifying interspecific competition and reducing the competitiveness of D. odorifera [72]. In addition, our results showed that cotreatment of mixed plantations and N application reduced the LA, LT, and LV of D. odorifera while increasing the LTD, indicating that N treatment induced changes in D. odorifera resistance under mixed culture conditions [73,74].
Understanding the relationships between plant leaf traits is necessary for understanding plant coordination strategies, but the effect of mixed stands on these relationships is still unclear [20]. Our results showed that E. urophylla × E. grandis and D. odorifera leaf morphological traits and physiological traits were negatively correlated overall (Figure 7). There appears to be a contradiction in nitrogen allocation between photosynthesis and leaf construction, indicating that plants have trade-offs between leaf investment and output [75]. Mixed planting did not change the strong positive correlation between the leaf physiological traits of E. urophylla × E. grandis but significantly weakened the correlation between the leaf physiological traits of D. odorifera (Figure 7), indicating that mixed planting caused photosynthetic physiological disorders in D. odorifera [76,77]. In addition, the correlation between the leaf morphological traits of E. urophylla × E. grandis was enhanced in the mixed plantation, which optimized leaf function and might be an important manifestation of improving the competitiveness of E. urophylla × E. grandis [68,78,79].
3.2. Competition for N Promotes the Growth of E. urophylla × E. grandis and Inhibits the Growth of D. odorifera
It has been shown that mixed culture has major productivity advantages [54,80]. However, this study showed that mixed cultivation had opposite effects on the growth of E. urophylla × E. grandis and D. odorifera. Compared to that in the monoculture system, the significant increase in aboveground biomass of E. urophylla × E. grandis in the mixed system contributed the majority (PLER > 0.5), but the aboveground biomass of D. odorifera in the mixed system decreased (PLER < 0.5) compared to that in the monoculture system. This result is also supported by the data on plant height, ground diameter, branch number, and plant biomass (Figure 5 and Figure 6). On the one hand, this difference appears to be the result of differences in the ability to use light, space, and soil resources among different tree species [81,82], and these differences result in reduced or facilitated competitiveness among tree species in mixed systems [83]. In this study, E. urophylla × E. grandis exhibited a taller plant height (Figure 5A), a greater number of branches (Figure 5E), and greater root biomass (Figure 5H). These characteristics allowed for improved light, water, and fertilizer access; enhanced competitiveness (Figure 5L); and promoted growth. On the other hand, interspecific relationships based on complementary effects are largely influenced by N availability [84]. Yao et al. reported that leguminous plants transfer N to eucalyptus trees through root contact, as shown by the 15N isotope labeling method [35,54]. This phenomenon has been observed in mixed plant species such as wheat and faba bean [85], maize and soybean [86], and Acacia mangium and Eucalyptus [55]. This enhances the N uptake of Eucalyptus plants and fully utilizes the N fixation ability of leguminous plants, improving the N utilization efficiency of the entire mixed system [87]. Additionally, the mixed system may redistribute N between different species through mycorrhizal mediation, which could be a significant factor in the differences in growth between Eucalyptus and leguminous plants [88,89].
Plants need adequate N for growth and development. This study revealed that N fertilization effectively improved soil N availability, resulting in increased height, diameter, branch number, and biomass in both the monoculture E. urophylla × E. grandis and D. odorifera (Figure 5 and Figure 6). Notably, compared to the control without N application, N fertilization reduced the above-ground biomass of the entire mixed cultivation system. This was primarily due to the significant decrease in the aboveground biomass of the mixed D. odorifera plants with N addition (Figure 6G). This result is consistent with the stress gradient hypothesis (SGH), which supports the shift from facilitation to competition in plant interactions as survival pressure decreases [90]. Compared to those in the monocultures with the same N application rate, the higher root/shoot ratios in the mixed E. urophylla × E. grandis and D. odorifera treatment groups likely indicated that more biomass was allocated to the roots to compete for N (Figure 5 and Figure 6J). Moreover, the competition index (CRED) for E. urophylla × E. grandis was much greater than the CRDE for D. odorifera in the mixed treatment (Figure 5 and Figure 6L), indicating that E. urophylla × E. grandis is more competitive. Additionally, research has shown that when forest growth conditions improve beyond the stress gradient range, the complementarity of certain species combinations may increase [91]. This may explain the decrease in total biomass for mixed E. urophylla × E. grandis and the increase in biomass for D. odorifera under the N3 treatment (Figure 5 and Figure 6).
3.3. Growth-Related Leaf Traits and Possible Regulatory Pathways in E. urophylla × E. grandis and D. odorifera
According to the classical “leaf economic spectrum” theory, leaves mediate the effects of heterogeneous environments on tree growth and development [3,18,92]. This study revealed several leaf functional traits significantly associated with the growth of E. urophylla × E. grandis and D. odorifera. Mantel tests revealed significant correlations between leaf traits LNC, Pn, gs, Ci, WUE, and CE and growth indicators under both pure and mixed cultivation of E. urophylla × E. grandis (Figure 7A,B). RDA also revealed that Pn, LMF, Tr, Chl a, and PNUE significantly influenced the growth and biomass accumulation of E. urophylla × E. grandis (Figure 8A). This indicates that the growth of E. urophylla × E. grandis is strongly dependent on leaf N supply and related photosynthetic processes. These traits were significantly promoted by mixed culture and N application, thereby promoting the height, ground diameter, branching, and biomass of E. urophylla × E. grandis (Figure 8A). Leaf morphological traits have been reported to be related to plant adaptation to environmental changes [40,46]. In this study, the LMF was found to be significantly negatively correlated with the growth indices and biomass of E. urophylla × E. grandis (Figure 8A). According to the optimal allocation theory, the reduction in light resources under monoculture cultivation induced an increase in E. urophylla × E. grandis LMF to mitigate light competition pressure by enhancing potential resource absorption capacity [47]. However, in mixed cultivation, the light resources available for E. urophylla × E. grandis may have improved, promoting plant growth (Figure 8A) [48]. Furthermore, structural equation modeling indicated that LNC, leaf pigment content, and other leaf physiological traits directly affect the growth of E. urophylla × E. grandis, but leaf morphological traits may indirectly influence the growth of E. urophylla × E. grandis by directly negatively regulating LNC and leaf pigment content (p < 0.05).
For D. odorifera, whether in monoculture or mixed cultivation, the Pn was still the most important leaf trait affecting plant growth (Figure 7C,D and Figure 8B). Furthermore, the RDA indicated that Tr, PNUE, and Chl a+b were significantly negatively correlated with biomass in D. odorifera, consistent with the results for E. urophylla × E. grandis. However, mixed cultivation reduced these indicators, which inhibited the growth of D. odorifera (Figure 9B). Moreover, we discovered that physiological indicators related to photosynthesis (Pn, gs, Tr, Ci, WUE, CE, and PNUE) directly regulate the growth (1.260) and biomass (1.075) of D. odorifera and have the greatest overall effect (Figure 9B). Therefore, a decrease in leaf photosynthetic efficiency is likely the cause of the inhibition of D. odorifera growth in mixed cultivation. In addition, for both E. urophylla × E. grandis and D. odorifera, the leaf N content, pigment content, and other physiological traits all had opposite effects on plant growth compared to leaf morphological traits (Figure 9). This discovery confirms the trade-off between leaf construction and output, which also determines plant growth [47].
4. Materials and Methods
4.1. Trees and Soil
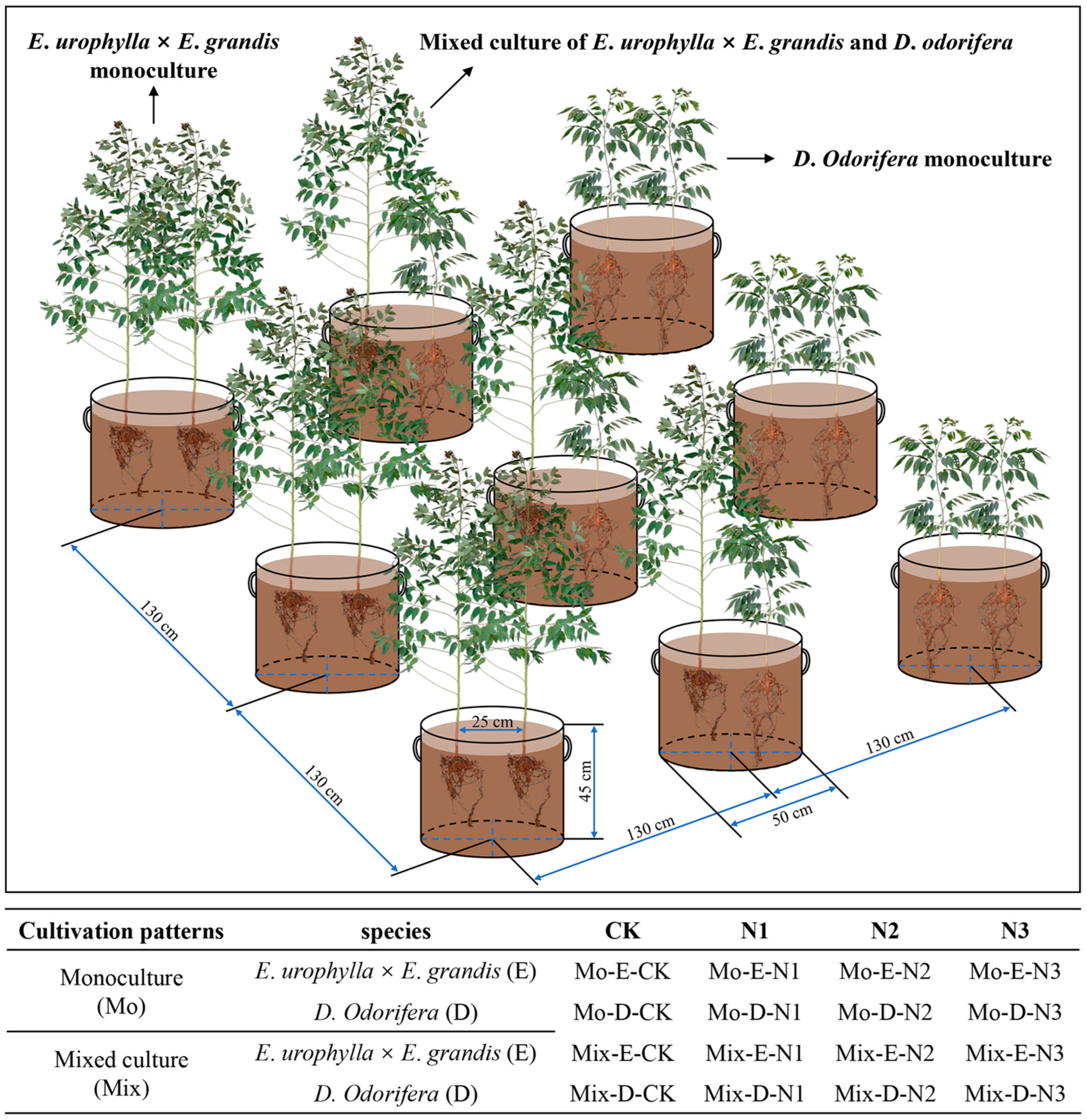
In this experiment, three-month-old E. urophylla × E. grandis (mean height: 44.62 cm) and one-year-old D. odorifera (mean height: 47.68 cm) plants were used because they had similar heights. These seedlings were obtained from the Guangxi Bagui Seedling Company (Nanning, Guangxi, China). The soil was collected at the Guangxi Gaofeng State-Owned Forest Farm. The acidic red soil in the 0–30 cm layer was air-dried and subsequently sieved through a 1 cm mesh to eliminate coarse impurities. The soil pH was 4.81, and the N content was 0.675 g kg−1. To ensure soil ventilation, perlite was added to the soil, and the substrates were mixed well (soil–perlite = 13:2 (v:v)). Nonwoven seedling bags (r = 25 cm and h = 45 cm) were used for culture containers, and each pot was filled with approximately 55 kg of soil (Figure 10).
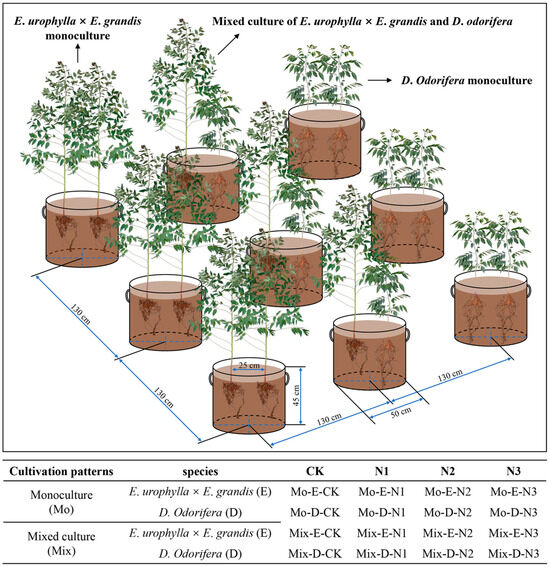
Figure 10.
Diagram of the mixed plantation, monoculture, and experimental treatments.
4.2. Experimental Design
The cultivation experiment was conducted from March to September 2021 at the nursery of Guangxi University, College of Forestry (22°51′4.8″ N, 108°17′30.3″ E). During cultivation, the air temperature variation ranged from 25–40 °C, and the humidity ranged from 50–80%. The sunshine duration of the whole year and the cultivation period were 1654.7 h and 937 h, respectively. The experiment was conducted in a randomized group design with three species combination patterns (E. urophylla × E. grandis monoculture, D. odorifera monoculture, and mixed culture of E. urophylla × E. grandis and D. odorifera), and two seedlings were planted in each pot (Figure 10). In each cultivation pattern, four N application levels (no urea application (CK), 3 g urea pot−1 (N1), 6 g urea pot−1 (N2), and 12 g urea pot−1 (N3)) were set separately. In total, this study was set up with 16 treatments (2 species × 2 cultivation patterns × 4 N addition levels), and each treatment contained 5 replicates. Urea (CH4N2O) was used as an artificial nitrogen source and applied to the soil as a solution 1 week after tree planting. Thereafter, each pot was given an equal amount of water each day to ensure the growth of the plants.
4.3. Leaf Functional Traits
After 180 days of N treatment, the Pn, gs, Tr, and Ci of the mature leaves of E. urophylla × E. grandis and D. odorifera were measured using a Li-6800 portable photosynthesis system (LI-COR, Lincoln, NE, USA) from 9:00 to 11:00 on a sunny day. According to a previous study, the light intensity of the artificial light source was set at 1200 μmol m−2 s−1 (E. urophylla × E. grandis) and 1000 μmol m−2 s−1 (D. odorifera), and the ambient CO2 concentration was 380 μmol (CO2) mol−1. The leaf chamber temperature was 31.3 ± 0.6 °C, and the ambient atmospheric pressure was 99.97 ± 0.02 kPa. WUE and CE were calculated by the ratios of Pn to Tr and Pn to Ci, respectively [63]. Photosynthetic N use sufficiency (PNUE) was calculated as the ratio of the net photosynthetic rate to the leaf nitrogen concentration. The leaf pigments were extracted using an 80% acetone solution, and the absorbances of the extracts at 470, 646, and 663 nm were measured using a Libra S22 UV/Vis spectrophotometer (Biochrom Ltd., Cambridge, UK). Leaf chlorophyll a (Chl a), chlorophyll b (Chl b), chlorophyll a+b (Chl a+b), and carotenoid (Car) contents were calculated using the equations deduced by Lichtenthaler [93].
Additionally, approximately 2400 leaves were collected from the upper, middle, and lower parts of the plants in the 12 treatments. To prevent errors caused by leaf water dissipation, the LT and LFM of each leaf were measured immediately after the leaf was removed. The leaf thickness was measured three times along the main vein using a spiral micrometer with an accuracy of 0.01 mm, and the mean value was considered the leaf thickness. The leaves were scanned using a scanner, and subsequently, the LA was determined through the use of the image processing package (Fiji) in ImageJ2 software (v1.53t National Institutes of Health, Bethesda, MD, USA). Finally, the LDM was determined using the same electronic balance after drying to a constant weight at 60 °C. LV was obtained by multiplying LA by LT; SLA was calculated by the ratio of LA to LDM [94]; LDMC was expressed by the ratio of LDM to LFM; and LTD was calculated by dividing LDM by LV [12]. From the whole-leaf perspective, the LMF at the branching level was expressed as the ratio of total leaf dry weight to total dry weight of leaves and branches [40]. Finally, the leaf samples were digested with H2SO4-H2O2, and the total N content of the leaves was determined through micro-Kjeldahl methods.
4.4. Plant Growth and Biomass
Plant height, ground diameter, and branch number were measured for each plant in the different treatments 180 days after N application. Plant height was defined as the vertical distance from the ground to the point of growth of the main stem. The ground diameter was defined as the diameter of the main stem 10 cm from the ground. Leaves, stems, branches, primary roots, and lateral roots of E. urophylla × E. grandis and D. odorifera seedlings from each treatment were separated 180 days after the N application. The plant samples were fixed at 95 °C for 2 h and then heated at 60 °C to a constant weight, after which the results were recorded. The shoot biomass was calculated by adding the leaf, branch, and stem biomass of the seedlings, and the root/shoot ratio is the ratio of seedling root biomass to aboveground biomass.
4.5. Statistics and Analysis
The partial land equivalent ratio (PLER) was calculated with reference to the following equation [95]:
where Bmix and Bmo represent the aboveground biomass (g plot−1) under mixed stand and monoculture cultivation, respectively.
The competitive ratio (CR) was calculated based on the following equation [96]:
where CRED indicates the competitive ratio of E. urophylla × E. grandis relative to that of D. odorifera and CRDE indicates the competitive ratio of D. odorifera relative to that of E. urophylla × E. grandis. BME and BE indicate the biomass of the whole plant (g plot−1) of Eucalyptus in mixed systems and monocultures, respectively; BMD and BD indicate the biomass of the whole plant (g plot−1) of D. odorifera in mixed stands and monoculture, respectively; and ZME and ZMD indicate the proportion of plants in mixed cultivation (ZME = 0.5 and ZMD = 0.5) of E. urophylla × E. grandis and D. odorifera, respectively. The capital letters “E” and “D” are used to represent E. urophylla × E. grandis and D. odorifera, respectively, in this study.
To explore the effects of different treatments and their interactions on plants, the data from the experiment were subjected to one-way and two-way ANOVA with a significance test (Duncan’s test, p < 0.05) using SPSS version 19.0® (SPSS Corp., Chicago, IL, USA). The Mantel test in R (version 4.3.1) and redundancy analysis (RDA) in Canoco 5.0 (Wageningen University and Research, Wageningen, The Netherlands) were used to predict the relationships between leaf functional traits and biomass in E. urophylla × E. grandis and D. odorifera. The potential correlation between leaf physiological and morphological traits was explored using correlation analysis in R (version 4.3.1). In addition, partial least squares structural equation modeling (PLS-SEM) was used to analyze the regulatory effects of leaf traits on plant growth. SmartPLS 4.0 (SmartPLS GmbH, Inc., Oststeinbek, Germany) was used to construct formative indicator models. A path-based weighting scheme with a maximum of 1000 iterations was chosen. We evaluated the significance of the paths between each latent variable using significance tests and adjusted for nonsignificant paths. Finally, we evaluated the structural models using the coefficient of determination (R2), effect size (f2), and cross-validation redundancy (Q2). Chin (1998) used R2 to measure the correlation between the explained variance of a latent variable and its total variance [97]. High, medium, and low explanations are denoted by R2 values of 0.670, 0.333, and 0.190, respectively. f2 was used to evaluate the structural equation modeling for the degree of influence of each pathway [98]. The predictive relevance of the structural model was evaluated using the nonparametric Stone–Geisser test, and Q2 was used to determine the predictive relevance of the model for a given structure [99,100].
5. Conclusions
This study investigated the N response and growth characteristics of E. urophylla × E. grandis and legume tree species on mixed plantations based on leaf functional traits. The results of multiple analyses indicated that the differences in nutrient acquisition characteristics and N competitive ability of the tree species themselves led to different responses of E. urophylla × E. grandis and D. odorifera leaf N content, physiological traits, and morphological traits to N application and mixed cultivation. This ultimately promoted E. urophylla × E. grandis growth but inhibited D. odorifera growth under the combined treatment of mixed cultivation and N application. Notably, leaf N content and physiological indicators related to photosynthesis directly determine seedling growth, while leaf morphological traits participate in the trade-off between seedling resource acquisition and indirect effects on seedling growth. The results of this study can help to understand the N utilization characteristics of trees in mixed stands and ultimately provide a reference for fertilization decisions and the selection of mixed tree species in the forestry production process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13070988/s1, Table S1: List of leaf functional trait indicators analyzed in this paper and their descriptions. Table S2: Effects of cultivation pattern, N addition, and their interactions on the leaf traits of E. urophylla × E. grandis and D. odorifera. Table S3: The r values and p-values of Pearson’s correlation analysis of leaf traits of E. urophylla × E. grandis in monoculture. Table S4: The t values and standard error of Pearson’s correlation analysis of leaf traits of E. urophylla × E. grandis in monoculture. Table S5: The r values and p-values of Pearson’s correlation analysis of leaf traits of E. urophylla × E. grandis in mixed culture. Table S6: The t values and standard error of Pearson’s correlation analysis of leaf traits of E. urophylla × E. grandis in mixed culture. Table S7: The r values and p-values of Pearson’s correlation analysis of leaf traits of D. odorifera in monoculture. Table S8: The t values and standard error of Pearson’s correlation analysis of leaf traits of D. odorifera in monoculture. Table S9: The r values and p-values of Pearson’s correlation analysis of leaf traits of D. odorifera in mixed culture. Table S10: The t values and standard error of Pearson’s correlation analysis of leaf traits of D. odorifera in mixed culture.
Author Contributions
Conceptualization, Methodology, Software, Formal analysis, Writing—Original Draft, H.Z.; Validation, Visualization, Writing—Review and Editing, Y.L.; Validation, Investigation, C.J.; Investigation, Y.C.; Investigation, J.D.; Methodology, M.L.; Data Curation, Y.H.; Conceptualization, Resources, Writing—Review and Editing, Project administration, S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant numbers 32260382 and 31460196.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article (and its Supplementary Material files).
Acknowledgments
The authors would like to express their gratitude to the editor and reviewers for providing conducive feedback to improve this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
LNC: leaf nitrogen content; Pn: net photosynthetic rate; PNUE: photosynthetic nitrogen use sufficiency; gs: stomatal conductance; Tr: transpiration rate; Ci: intercellular carbon dioxide concentration; WUE: water-use efficiency; CE: Rubisco carboxylation efficiency; Chl a: chlorophyll a; Chl b: chlorophyll b; Chl a+b: chlorophyll a and chlorophyll b; Car: carotenoids; LA: leaf area; LT: leaf thickness; LV: leaf volume; LDM: leaf dry mass; LFM: leaf fresh mass; SLA: specific leaf area; LTD: leaf tissue density; LDMC: leaf dry mass content; LMF: leaf mass fraction; LER: land equivalent ratio; PLER: partial land equivalent ratio; and CR: competitive ratio.
References
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Wang, Z.; Townsend, P.A.; Kruger, E.L. Leaf spectroscopy reveals divergent inter- and intra-species foliar trait covariation and trait-environment relationships across NEON domains. New Phytol. 2022, 235, 923–938. [Google Scholar] [CrossRef] [PubMed]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Guo, W.; Xu, W.; Wei, Y.; Wang, R. Leaf morphology correlates with water and light availability: What consequences for simple and compound leaves? Prog. Nat. Sci. 2009, 19, 1789–1798. [Google Scholar] [CrossRef]
- Hassiotou, F.; Renton, M.; Ludwig, M.; Evans, J.R.; Veneklaas, E.J. Photosynthesis at an extreme end of the leaf trait spectrum: How does it relate to high leaf dry mass per area and associated structural parameters? J. Exp. Bot. 2010, 61, 3015–3028. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.L.; Yu, G.R.; He, N.P.; Wang, Q.F.; Zhao, N.; Xu, Z.W. Latitudinal variation of leaf morphological traits from species to communities along a forest transect in eastern China. J. Geogr. Sci. 2016, 26, 15–26. [Google Scholar] [CrossRef]
- Poorter, H.; Jagodzinski, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niklas, K.J.; Wang, L.; Yu, K.; Li, Y.; Shi, P. Influence of leaf age on the scaling relationships of lamina mass vs. area. Front. Plant Sci. 2022, 13, 860206. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- Migliavacca, M.; Perez-Priego, O.; Rossini, M.; El-Madany, T.S.; Moreno, G.; van der Tol, C.; Rascher, U.; Berninger, A.; Bessenbacher, V.; Burkart, A.; et al. Plant functional traits and canopy structure control the relationship between photosynthetic CO2 uptake and far-red sun-induced fluorescence in a Mediterranean grassland under different nutrient availability. New Phytol. 2017, 214, 1078–1091. [Google Scholar] [CrossRef]
- Bucher, S.F.; Auerswald, K.; Grün-Wenzel, C.; Higgins, S.I.; Römermann, C. Abiotic site conditions affect photosynthesis rates by changing leaf functional traits. Basic Appl. Ecol. 2021, 57, 54–64. [Google Scholar] [CrossRef]
- Craine, J.M.; Towne, E.G. High leaf tissue density grassland species consistently more abundant across topographic and disturbance contrasts in a North American tallgrass prairie. Plant Soil 2010, 337, 193–203. [Google Scholar] [CrossRef]
- Tang, J.; Sun, B.; Cheng, R.; Shi, Z.; Da, L.; Liu, S.; Centritto, M. Effects of soil nitrogen (N) deficiency on photosynthetic N-use efficiency in N-fixing and non-N-fixing tree seedlings in subtropical China. Sci. Rep. 2019, 9, 4604. [Google Scholar] [CrossRef] [PubMed]
- Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Treatment of nitric oxide supplemented with nitrogen and sulfur regulates photosynthetic performance and stomatal behavior in mustard under salt stress. Physiol. Plant. 2020, 168, 490–510. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Li, L.; Li, Q.; Mao, H.; Wang, L.; Bie, Y.; Zeng, X.; Liao, L.; Wang, X.; Deng, H.; et al. Identification of photosynthesis characteristics and chlorophyll metabolism in leaves of Citrus Cultivar (Harumi) with varying degrees of chlorosis. Int. J. Mol. Sci. 2023, 24, 8394. [Google Scholar] [CrossRef] [PubMed]
- Iniguez, C.; Aguilo-Nicolau, P.; Galmes, J. Improving photosynthesis through the enhancement of Rubisco carboxylation capacity. Biochem. Soc. Trans. 2021, 49, 2007–2019. [Google Scholar] [CrossRef] [PubMed]
- Medrano, H.; Flexas, J.; Galmés, J. Variability in water use efficiency at the leaf level among Mediterranean plants with different growth forms. Plant Soil 2009, 317, 17–29. [Google Scholar] [CrossRef]
- Donovan, L.A.; Maherali, H.; Caruso, C.M.; Huber, H.; de Kroon, H. The evolution of the worldwide leaf economics spectrum. Trends Ecol. Evol. 2011, 26, 88–95. [Google Scholar] [CrossRef]
- Andersson, I.; Backlund, A. Structure and function of Rubisco. Plant Physiol. Biochem. 2008, 46, 275–291. [Google Scholar] [CrossRef]
- Onoda, Y.; Wright, I.J.; Evans, J.R.; Hikosaka, K.; Kitajima, K.; Niinemets, U.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef]
- Evans, J.R.; Clarke, V.C. The nitrogen cost of photosynthesis. J. Exp. Bot. 2019, 70, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, G.; Zhang, Y.; Qin, S.; Dong, J.; Cui, Y.; Liu, X.; Zheng, P.; Wang, R. Leaf functional traits of two species affected by nitrogen addition rate and period not nitrogen compound type in a meadow grassland. Front. Plant Sci. 2022, 13, 841464. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Z.; Zhang, G.; Liu, Q.; Zheng, P.; Wang, R. Tipping point of plant functional traits of Leymus chinensis to nitrogen addition in a temperate grassland. Front. Plant Sci. 2022, 13, 982478. [Google Scholar] [CrossRef]
- Zheng, L.L.; Zhao, Q.; Yu, Z.Y.; Zhao, S.Y.; Zeng, D.H. Altered leaf functional traits by nitrogen addition in a nutrient-poor pine plantation: A consequence of decreased phosphorus availability. Sci. Rep. 2017, 7, 7415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Adams, H.D.; Wang, A.; Wu, J.; Jin, C.; Guan, D.; Yuan, F. Responses of woody plant functional traits to nitrogen addition: A meta-analysis of leaf economics, gas exchange, and hydraulic traits. Front. Plant Sci. 2018, 9, 683. [Google Scholar] [CrossRef]
- Zhou, X.; Xin, J.; Huang, X.; Li, H.; Li, F.; Song, W. Linking leaf functional traits with soil and climate factors in forest ecosystems in China. Plants 2022, 11, 3545. [Google Scholar] [CrossRef]
- Bristow, M.; Vanclay, J.K.; Brooks, L.; Hunt, M. Growth and species interactions of Eucalyptus pellita in a mixed and monoculture plantation in the humid tropics of north Queensland. For. Ecol. Manag. 2006, 233, 285–294. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, H.; Wen, Y.; Goodale, U.M.; Zhu, Y.; Yu, S.; Li, C.; Li, X. Intensive management and declines in soil nutrients lead to serious exotic plant invasion in Eucalyptus plantations under successive short-rotation regimes. Land Degrad. Dev. 2019, 31, 297–310. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, J.; Qin, Z.; Wang, H.; Qin, Q.; Zhao, Z. Soil fertility evolution characteristics in main Eucalyptus producing areas of Guangxi: 1993–2018. Chin. Agric. Sci. Bull. 2021, 37, 94–99. [Google Scholar]
- Li, X.; Ye, D.; Liang, H.; Zhu, H.; Qin, L.; Zhu, Y.; Wen, Y. Effects of successive rotation regimes on carbon stocks in Eucalyptus plantations in subtropical China measured over a full rotation. PLoS ONE 2015, 10, e0132858. [Google Scholar] [CrossRef]
- Moreau, D.; Bardgett, R.D.; Finlay, R.D.; Jones, D.L.; Philippot, L. A plant perspective on nitrogen cycling in the rhizosphere. Funct. Ecol. 2019, 33, 540–552. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, H.; Chen, F.L.; Ouyang, Z.Y.; Wang, Y.; Wu, Y.F.; Lan, J.; Fu, M.; Xiang, X.W. Changes in soil quality after converting to plantations in southern China. Solid Earth 2015, 6, 115–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Geographical spatial distribution and productivity dynamic change of eucalyptus plantations in China. Sci. Rep. 2021, 11, 19764. [Google Scholar] [CrossRef] [PubMed]
- Peix, A.; Ramírez-Bahena, M.H.; Velázquez, E.; Bedmar, E.J. Bacterial associations with legumes. Crit. Rev. Plant Sci. 2015, 34, 17–42. [Google Scholar] [CrossRef]
- Yao, X.Y.; Li, Y.F.; Liao, L.N.; Sun, G.; Wang, H.X.; Ye, S.M. Enhancement of nutrient absorption and interspecific nitrogen transfer in a Eucalyptus urophylla × eucalyptus grandis and Dalbergia odorifera mixed plantation. For. Ecol. Manag. 2019, 449, 117465. [Google Scholar] [CrossRef]
- Xu, Y.X.; Ren, S.Q.; Liang, Y.F.; Du, A.; Li, C.; Wang, Z.C.; Zhu, W.K.; Wu, L.C. Soil nutrient supply and tree species drive changes in soil microbial communities during the transformation of a multi-generation Eucalyptus plantation. Appl. Soil Ecol. 2021, 166, 103991. [Google Scholar] [CrossRef]
- Mathan, J.; Bhattacharya, J.; Ranjan, A. Enhancing crop yield by optimizing plant developmental features. Development 2016, 143, 3283–3294. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Fang, Q.; Peng, S.; Li, Y. Genotypic variation of plant biomass under nitrogen deficiency is positively correlated with conservative economic traits in wheat. J. Exp. Bot. 2022, 73, 2175–2189. [Google Scholar] [CrossRef] [PubMed]
- Lusk, C.H.; Grierson, E.R.P.; Laughlin, D.C. Large leaves in warm, moist environments confer an advantage in seedling light interception efficiency. New Phytol. 2019, 223, 1319–1327. [Google Scholar] [CrossRef]
- Yin, Q.L.; Tian, T.T.; Han, X.H.; Xu, J.S.; Chai, Y.F.; Mo, J.; Lei, M.L.; Wang, L.; Yue, M. The relationships between biomass allocation and plant functional trait. Ecol. Indic. 2019, 102, 302–308. [Google Scholar] [CrossRef]
- Huang, W.W.; Reddy, G.V.P.; Li, Y.Y.; Larsen, J.B.; Shi, P.J. Increase in absolute leaf water content tends to keep pace with that of leaf dry mass-evidence from bamboo plants. Symmetry 2020, 12, 1345. [Google Scholar] [CrossRef]
- Ravi, V.; Pushpaleela, A.; Raju, S.; Gangadharan, B.; More, S.J. Evaluation of photosynthetic efficiency of yam bean (Pachyrhizus erosus L.) at saturating photon flux density under elevated carbon dioxide. Physiol. Mol. Biol. Plants 2020, 26, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Arias-Rios, J.A.; El Mujtar, V.A.; Pastorino, M.; Marchelli, P. Genetic variation of leaf pigment content in a southern beech. Trees-Struct. Funct. 2022, 36, 1823–1836. [Google Scholar] [CrossRef]
- Capo-Bauca, S.; Whitney, S.; Iniguez, C.; Serrano, O.; Rhodes, T.; Galmes, J. The trajectory in catalytic evolution of Rubisco in Posidonia seagrass species differs from terrestrial plants. Plant Physiol. 2023, 191, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; Trueba, S.; Coste, S.; Ducouret, E.; Tysklind, N.; Heuertz, M.; Bonal, D.; Burban, B.; Herault, B.; Derroire, G. Seasonal variation of leaf thickness: An overlooked component of functional trait variability. Plant. Biol. 2022, 24, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, X. Limiting resource and leaf functional traits jointly determine distribution patterns of leaf intrinsic water use efficiency along aridity gradients. Front. Plant Sci. 2022, 13, 909603. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Violle, C.; Bourget, M.Y.; Scherer-Lorenzen, M.; Fort, F. Allocation, morphology, physiology, architecture: The multiple facets of plant above- and below-ground responses to resource stress. New Phytol. 2018, 219, 1338–1352. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Cadotte, M.W.; Jin, G. Size- and environment-driven seedling survival and growth are mediated by leaf functional traits. Proc. Biol. Sci. 2022, 289, 20221400. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, R.; DiManno, N.M. Detecting terrestrial nutrient limitation: A global meta-analysis of foliar nutrient concentrations after fertilization. Front. Earth Sci. 2016, 4, 23. [Google Scholar] [CrossRef]
- Yuan, Z.Y.; Chen, H.Y. Negative effects of fertilization on plant nutrient resorption. Ecology 2015, 96, 373–380. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Yu, H.; Le Roux, J.J.; Jiang, Z.; Sun, F.; Peng, C.; Li, W. Soil nitrogen dynamics and competition during plant invasion: Insights from Mikania micrantha invasions in China. New Phytol. 2021, 229, 3440–3452. [Google Scholar] [CrossRef] [PubMed]
- Reyer, C.; Lasch, P.; Mohren, G.M.J.; Sterck, F.J. Inter-specific competition in mixed forests of Douglas-fir (Pseudotsuga menziesii) and common beech (Fagus sylvatica) under climate change—A model-based analysis. Ann. For. Sci. 2010, 67, 805. [Google Scholar] [CrossRef]
- Yao, X.; Goodale, U.M.; Yang, M.; Liao, L.; Yu, S.; Wang, S.; Ye, S. Bidirectional nitrogen transfer and plant growth in a mixed plantation of N2-fixing species and Eucalyptus urophylla × E. grandis under different N applications. Forests 2021, 12, 1171. [Google Scholar] [CrossRef]
- Oliveira, I.R.; Bordron, B.; Laclau, J.P.; Paula, R.R.; Ferraz, A.V.; Gonçalves, J.L.M.; le Maire, G.; Bouillet, J.P. Nutrient deficiency enhances the rate of short-term belowground transfer of nitrogen from to trees in mixed-species plantations. For. Ecol. Manag. 2021, 491, 119192. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, T.; Lu, X.; Ellsworth, D.S.; BassiriRad, H.; You, C.; Wang, D.; He, P.; Deng, Q.; Liu, H.; et al. Global response patterns of plant photosynthesis to nitrogen addition: A meta-analysis. Glob. Chang. Biol. 2020, 26, 3585–3600. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhou, J.J. Growth performance, photosynthesis, and root characteristics are associated with nitrogen use efficiency in six poplar species. Environ. Exp. Bot. 2019, 164, 40–51. [Google Scholar] [CrossRef]
- Bassi, D.; Menossi, M.; Mattiello, L. Nitrogen supply influences photosynthesis establishment along the sugarcane leaf. Sci. Rep. 2018, 8, 2327. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Sánchez, M.G.; Gómez-Merino, F.C.; Tejeda-Sartorius, O.; Trejo-Téllez, L.I. Nitrogen nutrition differentially affects concentrations of photosynthetic pigments and antioxidant compounds in Mexican Marigold (Tagetes erecta L.). Agriculture 2023, 13, 517. [Google Scholar] [CrossRef]
- Livingston, N.J.; Guy, R.D.; Sun, Z.J.; Ethier, G.J. The effects of nitrogen stress on the stable carbon isotope composition, productivity and water use efficiency of white spruce (Picea glauca (Moench) Voss) seedlings. Plant Cell Environ. 2002, 22, 281–289. [Google Scholar] [CrossRef]
- Chou, S.R.; Chen, B.; Chen, J.; Wang, M.M.; Wang, S.Q.; Croft, H.; Shi, Q. Estimation of leaf photosynthetic capacity from the photochemical reflectance index and leaf pigments. Ecol. Indic. 2020, 110, 105867. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Prieto, I.; Armas, C.; Casanoves, F.; Dieme, J.S.; Diouf, M.; Yossi, H.; Kaya, B.; Pugnaire, F.I.; Rusch, G.M. Higher leaf nitrogen content is linked to tighter stomatal regulation of transpiration and more efficient water use across dryland trees. New Phytol. 2022, 235, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.Y.; Chen, L.S.; Huang, Z.R. The photosynthetic performance of two Citrus species under long-term aluminum treatment. Photosynthetica 2020, 58, 228–235. [Google Scholar] [CrossRef]
- Robinson, D.E.; Wagner, R.G.; Bell, F.W.; Swanton, C.J. Photosynthesis, nitrogen-use efficiency, and water-use efficiency of jack pine seedlings in competition with four boreal forest plant species. Can. J. For. Res. 2001, 31, 2014–2025. [Google Scholar] [CrossRef]
- Reynolds, P.E.; Simpson, J.A.; Thevathasan, N.V.; Gordon, A.M. Effects of tree competition on corn and soybean photosynthesis, growth, and yield in a temperate tree-based agroforestry intercropping system in southern Ontario, Canada. Ecol. Eng. 2007, 29, 362–371. [Google Scholar] [CrossRef]
- Zou, Y.X.; Li, B.Y.; Peñuelas, J.; Sardans, J.; Yu, H.; Chen, X.P.; Deng, X.Y.; Cheng, D.L.; Zhong, Q.L. Response of functional traits in Machilus pauhoi to nitrogen addition is influenced by differences of provenances. For. Ecol. Manag. 2022, 513, 120207. [Google Scholar] [CrossRef]
- Freschet, G.T.; Kichenin, E.; Wardle, D.A.; de Bello, F. Explaining within-community variation in plant biomass allocation: A balance between organ biomass and morphology above vs below ground? J. Veg. Sci. 2015, 26, 431–440. [Google Scholar] [CrossRef]
- Takigahira, H.; Yamawo, A. Competitive responses based on kin-discrimination underlie variations in leaf functional traits in Japanese beech (Fagus crenata) seedlings. Evol. Ecol. 2019, 33, 521–531. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Scalon, M.C.; Bohn, A.; Coelho, G.C.; Meister, L.; Alves, R.D.; Secco, R.T.; Zwiener, V.P.; Marcilio-Silva, V.; Trindade, W.C.F.; Marques, M.C.M. Relationship between growth trajectories and functional traits for woody trees in a secondary tropical forest. Front. For. Glob. Chang. 2022, 5, 754656. [Google Scholar] [CrossRef]
- Hodgson, J.G.; Montserrat-Marti, G.; Charles, M.; Jones, G.; Wilson, P.; Shipley, B.; Sharafi, M.; Cerabolini, B.E.; Cornelissen, J.H.; Band, S.R.; et al. Is leaf dry matter content a better predictor of soil fertility than specific leaf area? Ann. Bot. 2011, 108, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.L.; Guo, Z.; Zhang, P.F.; Du, G.Z. Shift in community functional composition following nitrogen fertilization in an alpine meadow through intraspecific trait variation and community composition change. Plant Soil 2018, 431, 289–302. [Google Scholar] [CrossRef]
- Bussotti, F. Functional leaf traits, plant communities and acclimation processes in relation to oxidative stress in trees: A critical overview. Glob. Chang. Biol. 2008, 14, 2727–2739. [Google Scholar] [CrossRef]
- Perez-Ramos, I.M.; Matias, L.; Gomez-Aparicio, L.; Godoy, O. Functional traits and phenotypic plasticity modulate species coexistence across contrasting climatic conditions. Nat. Commun. 2019, 10, 2555. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.X.; Shangguan, Z.P. Spatial patterns of photosynthetic characteristics and leaf physical traits of plants in the Loess Plateau of China. Plant Ecol. 2007, 191, 279–293. [Google Scholar] [CrossRef]
- Hikosaka, K. Interspecific difference in the photosynthesis-nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Dietze, M. Scale dependence in the effects of leaf ecophysiological traits on photosynthesis: Bayesian parameterization of photosynthesis models. New Phytol. 2013, 200, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Leigh, A.; Rayburg, S. Relationships among leaf traits of Australian arid zone plants: Alternative modes of thermal protection. Aust. J. Bot. 2012, 60, 471–483. [Google Scholar] [CrossRef]
- Adler, P.B.; Fajardo, A.; Kleinhesselink, A.R.; Kraft, N.J. Trait-based tests of coexistence mechanisms. Ecol. Lett. 2013, 16, 1294–1306. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Castro-Izaguirre, N.; Baruffol, M.; Brezzi, M.; Lang, A.; Li, Y.; Hardtle, W.; von Oheimb, G.; Yang, X.; et al. Impacts of species richness on productivity in a large-scale subtropical forest experiment. Science 2018, 362, 80–83. [Google Scholar] [CrossRef]
- Nunes, L.; Gower, S.T.; Monteiro, M.L.; Lopes, D.; Rego, F.C. Growth dynamics and productivity of pure and mixed Castanea sativa Mill. and Pseudotsuga menziesii (Mirb.) Franco plantations in northern Portugal. Iforest 2013, 7, 92–102. [Google Scholar] [CrossRef]
- Williams, L.J.; Paquette, A.; Cavender-Bares, J.; Messier, C.; Reich, P.B. Spatial complementarity in tree crowns explains overyielding in species mixtures. Nat. Ecol. Evol. 2017, 1, 0063. [Google Scholar] [CrossRef] [PubMed]
- Amazonas, N.T.; Forrester, D.I.; Silva, C.C.; de Almeida, D.R.A.; Oliveira, R.S.; Rodrigues, R.R.; Brancalion, P.H.S. Light- and nutrient-related relationships in mixed plantations of Eucalyptus and a high diversity of native tree species. New For. 2021, 52, 807–828. [Google Scholar] [CrossRef]
- Forrester, D.I. The spatial and temporal dynamics of species interactions in mixed-species forests: From pattern to process. For. Ecol. Manag. 2014, 312, 282–292. [Google Scholar] [CrossRef]
- Xiao, Y.B.; Li, L.; Zhang, F.S. Effect of root contact on interspecific competition and N transfer between wheat and fababean using direct and indirect 15N techniques. Plant Soil 2004, 262, 45–54. [Google Scholar] [CrossRef]
- Hamel, C.; Barrantes-Cartn, U.; Furlan, V.; Smith, D.L. Endomycorrhizal fungi in nitrogen transfer from soybean to maize. Plant Soil 1991, 138, 33–40. [Google Scholar] [CrossRef]
- Lan, Y.; Liao, L.; Yao, X.; Ye, S. Synergistic effects of nitrogen and plant growth-promoting rhizobacteria inoculation on the growth, physiological traits and nutrient absorption of intercropped Eucalyptus urophylla × Eucalyptus grandis and Dalbergia odorifera. Trees 2022, 37, 319–330. [Google Scholar] [CrossRef]
- Pena, R.; Tejedor, J.; Zeller, B.; Dannenmann, M.; Polle, A. Interspecific temporal and spatial differences in the acquisition of litter-derived nitrogen by ectomycorrhizal fungal assemblages. New Phytol. 2013, 199, 520–528. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cornelissen, J.H.C.; Wang, P.; Dong, M.; Ou, J. Nitrogen transfer from one plant to another depends on plant biomass production between conspecific and heterospecific species via a common arbuscular mycorrhizal network. Environ. Sci. Pollut. Res. Int. 2019, 26, 8828–8837. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Bertness, M.D.; Altieri, A.H. Global shifts towards positive species interactions with increasing environmental stress. Ecol. Lett. 2013, 16, 695–706. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J. A review of processes behind diversity-productivity relationships in forests. Curr. Rep. 2016, 2, 45–61. [Google Scholar] [CrossRef]
- Feng, H.; Guo, J.; Peng, C.; Kneeshaw, D.; Roberge, G.; Pan, C.; Ma, X.; Zhou, D.; Wang, W. Nitrogen addition promotes terrestrial plants to allocate more biomass to aboveground organs: A global meta-analysis. Glob. Chang. Biol. 2023, 29, 3970–3989. [Google Scholar] [CrossRef] [PubMed]
- Lichtenhaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Araujo, I.; Morandi, P.S.; Muller, A.O.; Mariano, L.H.; Alvarez, F.; da Silva, I.V.; Marimon, B.; Marimon, B.S. Leaf functional traits and monodominance in Southern Amazonia tropical forests. Plant Ecol. 2022, 223, 185–200. [Google Scholar] [CrossRef]
- Zhang, W.P.; Li, Z.X.; Gao, S.N.; Yang, H.; Xu, H.S.; Yang, X.; Fan, H.X.; Su, Y.; Surigaoge; Weiner, J.; et al. Resistance vs. surrender: Different responses of functional traits of soybean and peanut to intercropping with maize. Field Crops Res. 2023, 291, 108779. [Google Scholar] [CrossRef]
- Xu, B.; Xu, W.; Huang, J.; Shan, L.; Li, F. Biomass production and relative competitiveness of a C3 legume and a C4 grass co-dominant in the semiarid Loess Plateau of China. Plant Soil 2011, 347, 25–39. [Google Scholar] [CrossRef]
- Chin, W.W. The Partial Least Squares Approach to Structural Equation Modeling; Lawrence Erlbaum Associates: Mahwah, NJ, USA; London, UK, 1998. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 1977; p. 469. [Google Scholar]
- Urbach, N.; Ahlemann, F. Structural equation modeling in information systems research using Partial Least Squares. J. Inf. Technol. Theory Appl. 2010, 11, 5–40. [Google Scholar]
- Hair, J.F.; Risher, J.J.; Sarstedt, M.; Ringle, C.M. When to use and how to report the results of PLS-SEM. Eur. Bus. Rev. 2019, 31, 2–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).