Exogenous Spermidine Alleviated Low-Temperature Damage by Affecting Polyamine Metabolism and Antioxidant Levels in Apples
Abstract
:1. Introduction
2. Results
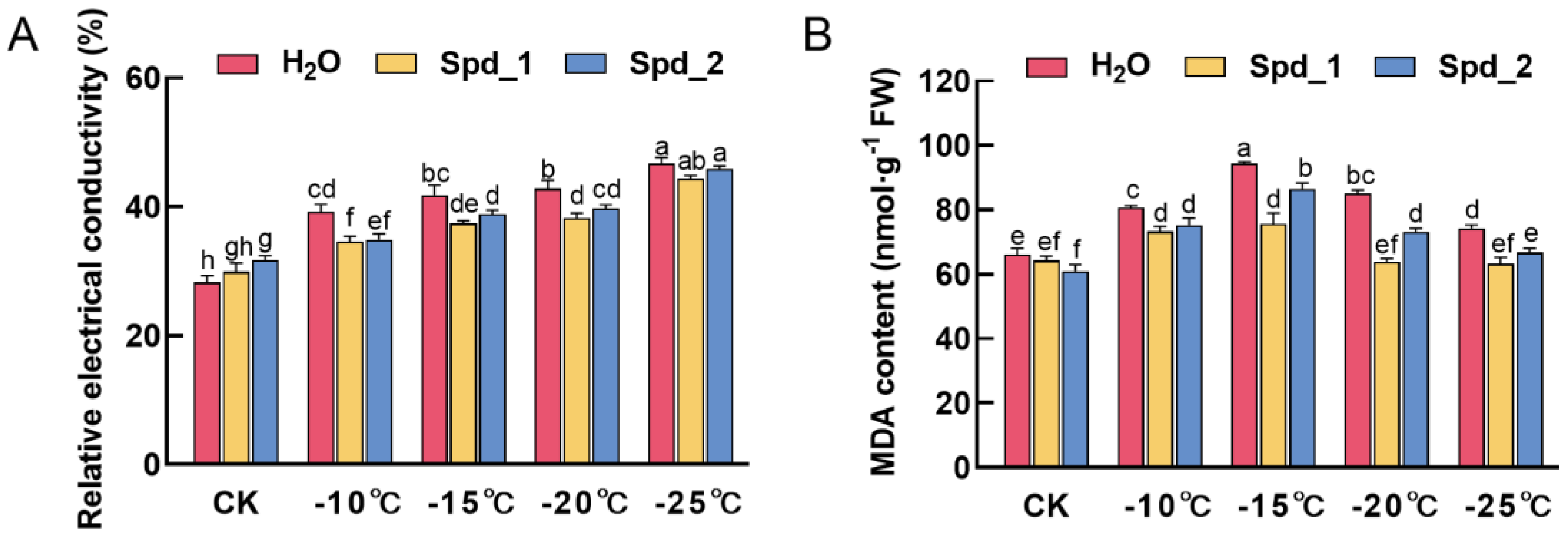
2.1. Effect of Exogenous Spd on Membrane Damage of Apple under Low-Temperature Stress
2.2. Effects of Exogenous Spd on ROS Metabolism of Apple under Low-Temperature Stress
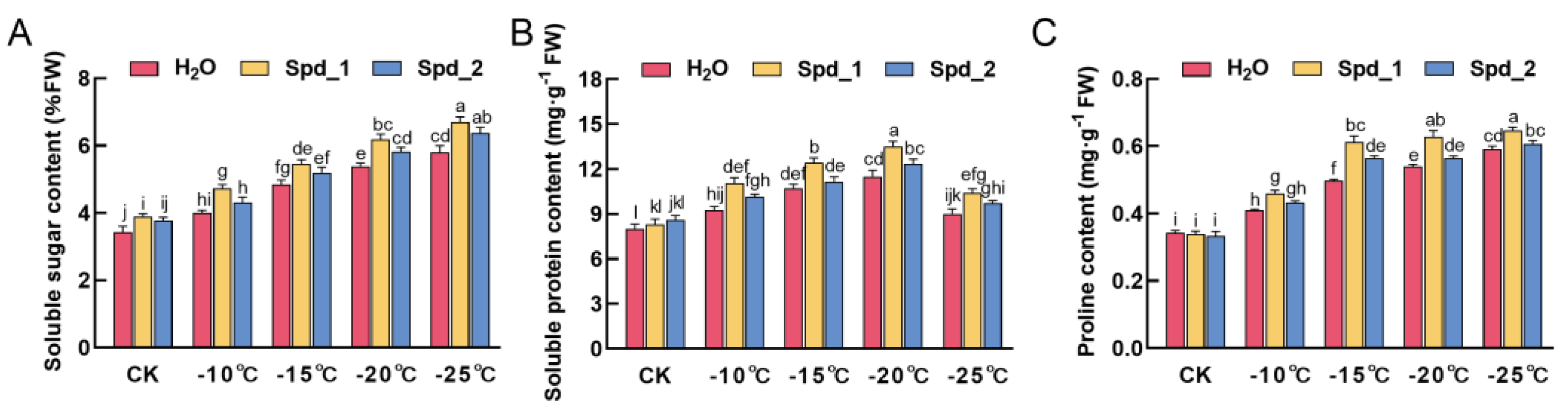
2.3. Effects of Exogenous Spd on Osmotic Adjustment Substance Content of Apple under Low-Temperature Stress
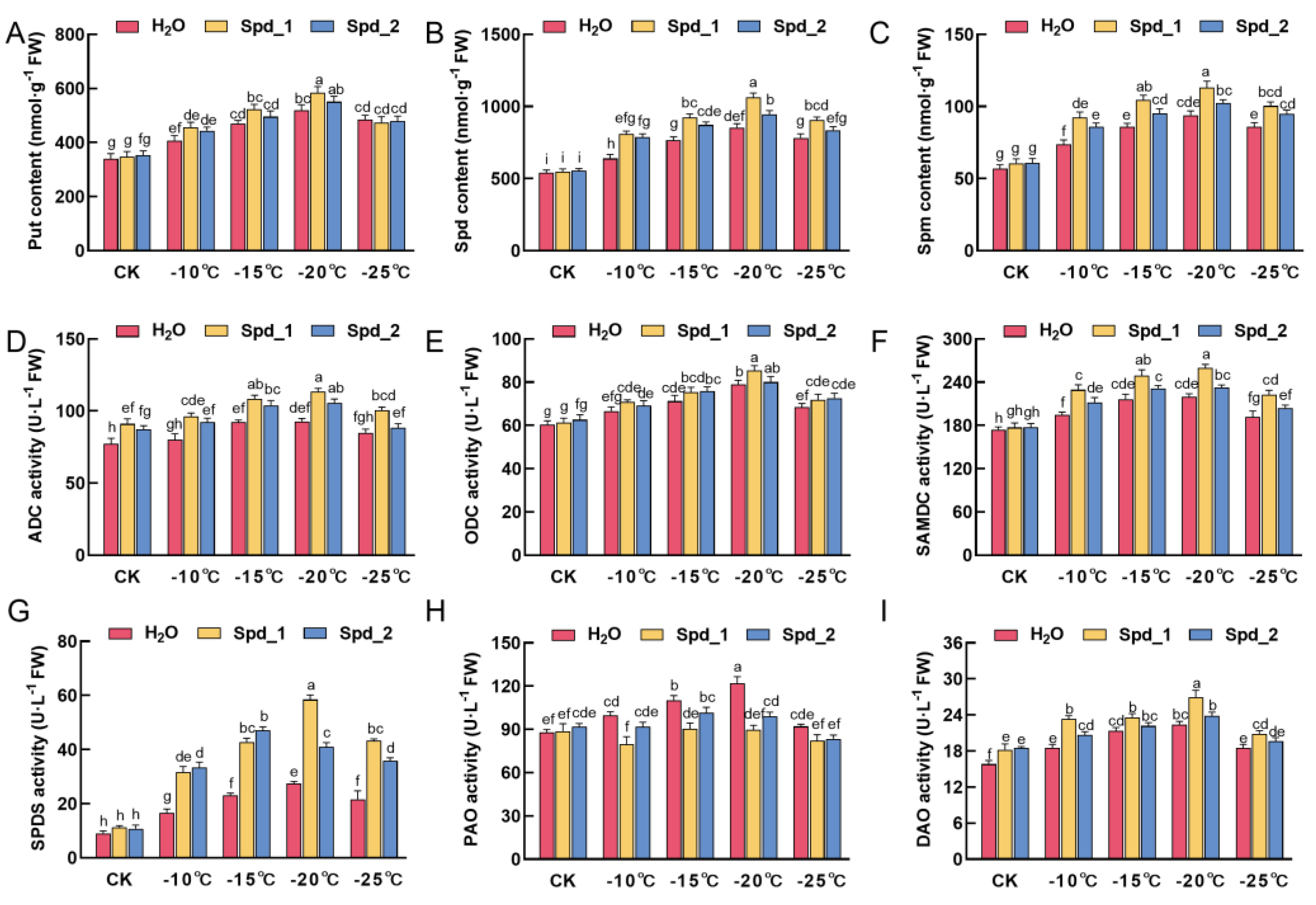
2.4. Effects of Exogenous Spd on PAs Metabolism in Apple under Low-Temperature Stress
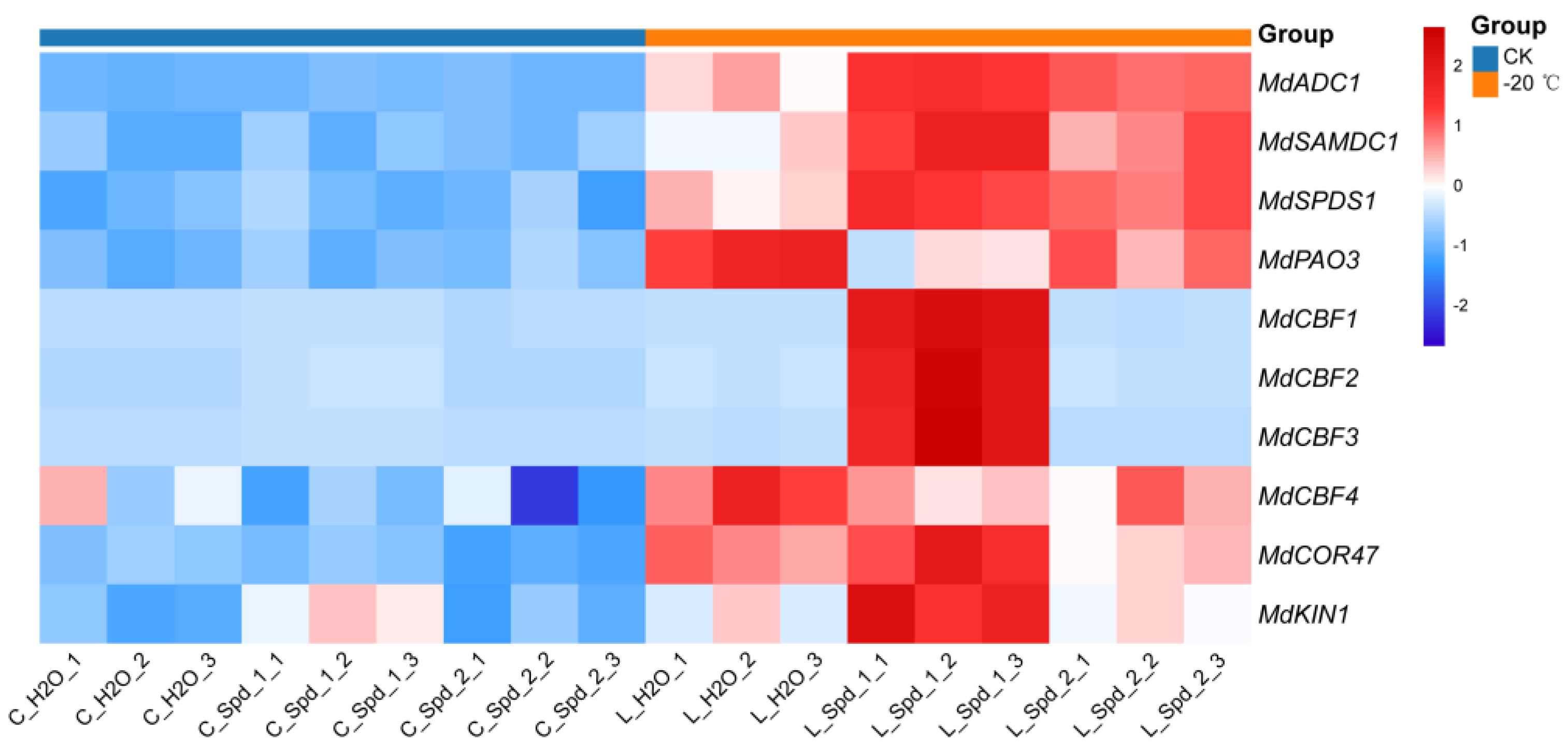
2.5. Effects of Exogenous Spd on PAs Metabolism and Cold Resistance Related Gene Expression in Apple under Low-Temperature Stress
2.6. Correlation Analysis and Principal Component Analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Determination of Physiological Indexes Related to Stress Resistance
4.3. Determination of Put, Spd, and Spm Content
4.4. Determination of Key Enzymes in Polyamine Metabolism by ELISA
4.5. RNA Extraction and qRT-PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Campoy, J.A.; Ruiz, D.; Egea, J. Dormancy in temperate fruit trees in a global warming context: A review. Sci. Hortic. 2011, 130, 357–372. [Google Scholar] [CrossRef]
- Salama, A.M.; Ezzat, A.; El-Ramady, H.; Alam-Eldein, S.M.; Okba, S.K.; Elmenofy, H.M.; Hassan, I.F.; Holb, I.J. Temperate fruit trees under climate change: Challenges for dormancy and chilling requirements in warm winter regions. Horticulturae 2021, 7, 86. [Google Scholar] [CrossRef]
- Xu, G.; He, M.; Zhao, D.; Lyu, D.; Qin, S. Physiological and structural changes in apple tree branches of different varieties during dormancy. Horticulturae 2023, 9, 947. [Google Scholar] [CrossRef]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Wu, H.; Gong, X.; Moriguchi, T. Polyamines function in stress tolerance: From synthesis to regulation. Front. Plant Sci. 2015, 6, 827. [Google Scholar] [CrossRef] [PubMed]
- Napieraj, N.; Janicka, M.; Reda, M. Interactions of polyamines and phytohormones in plant response to abiotic stress. Plants 2023, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Huang, K.; Batool, M.; Idrees, F.; Afzal, R.; Haroon, M.; Noushahi, H.A.; Wu, W.; Hu, Q.; Lu, X.; et al. Versatile roles of polyamines in improving abiotic stress tolerance of plants. Front. Plant Sci. 2022, 13, 1003155. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.; Mandal, S.; Gupta, S.K.; Anand, U.; Ghorai, M.; Mundhra, A.; Rahman, M.H.; Ray, P.; Mitra, S.; Ray, D.; et al. Role of polyamines in molecular regulation and cross-talks against drought tolerance in plants. Plant Growth Regul. 2023, 42, 4901–4917. [Google Scholar] [CrossRef]
- Saha, J.; Brauer, E.K.; Sengupta, A.; Popescu, S.C.; Gupta, K.; Gupta, B. Polyamines as redox homeostasis regulators during salt stress in plants. Front. Environ. Sci. 2015, 3, 21. [Google Scholar] [CrossRef]
- Alcázar, R.; Cuevas, J.C.; Planas, J.; Zarza, X.; Bortolotti, C.; Carrasco, P.; Salinas, J.; Tiburcio, A.F.; Altabella, T. Integration of polyamines in the cold acclimation response. Plant Sci. 2011, 180, 31–38. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Zheng, Y.; Zheng, Y.; Li, S.; Shao, J.; Luo, J.; Hu, J.; Xu, S. Polyamines are involved in chilling tolerance in tobacco (Nicotiana tabacum) seedlings. Plant Growth Regul. 2019, 89, 153–166. [Google Scholar] [CrossRef]
- Amini, S.; Maali-Amiri, R.; Kazemi-Shahandashti, S.S.; López-Gómez, M.; Sadeghzadeh, B.; Sobhani-Najafabadi, A.; Kariman, K. Effect of cold stress on polyamine metabolism and antioxidant responses in chickpea. J. Plant Physiol. 2021, 258–259, 153387. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, B.; Li, W.; Song, H.; Yu, Y.; Chen, J. Polyamines enhance chilling tolerance of cucumber (Cucumis sativus L.) through modulating antioxidative system. Sci. Hortic. 2009, 122, 200–208. [Google Scholar] [CrossRef]
- Cao, D.; Huang, Y.; Mei, G.; Zhang, S.; Wu, H.; Zhao, T. Spermidine enhances chilling tolerance of kale seeds by modulating ROS and phytohormone metabolism. PLoS ONE 2023, 18, e0289563. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, P.; Li, X.; Zhou, D.; Cai, X.; Hu, X.; Hu, S. Regulation of cold resistance by the polyamine biosynthetic gene SlSPDS2 via modulating the antioxidant enzyme system and osmotic regulatory substances in Solanum lycopersicum. Environ. Exp. Bot. 2023, 216, 105531. [Google Scholar] [CrossRef]
- Xu, G.; Li, L.; Zhou, J.; Lyu, D.; Zhao, D.; Qin, S. Comparison of transcriptome and metabolome analysis revealed differences in cold resistant metabolic pathways in different apple cultivars under low temperature stress. Hortic. Plant J. 2023, 9, 183–198. [Google Scholar] [CrossRef]
- Turkyilmaz Unal, B.; Mentis, O.; Akyol, E. Effects of exogenous salicylic acid on antioxidant activity and proline accumulation in apple (Malus domestica L.). Hortic. Environ. Biotechnol. 2015, 56, 606–611. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Liu, W.; Wang, N.; Qu, C.; Jiang, S.; Fang, H.; Zhang, Z.; Chen, X. Methyl jasmonate enhances apple’ cold tolerance through the JAZ–MYC2 pathway. Plant Cell Tiss. Org. 2019, 136, 75–84. [Google Scholar] [CrossRef]
- Lu, X.; Dai, P.; Ma, H.; Lyu, D. Regulatory effect of exogenous γ-aminobutyric acid on respiratory rate through the γ-aminobutyric acid shunt in Malus baccata (L.) Borkh. roots under suboptimal low root-zone temperature. Horticulturae 2023, 9, 268. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Raziq, A.; Mohi Ud Din, A.; Anwar, S.; Wang, Y.; Jahan, M.S.; He, M.; Ling, C.G.; Sun, J.; Shu, S.; Guo, S. Exogenous spermidine modulates polyamine metabolism and improves stress responsive mechanisms to protect tomato seedlings against salt stress. Plant Physiol. Biochem. 2022, 187, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Pan, X.; Jiang, Q.; Xi, Z. Exogenous putrescine alleviates drought stress by altering reactive oxygen species scavenging and biosynthesis of polyamines in the seedlings of Cabernet sauvignon. Front. Plant Sci. 2021, 12, 767992. [Google Scholar] [CrossRef] [PubMed]
- Jalili, I.; Ebadi, A.; Askari, M.A.; KalatehJari, S.; Aazami, M.A. Foliar application of putrescine, salicylic acid, and ascorbic acid mitigates frost stress damage in Vitis vinifera cv. ‘Giziluzum’. BMC Plant Biol. 2023, 23, 135. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.; Zhu, J.; Boldizsár, Á.; Vágújfalvi, A.; Burke, A.; Garland-Campbell, K.; Galiba, G.; Dubcovsky, J. Large deletions in the CBF gene cluster at the Fr-B2 locus are associated with reduced frost tolerance in wheat. Theor. Appl. Genet. 2013, 126, 2683–2697. [Google Scholar] [CrossRef]
- Guo, X.; Liu, D.; Chong, K. Cold signaling in plants: Insights into mechanisms and regulation. J. Integr. Plant Biol. 2018, 60, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Wang, G.; Xu, H.; Liu, J.; Luo, J.; Shen, Y. Exogenous spermidine improves chilling tolerance in sweet corn seedlings by regulation on abscisic acid, ROS and Ca2+ pathways. J. Plant Biol. 2021, 64, 487–499. [Google Scholar] [CrossRef]
- Chang, N.; Zhou, Z.; Li, Y.; Zhang, X. Exogenously applied Spd and Spm enhance drought tolerance in tea plants by increasing fatty acid desaturation and plasma membrane H+-ATPase activity. Plant Physiol. Biochem. 2022, 170, 225–233. [Google Scholar]
- Devireddy, A.R.; Tschaplinski, T.J.; Tuskan, G.A.; Muchero, W.; Chen, J.G. Role of reactive oxygen species and hormones in plant responses to temperature changes. Int. J. Mol. Sci. 2021, 22, 8843. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Fujita, M. Exogenous spermidine alleviates low temperature injury in mung bean (Vigna radiata L.) seedlings by modulating ascorbate-glutathione and glyoxalase pathway. Int. J. Mol. Sci. 2015, 16, 30117–30132. [Google Scholar] [CrossRef]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.M.; Qian, P.; Xin, W.; Li, H.Y.; Burritt, D.J.; Fujita, M.; Tran, L.S. Hydrogen peroxide priming modulates abiotic oxidative stress tolerance: Insights from ROS detoxification and scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small amines with large effects on plant abiotic stress tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Zeng, Y.H.; Zhang, Y.P.; Xiang, J.; Hui, W.U.; Chen, H.Z.; Zhang, Y.K.; Zhu, D.F. Effects of chilling tolerance induced by spermidine pretreatment on antioxidative activity, endogenous hormones and ultrastructure of indica-japonica hybrid rice seedlings. J. Integr. Agric. 2016, 15, 295–308. [Google Scholar] [CrossRef]
- Chen, J.; Fang, J.; Guo, Z.; Lu, S. Polyamines and antioxidant defense system are associated with cold tolerance in centipedegrass. Front. Agric. Sci. Eng. 2018, 5, 129–138. [Google Scholar] [CrossRef]
- Diao, Q.; Song, Y.; Qi, H. Exogenous spermidine enhances chilling tolerance of tomato (Solanum lycopersicum L.) seedlings via involvement in polyamines metabolism and physiological parameter levels. Acta Physiol. Plant. 2015, 37, 230. [Google Scholar] [CrossRef]
- Manasa, S.L.; Panigrahy, M.; Panigrahi, K.C.; Rout, G.R. Overview of cold stress regulation in plants. Bot. Rev. 2022, 88, 359–387. [Google Scholar] [CrossRef]
- Niu, R.; Zhao, X.; Wang, C.; Wang, F. Physiochemical responses and ecological adaptations of peach to low-temperature stress: Assessing the cold resistance of local peach varieties from Gansu, China. Plants 2023, 12, 4183. [Google Scholar] [CrossRef] [PubMed]
- Jankovska-Bortkevič, E.; Gavelienė, V.; Šveikauskas, V.; Mockevičiūtė, R.; Jankauskienė, J.; Todorova, D.; Sergiev, I.; Jurkonienė, S. Foliar application of polyamines modulates winter oilseed rape responses to increasing cold. Plants 2020, 9, 179. [Google Scholar] [CrossRef]
- Gupta, K.; Dey, A.; Gupta, B. Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 2013, 35, 2015–2036. [Google Scholar] [CrossRef]
- Gondor, O.K.; Szalai, G.; Kovács, V.; Janda, T.; Pál, M. Relationship between polyamines and other cold-induced response mechanisms in different cereal species. J. Agron. Crop Sci. 2016, 202, 217–230. [Google Scholar] [CrossRef]
- Sheteiwy, M.; Shen, H.; Xu, J.; Guan, Y.; Song, W.; Hu, J. Seed polyamines metabolism induced by seed priming with spermidine and 5-aminolevulinic acid for chilling tolerance improvement in rice (Oryza sativa L.) seedlings. Environ. Exp. Bot. 2017, 137, 58–72. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wen, W.; Shi, Z.; Gu, Q.; Ahammed, G.; Cao, K.; Jahan, M.; Shu, S.; Wang, J.; et al. Hydrogen peroxide mediates spermidine-induced autophagy to alleviate salt stress in cucumber. Autophagy 2021, 17, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Janse van Rensburg, H.C.; Limami, A.M.; Van den Ende, W. Spermine and spermidine priming against Botrytis cinerea modulates ROS dynamics and metabolism in Arabidopsis. Biomolecules 2021, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Parvin, S.; Lee, O.R.; Sathiyaraj, G.; Khorolragchaa, A.; Kim, Y.J.; Yang, D.C. Spermidine alleviates the growth of saline-stressed ginseng seedlings through antioxidative defense system. Gene 2014, 537, 70–78. [Google Scholar] [CrossRef]
- Szalai, G.; Janda, K.; Darko, E.; Janda, T.; Peeva, V.; Pal, M. Comparative analysis of polyamine metabolism in wheat and maize plants. Plant Physiol. Biochem. 2017, 112, 239–250. [Google Scholar] [CrossRef]
- Zhang, X.; Xia, Y.; Li, S.; Cao, L.; Zhou, Z.; Chang, N.; Li, Y. The arginine decarboxylase gene CsADC1, associated with the polyamine pathway, plays an important role in tea cold tolerance. Environ. Exp. Bot. 2023, 214, 105473. [Google Scholar] [CrossRef]
- Kou, S.; Chen, L.; Tu, W.; Scossa, F.; Wang, Y.; Liu, J.; Fernie, A.R.; Song, B.; Xie, C. The arginine decarboxylase gene ADC1, associated to the putrescine pathway, plays an important role in potato cold-acclimated freezing tolerance as revealed by transcriptome and metabolome analyses. Plant J. 2018, 96, 1283–1298. [Google Scholar] [CrossRef]
- Jiao, P.; Jin, S.; Chen, N.; Wang, C.; Liu, S.; Qu, J.; Guan, S.; Ma, Y. Improvement of cold tolerance in maize (Zea mays L.) using agrobacterium-mediated transformation of ZmSAMDC gene. GM Crops Food 2022, 13, 131–141. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, M.; Yu, G.; Wang, D.; Xu, Z.; Liang, L.; Xiao, J.; Xie, Y.; Tang, Y.; Sun, G.; et al. CaSPDS, a spermidine synthase gene from pepper (Capsicum annuum L.), plays an important role in response to cold stress. Int. J. Mol. Sci. 2023, 24, 5013. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, L.; Xiao, J.; Xie, Y.; Zhu, L.; Xue, X.; Xu, L.; Zhou, P.; Ran, J.; Huang, Z.; et al. Genome-Wide identification of polyamine oxidase (PAO) family genes: Roles of CaPAO2 and CaPAO4 in the cold tolerance of pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2022, 23, 9999. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, H.; Ma, C.; Zhang, Y.; Polle, A.; Rennenberg, H.; Cheng, X.; Luo, Z.B. Overexpression of bacterial γ-glutamylcysteine synthetase mediates changes in cadmium influx, allocation and detoxification in poplar. New Phytol. 2015, 205, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wan, H.; He, J.; Lyu, D.; Li, H. Integration of cadmium accumulation, subcellular distribution, and physiological responses to understand cadmium tolerance in apple rootstocks. Front. Plant Sci. 2017, 8, 966. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, B.; Zhao, X.; Wang, P.; Lyu, D.; Qin, S. Auxin participates in the regulation of the antioxidant system in Malus baccata Borkh. roots under sub-low temperature by exogenous sucrose application. Horticulturae 2023, 9, 297. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Luo, J.; Ma, C.; Li, S.; Qu, L.; Gai, Y.; Jiang, X.; Janz, D.; Polle, A.; et al. A transcriptomic network underlies microstructural and physiological responses to cadmium in Populus × canescens. Plant Physiol. 2013, 162, 424–439. [Google Scholar] [CrossRef]
- Duan, J.; Li, J.; Guo, S.; Kang, Y. Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J. Plant Physiol. 2008, 165, 1620–1635. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, M.; Zhou, J.; Lyu, D.; Xu, G.; Qin, S. Exogenous Spermidine Alleviated Low-Temperature Damage by Affecting Polyamine Metabolism and Antioxidant Levels in Apples. Plants 2024, 13, 1100. https://doi.org/10.3390/plants13081100
He M, Zhou J, Lyu D, Xu G, Qin S. Exogenous Spermidine Alleviated Low-Temperature Damage by Affecting Polyamine Metabolism and Antioxidant Levels in Apples. Plants. 2024; 13(8):1100. https://doi.org/10.3390/plants13081100
Chicago/Turabian StyleHe, Meiqi, Jia Zhou, Deguo Lyu, Gongxun Xu, and Sijun Qin. 2024. "Exogenous Spermidine Alleviated Low-Temperature Damage by Affecting Polyamine Metabolism and Antioxidant Levels in Apples" Plants 13, no. 8: 1100. https://doi.org/10.3390/plants13081100
APA StyleHe, M., Zhou, J., Lyu, D., Xu, G., & Qin, S. (2024). Exogenous Spermidine Alleviated Low-Temperature Damage by Affecting Polyamine Metabolism and Antioxidant Levels in Apples. Plants, 13(8), 1100. https://doi.org/10.3390/plants13081100




