Antibiotic Resistance in Plant Pathogenic Bacteria: Recent Data and Environmental Impact of Unchecked Use and the Potential of Biocontrol Agents as an Eco-Friendly Alternative
Abstract
:1. Introduction
2. Plant Bacterial Pathogens and Their Control by Antibiotics
2.1. Plant Pathogens and Diseases
2.1.1. Pseudomonas spp.
2.1.2. Ralstonia spp.
2.1.3. Agrobacterium spp.
2.1.4. Xanthomonas spp.
2.1.5. Pectobacterium spp.
2.2. Antibiotics in Plant Pathogen Control
2.3. Antibiotic Resistance Profile of Plant Pathogens
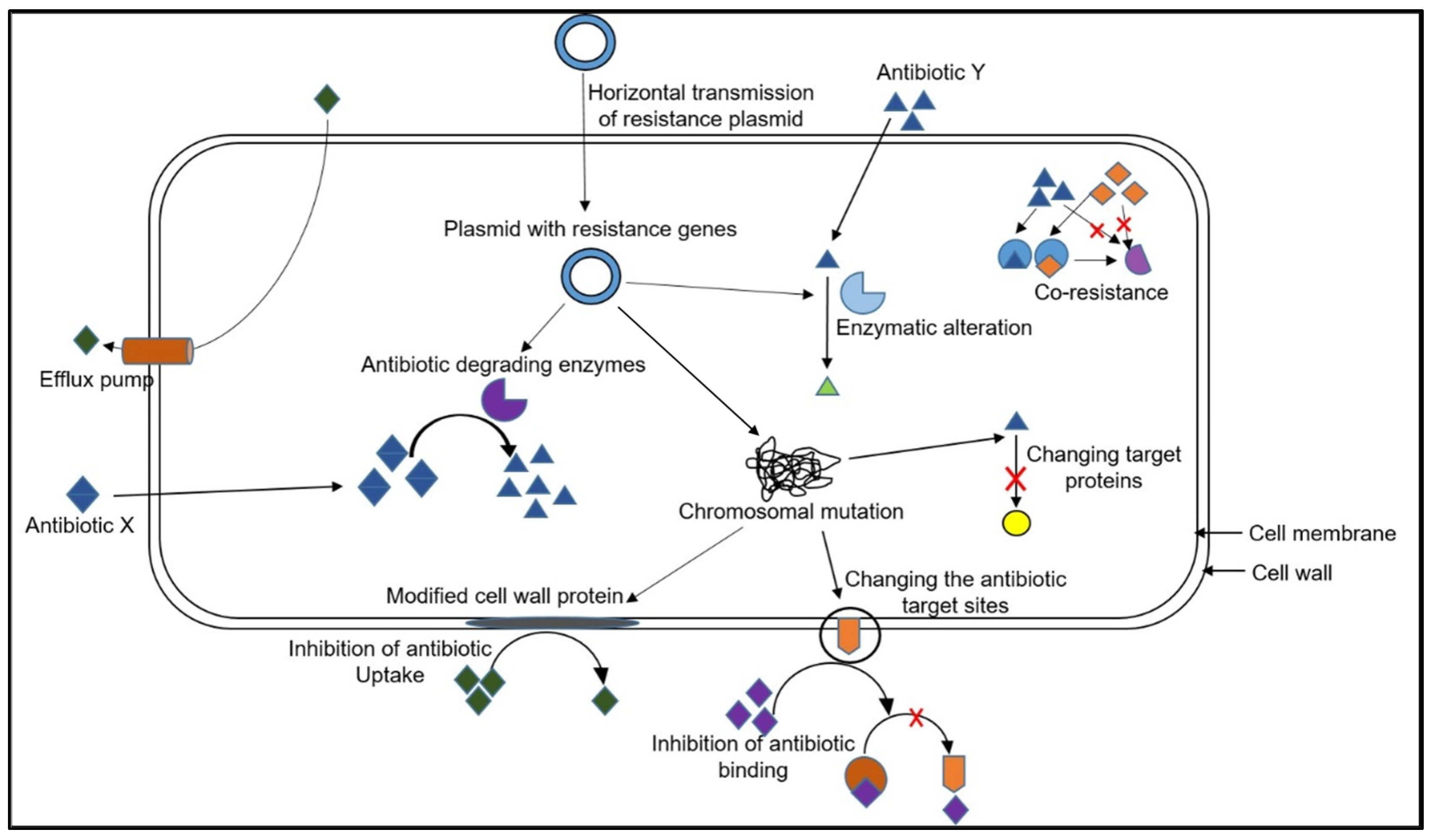
3. Mechanisms of Antibiotic Resistance
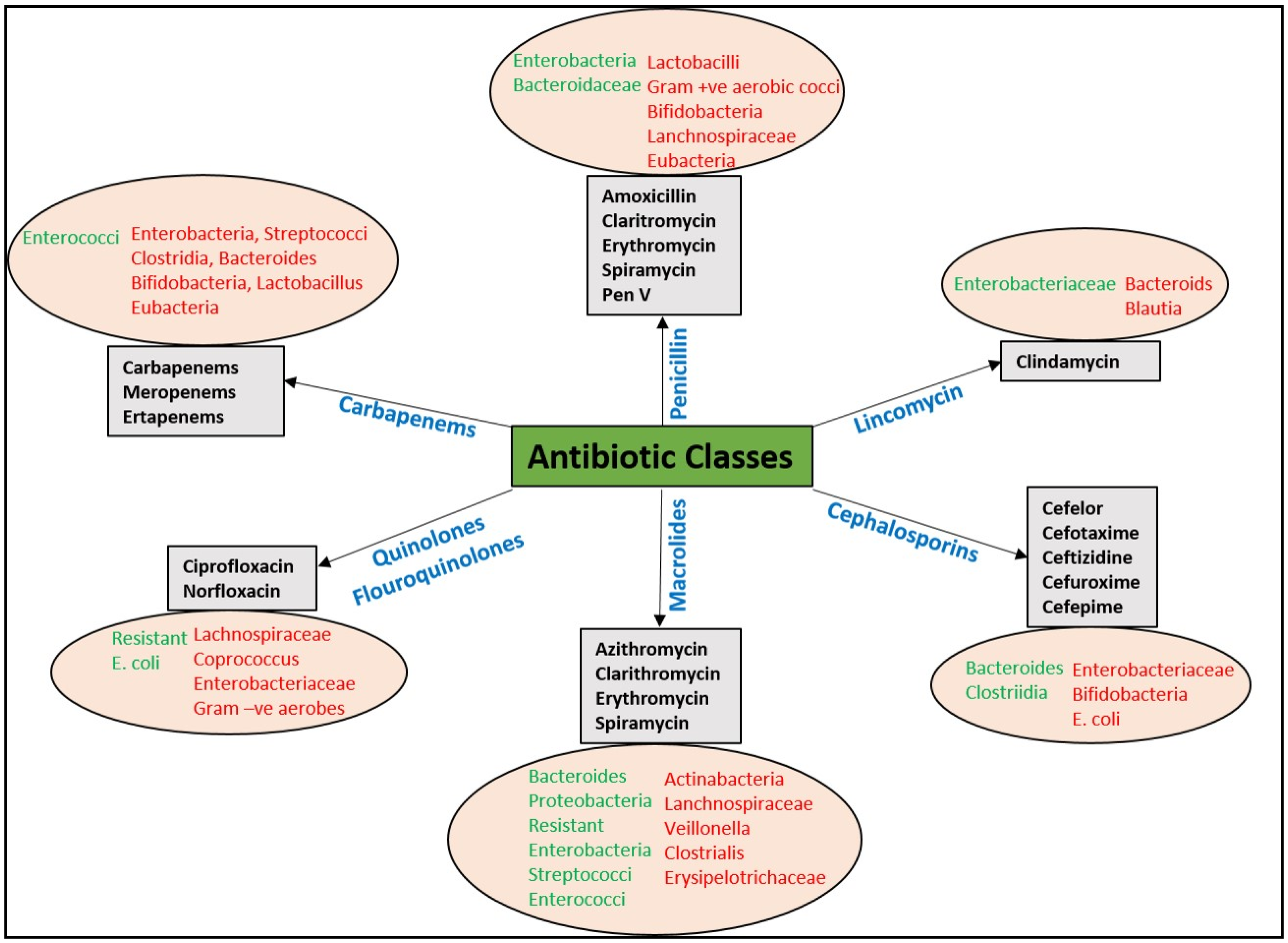
3.1. Mechanism of Spread of Antibiotic Resistance to Food-Borne Pathogens
3.2. Impacts of Antibiotic Resistance
3.2.1. Impact on Public Health
3.2.2. Impact on Environment
4. Biocontrol Agents to Control Plant Pathogens Rather Than Antibiotics
4.1. Use of Endophytes
4.1.1. Use of Bacterial Endophytes
4.1.2. Use of Fungal Endophytes
4.2. Use of Viral Vectors to Encounter Pathogens
4.3. Use of Genetically Modified Organisms
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Verhaegen, M.; Bergot, T.; Liebana, E.; Stancanelli, G.; Streissl, F.; Mingeot-Leclercq, M.P.; Mahillon, J.; Bragard, C. On the Use of Antibiotics to Control Plant Pathogenic Bacteria: A Genetic and Genomic Perspective. Front. Microbiol. 2023, 14, 1221478. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yu, K.; He, Y. The Dynamics and Transmission of Antibiotic Resistance Associated with Plant Microbiomes. Environ. Int. 2023, 176, 107986. [Google Scholar] [CrossRef] [PubMed]
- Sundin, G.W.; Wang, N. Antibiotic Resistance in Plant-Pathogenic Bacteria. Annu. Rev. Phytopathol. 2018, 56, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance. Microbiol. Spectr. 2016, 4, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, S.E. The Relationship between Antimicrobial Resistance and Patient Outcomes: Mortality, Length of Hospital Stay, and Health Care Costs. Clin. Infect. Dis. 2006, 42, S82–S89. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Future Health and Wealth of Nations; Wellcome Collection: London, UK, 2014. [Google Scholar]
- Martins, P.M.M.; Merfa, M.V.; Takita, M.A.; De Souza, A.A. Persistence in Phytopathogenic Bacteria: Do We Know Enough? Front. Microbiol. 2018, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- He, D.C.; He, M.H.; Amalin, D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological Control of Plant Diseases: An Evolutionary and Eco-Economic Consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Porto de Souza Vandenberghe, L.; Marcela Blandon Garcia, L.; Rodrigues, C.; Cândido Camara, M.; Vinícius de Melo Pereira, G.; de Oliveira, J.; Ricardo Soccol, C. Potential Applications of Plant Probiotic Microorganisms in Agriculture and Forestry. AIMS Microbiol. 2017, 3, 629–648. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Abril-Urias, P.; Escobar, C. Biological Control of Plant-Parasitic Nematodes by Filamentous Fungi Inducers of Resistance: Trichoderma, Mycorrhizal and Endophytic Fungi. Front. Microbiol. 2020, 11, 992. [Google Scholar] [CrossRef]
- Rahman, M.; Wadud, M.; Islam, T.; Hussain, M.; Bristy, E.; Tuhin, A. Evaluation of Antibacterial Activity of Piper Betel Leaves and Nigella Sativa Seeds against Multidrug Resistant Food and Water Borne Pathogenic Bacteria: An in Vitro Study Model. Microbiol. Res. J. Int. 2018, 22, 1–11. [Google Scholar] [CrossRef]
- Shalahuddin Millat, M.; Islam, S.; Hussain, M.S.; Rahman Moghal, M.M.; Islam, T. Anti-Bacterial Profiling of Launaea sarmentosa (Willd.) and Bruguiera cylindrical (L.): Two Distinct Ethno Medicinal Plants of Bangladesh. Eur. J. Exp. Biol. 2017, 7, 1–5. [Google Scholar] [CrossRef]
- Rabbee, M.F.; Sarafat Ali, M.; Choi, J.; Hwang, B.S.; Jeong, S.C.; Baek, K. Hyun Bacillus velezensis: A Valuable Member of Bioactive Molecules within Plant Microbiomes. Molecules 2019, 24, 1046. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Ozymko, Z.; De Jager, V.; Siwinska, J.; Smolarska, A.; Ossowicki, A.; Narajczyk, M.; Lojkowska, E. Genomic, Proteomic and Morphological Characterization of Two Novel Broad Host Lytic Bacteriophages ΦPD10.3 and ΦPD23.1 Infecting Pectinolytic Pectobacterium spp. and Dickeya spp. PLoS ONE 2015, 10, e0119812. [Google Scholar] [CrossRef] [PubMed]
- Umrao, P.D.; Kumar, V.; Kaistha, S.D. Biocontrol Potential of Bacteriophage ɸsp1 against Bacterial Wilt-Causing Ralstonia solanacearum in Solanaceae Crops. Egypt. J. Biol. Pest Control. 2021, 31, 1–12. [Google Scholar] [CrossRef]
- Chutulo, E.C.; Chalannavar, R.K. Endophytic Mycoflora and Their Bioactive Compounds from Azadirachta Indica: A Comprehensive Review. J. Fungi 2018, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.S. Bioactive Metabolites from Tomato Endophytic Fungi with Antibacterial Activity against Tomato Bacterial Spot Disease. Rhizosphere 2021, 17, 100292. [Google Scholar] [CrossRef]
- Islam, T.; Rabbee, M.F.; Choi, J.; Baek, K.H. Biosynthesis, Molecular Regulation, and Application of Bacilysin Produced by Bacillus Species. Metabolites 2022, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- van Esse, H.P.; Reuber, T.L.; van der Does, D. Genetic Modification to Improve Disease Resistance in Crops. N. Phytol. 2020, 225, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef]
- Mulet, M.; Gomila, M.; Busquets, A.; Sánchez, D.; Lalucat, J.; García-Valdés, E. Genome-Based Taxonomy of Species in the Pseudomonas syringae and Pseudomonas Lutea Phylogenetic Groups and Proposal of Pseudomonas maioricensis sp. nov., Isolated from Agricultural Soil. Microorganisms 2024, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Lipps, S.M.; Samac, D.A. Pseudomonas Viridiflava: An Internal Outsider of the Pseudomonas syringae Species Complex. Mol. Plant Pathol. 2022, 23, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.L.; Preston, G.M. Pseudomonas syringae: Enterprising Epiphyte and Stealthy Parasite. Microbiology 2018, 165, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Preston, G.M. Profiling the Extended Phenotype of Plant Pathogens. Mol. Plant Pathol. 2017, 18, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Bradley, C.A.; Allen, T.W.; Sisson, A.J.; Bergstrom, G.C.; Bissonnette, K.M.; Bond, J.; Byamukama, E.; Chilvers, M.I.; Collins, A.A.; Damicone, J.P.; et al. Soybean Yield Loss Estimates Due to Diseases in the United States and Ontario, Canada, from 2015 to 2019. Plant Health Prog. 2021, 22, 483–495. [Google Scholar] [CrossRef]
- Baltrus, D.A.; McCann, H.C.; Guttman, D.S. Evolution, Genomics and Epidemiology of Pseudomonas syringae. Mol. Plant Pathol. 2017, 18, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.F.; Guttman, D.S. Evolution of the Core Genome of Pseudomonas syringae, a Highly Clonal, Endemic Plant Pathogen. Appl. Environ. Microbiol. 2004, 70, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Tsiamis, G.; Mansfield, J.W.; Hockenhull, R.; Jackson, R.W.; Sesma, A.; Athanassopoulos, E.; Bennett, M.A.; Stevens, C.; Vivian, A.; Taylor, J.D.; et al. Cultivar-Specific Avirulence and Virulence Functions Assigned to AvrPphF in Pseudomonas syringae pv. phaseolicola, the Cause of Bean Halo-Blight Disease. EMBO J. 2000, 19, 3204–3214. [Google Scholar] [CrossRef] [PubMed]
- Guttman, D.S.; Greenberg, J.T. Functional Analysis of the Type III Effectors AvrRpt2 and AvrRpm1 of Pseudomonas syringae with the Use of a Single-Copy Genomic Integration System. Mol. Plant-Microbe Interact. 2007, 14, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Abramovitch, R.B.; Kim, Y.J.; Chen, S.; Dickman, M.B.; Martin, G.B. Pseudomonas Type III Effector AvrPtoB Induces Plant Disease Susceptibility by Inhibition of Host Programmed Cell Death. EMBO J. 2003, 22, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.S.H.; Morgan, R.L.; Sarkar, S.F.; Wang, P.W.; Guttman, D.S. Phylogenetic Characterization of Virulence and Resistance Phenotypes of Pseudomonas syringae. Appl. Environ. Microbiol. 2005, 71, 5182. [Google Scholar] [CrossRef] [PubMed]
- Hulin, M.T.; Vadillo Dieguez, A.; Cossu, F.; Lynn, S.; Russell, K.; Neale, H.C.; Jackson, R.W.; Arnold, D.L.; Mansfield, J.W.; Harrison, R.J. Identifying Resistance in Wild and Ornamental Cherry towards Bacterial Canker Caused by Pseudomonas syringae. Plant Pathol. 2022, 71, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, B.; López, M.M.; Biosca, E.G. Biocontrol of the Major Plant Pathogen Ralstonia solanacearum in Irrigation Water and Host Plants by Novel Waterborne Lytic Bacteriophages. Front. Microbiol. 2019, 10, 2813. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wei, Z.; Xu, J.; Chen, H.; Zhang, Y.; She, X.; Macho, A.P.; Ding, W.; Liao, B. Bacterial Wilt in China: History, Current Status, and Future Perspectives. Front. Plant Sci. 2017, 8, 1549. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elyousr, K.A.M.; Hassan, S.A. Biological Control of Ralstonia solanacearum (Smith), the Causal Pathogen of Bacterial Wilt Disease by Using Pantoea spp. Egypt. J. Biol. Pest Control 2021, 31, 1–8. [Google Scholar] [CrossRef]
- Devappa, V.; Archith, T.; Priya, N. Interaction of Ralstonia solanacearum and Meloidogyne Incognita on Tomato (Solanumlyco persicon L.). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 156–160. [Google Scholar] [CrossRef]
- Karim, Z.; Hossain, M.S.; Begum, M.M. Ralstonia solanacearum: A Threat to Potato Production in Bangladesh. Fundam. Appl. Agric. 2018, 3, 407. [Google Scholar] [CrossRef]
- Singh, D.; Chaudhary, G.; Yadav, D.K. Characterization and Diversity of Indian Isolates of Ralstonia solanacearum Inciting Bacterial Wilt of Tomato. Indian Phytopathol. 2021, 74, 425–429. [Google Scholar] [CrossRef]
- Coutinho, T.A.; Wingfield, M.J. Ralstonia solanacearum and R. Pseudosolanacearum on Eucalyptus: Opportunists or Primary Pathogens? Front. Plant Sci. 2017, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Prior, P.; Ailloud, F.; Dalsing, B.L.; Remenant, B.; Sanchez, B.; Allen, C. Genomic and Proteomic Evidence Supporting the Division of the Plant Pathogen Ralstonia solanacearum into Three Species. BMC Genom. 2016, 17, 90. [Google Scholar] [CrossRef]
- Safni, I.; Cleenwerck, I.; De Vos, P.; Fegan, M.; Sly, L.; Kappler, U. Polyphasic Taxonomic Revision of the Ralstonia solanacearum Species Complex: Proposal to Emend the Descriptions of Ralstonia solanacearum and Ralstonia syzygii and Reclassify Current R. Syzygii Strains as Ralstonia syzygii subsp. Syzygii subsp. nov., R.S. Int. J. Syst. Evol. Microbiol. 2014, 64, 3087–3103. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, B.; Vasse, J.; Le-Courtois, V.; Trigalet-Démery, D.; López, M.M.; Trigalet, A. Comparative Behavior of Ralstonia solanacearum Biovar 2 in Diverse Plant Species. Phytopathology 2007, 98, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Genin, S.; Denny, T.P. Pathogenomics of the Ralstonia solanacearum Species Complex. Annu. Rev. Phytopathol. 2011, 50, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Peeters, N.; Carrère, S.; Anisimova, M.; Plener, L.; Cazalé, A.C.; Genin, S. Repertoire, Unified Nomenclature and Evolution of the Type III Effector Gene Set in the Ralstonia solanacearum Species Complex. BMC Genom. 2013, 14, 859. [Google Scholar] [CrossRef] [PubMed]
- Deslandes, L.; Genin, S. Opening the Ralstonia solanacearum Type III Effector Tool Box: Insights into Host Cell Subversion Mechanisms. Curr. Opin. Plant Biol. 2014, 20, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of Two Plant-Growth Promoting Bacillus velezensis Isolates Against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef] [PubMed]
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical Pesticides and Human Health: The Urgent Need for a New Concept in Agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Yuliar; Asi Nion, Y.; Toyota, K. Recent Trends in Control Methods for Bacterial Wilt Diseases Caused by Ralstonia solanacearum. Microbes Environ. 2015, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.F.; Townsend, C.O. A Plant-Tumor of Bacterial Origin. Science 1907, 25, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Nester, E.W. Agrobacterium: Nature’s Genetic Engineer. Front. Plant Sci. 2015, 5, 730. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.; Hommais, F.; Nasser, W.; Reverchon, S. Plant–Phytopathogen Interactions: Bacterial Responses to Environmental and Plant Stimuli. Environ. Microbiol. 2017, 19, 1689–1716. [Google Scholar] [CrossRef] [PubMed]
- Costechareyre, D.; Rhouma, A.; Lavire, C.; Portier, P.; Chapulliot, D.; Bertolla, F.; Boubaker, A.; Dessaux, Y.; Nesme, X. Rapid and Efficient Identification of Agrobacterium Species by RecA Allele Analysis. Microb. Ecol. 2010, 60, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.; Bae, J.; Kang, B.; Lee, Y.; Kim, S.; Fuqua, C.; Kim, J. Simple and Economical Biosensors for Distinguishing Agrobacterium-Mediated Plant Galls from Nematode-Mediated Root Knots. Sci. Rep. 2019, 9, 17961. [Google Scholar] [CrossRef]
- Successful Detailed Tracking of Major Plant Disease’s Global Spread: USDA ARS. Available online: https://www.ars.usda.gov/news-events/news/research-news/2020/successful-detailed-tracking-of-major-plant-diseases-global-spread/ (accessed on 6 April 2024).
- Barton, I.S.; Fuqua, C.; Platt, T.G. Ecological and Evolutionary Dynamics of a Model Facultative Pathogen: Agrobacterium and Crown Gall Disease of Plants. Environ. Microbiol. 2018, 20, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Dessaux, Y.; Petit, A.; Farrand, S.K.; Murphy, P.J. Opines and Opine-Like Molecules Involved in Plant-Interactions. In The Rhizobiaceae: Molecular Biology of Model Plant-Associated Bacteria; Springer: Dordrecht, The Netherlands, 1998; pp. 173–197. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Eberhard, A.; Winans, S.C. Agrobacterium Tumefaciens Can Obtain Sulphur from an Opine That Is Synthesized by Octopine Synthase Using S-Methylmethionine as a Substrate. Mol. Microbiol. 2012, 84, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Pitzschke, A. Agrobacterium Infection and Plant Defense-Transformation Success Hangs by a Thread. Front. Plant Sci. 2013, 4, 519. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.Y.; Chou, S.J.; Müller, C.; Halkier, B.A.; Deeken, R.; Lai, E.M. Differential Roles of Glucosinolates and Camalexin at Different Stages of Agrobacterium-Mediated Transformation. Mol. Plant Pathol. 2018, 19, 1956–1970. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Thiour-Mauprivez, C.; Wisniewski-Dyé, F.; Kerzaon, I.; Comte, G.; Vial, L.; Lavire, C. Ecological Conditions and Molecular Determinants Involved in Agrobacterium Lifestyle in Tumors. Front. Plant Sci. 2019, 10, 978. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.P.; Cornforth, D.M.; Mideo, N. Evolution of Virulence in Opportunistic Pathogens: Generalism, Plasticity, and Control. Trends Microbiol. 2012, 20, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Mikonranta, L.; Friman, V.P.; Laakso, J. Life History Trade-Offs and Relaxed Selection Can Decrease Bacterial Virulence in Environmental Reservoirs. PLoS ONE 2012, 7, e43801. [Google Scholar] [CrossRef] [PubMed]
- Timilsina, S.; Potnis, N.; Newberry, E.A.; Liyanapathiranage, P.; Iruegas-Bocardo, F.; White, F.F.; Goss, E.M.; Jones, J.B. Xanthomonas Diversity, Virulence and Plant–Pathogen Interactions. Nat. Rev. Microbiol. 2020, 18, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.P.; Vorhölter, F.-J.; Potnis, N.; Jones, J.B.; Van Sluys, M.-A.; Bogdanove, A.J.; Dow, J.M. Pathogenomics of Xanthomonas: Understanding Bacterium–Plant Interactions. Nat. Rev. Microbiol. 2011, 9, 344–355. [Google Scholar] [CrossRef] [PubMed]
- An, S.-Q.; Potnis, N.; Dow, M.; Vorhölter, F.-J.; He, Y.-Q.; Becker, A.; Teper, D.; Li, Y.; Wang, N.; Bleris, L.; et al. Mechanistic Insights into Host Adaptation, Virulence and Epidemiology of the Phytopathogen Xanthomonas. FEMS Microbiol. Rev. 2020, 44, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.-A.; Arlat, M.; Boulanger, A.; Boureau, T.; Carrère, S.; Cesbron, S.; Chen, N.W.; Cociancich, S.; Darrasse, A.; Denancé, N.; et al. Using Ecology, Physiology, and Genomics to Understand Host Specificity in Xanthomonas. Annu. Rev. Phytopathol. 2016, 54, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Fargier, E.; Saux, M.F.-L.; Manceau, C. A Multilocus Sequence Analysis of Xanthomonas campestris Reveals a Complex Structure within Crucifer-Attacking Pathovars of This Species. Syst. Appl. Microbiol. 2011, 34, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.P.; Oka, G.U.; Alvarez-Martinez, C.E.; Bisson-Filho, A.W.; Dunger, G.; Hobeika, L.; Cavalcante, N.S.; Alegria, M.C.; Barbosa, L.R.; Salinas, R.K.; et al. Bacterial Killing via a Type IV Secretion System. Nat. Commun. 2015, 6, 6453. [Google Scholar] [CrossRef] [PubMed]
- Bayer-Santos, E.; Lima, L.d.P.; Ceseti, L.d.M.; Ratagami, C.Y.; de Santana, E.S.; da Silva, A.M.; Farah, C.S.; Alvarez-Martinez, C.E. Xanthomonas citri T6SS Mediates Resistance to Dictyostelium Predation and Is Regulated by an ECF σ Factor and Cognate Ser/Thr Kinase. Environ. Microbiol. 2018, 20, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.C.R.; Ferro, J.A.; Reinach, F.C.; Farah, C.S.; Furlan, L.R.; Quaggio, R.B.; Monteiro-Vitorello, C.B.; Van Sluys, M.A.; Almeida, N.A.; Alves, L.M.C.; et al. Comparison of the Genomes of Two Xanthomonas Pathogens with Differing Host Specificities. Nature 2002, 417, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, I.; Royer, M.; Barbe, V.; Carrere, S.; Koebnik, R.; Cociancich, S.; Couloux, A.; Darrasse, A.; Gouzy, J.; Jacques, M.-A.; et al. The Complete Genome Sequence of Xanthomonas Albilineans Provides New Insights into the Reductive Genome Evolution of the Xylem-Limited Xanthomonadaceae. BMC Genom. 2009, 10, 616. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, L.; Mwangi, M.; Abele, S.; Aritua, V.; Tushemereirwe, W.K.; Bandyopadhyay, R. Xanthomonas Wilt: A Threat to Banana Production in East and Central Africa. Plant Dis. 2009, 93, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Ainembabazi, J.H.; Tripathi, L.; Rusike, J.; Abdoulaye, T.; Manyong, V. Ex-Ante Economic Impact Assessment of Genetically Modified Banana Resistant to Xanthomonas Wilt in the Great Lakes Region of Africa. PLoS ONE 2015, 10, e0138998. [Google Scholar] [CrossRef] [PubMed]
- Behlau, F.; Fonseca, A.E.; Belasque, J. A Comprehensive Analysis of the Asiatic Citrus Canker Eradication Programme in São Paulo State, Brazil, from 1999 to 2009. Plant Pathol. 2016, 65, 1390–1399. [Google Scholar] [CrossRef]
- Christiano, R.S.C.; Dalla Pria, M.; Jesus Junior, W.C.; Amorim, L.; Bergamin Filho, A. Modelling the Progress of Asiatic Citrus Canker on Tahiti Lime in Relation to Temperature and Leaf Wetness. Eur. J. Plant Pathol. 2009, 124, 1–7. [Google Scholar] [CrossRef]
- Morales, G.; Moragrega, C.; Montesinos, E.; Llorente, I. Effects of Leaf Wetness Duration and Temperature on Infection of Prunus by Xanthomonas arboricola pv. pruni. PLoS ONE 2018, 13, e0193813. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Moragrega, C.; Montesinos, E.; Llorente, I. Environmental and Inoculum Effects on Epidemiology of Bacterial Spot Disease of Stone Fruits and Development of a Disease Forecasting System. Eur. J. Plant Pathol. 2018, 152, 635–651. [Google Scholar] [CrossRef]
- Zeng, W.; Melotto, M.; He, S.Y. Plant Stomata: A Checkpoint of Host Immunity and Pathogen Virulence. Curr. Opin. Biotechnol. 2010, 21, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, A.; Jauneau, A.; Auriac, M.-C.; Lauber, E.; Martinez, Y.; Chiarenza, S.; Leonhardt, N.; Berthomé, R.; Noël, L.D. Immunity at Cauliflower Hydathodes Controls Systemic Infection by Xanthomonas campestris pv. Campestris. Plant Physiol. 2017, 174, 700. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.H.; Cook, A.Z.; Parker, P.E.; Gottwald, T.R.; Graham, J.H. Short-Distance Dispersal of Splashed Bacteria of Xanthomonas citri subsp. citri from Canker-Infected Grapefruit Tree Canopies in Turbulent Wind. Plant Pathol. 2012, 61, 829–836. [Google Scholar] [CrossRef]
- Canteros, B.I.; Gochez, A.M.; Moschini, R.C. Management of Citrus Canker in Argentina, a Success Story. Plant Pathol. J. 2017, 33, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Nakato, V.; Mahuku, G.; Coutinho, T. Xanthomonas campestris pv. Musacearum: A Major Constraint to Banana, Plantain and Enset Production in Central and East Africa over the Past Decade. Mol. Plant Pathol. 2018, 19, 525. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Munkvold, G.P. Comparison of Seed Transmission and Survival of Xanthomonas axonopodis pv. phaseoli and Xanthomonas fuscans subsp. Fuscans in Common Bean Seeds. Plant Health Prog. 2013, 14, 41. [Google Scholar] [CrossRef]
- Dutta, B.; Schneider, R.W.; Robertson, C.L.; Walcott, R.R.; Dutta, R.W.S.B. Embryo Localization Enhances the Survival of Acidovorax Citrulli in Watermelon Seeds. Phytopathology 2016, 106, 330–338. [Google Scholar] [CrossRef]
- van der Wolf, J.M.; van der Zouwen, P.S.; van der Heijden, L. Flower Infection of Brassica Oleracea with Xanthomonas campestris pv. Campestris Results in High Levels of Seed Infection. Eur. J. Plant Pathol. 2013, 136, 103–111. [Google Scholar] [CrossRef]
- van der Wolf, J.; Kastelein, P.; da Silva Júnior, T.A.F.; Lelis, F.V.; van der Zouwen, P. Colonization of Siliques and Seeds of Rapid Cycling Brassica Oleracea Plants by Xanthomonas campestris pv. Campestris after Spray-Inoculation of Flower Clusters. Eur. J. Plant Pathol. 2019, 154, 445–461. [Google Scholar] [CrossRef]
- Comas, I.; Moya, A.; Azad, R.K.; Lawrence, J.G.; Gonzalez-Candelas, F. The Evolutionary Origin of Xanthomonadales Genomes and the Nature of the Horizontal Gene Transfer Process. Mol. Biol. Evol. 2006, 23, 2049–2057. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.C.; Paquola, A.C.M.; Varani, A.M.; Van Sluys, M.A.; Menck, C.F.M. Laterally Transferred Genomic Islands in Xanthomonadales Related to Pathogenicity and Primary Metabolism. FEMS Microbiol. Lett. 2008, 281, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Darrasse, A.; Darsonval, A.; Boureau, T.; Brisset, M.-N.; Durand, K.; Jacques, M.-A. Transmission of Plant-Pathogenic Bacteria by Nonhost Seeds without Induction of an Associated Defense Reaction at Emergence. Appl. Environ. Microbiol. 2010, 76, 6787. [Google Scholar] [CrossRef] [PubMed]
- Cazalet, C.; Gomez-Valero, L.; Rusniok, C.; Lomma, M.; Dervins-Ravault, D.; Newton, H.J.; Sansom, F.M.; Jarraud, S.; Zidane, N.; Ma, L.; et al. Analysis of the Legionella longbeachae Genome and Transcriptome Uncovers Unique Strategies to Cause Legionnaires’ Disease. PLoS Genet. 2010, 6, e1000851. [Google Scholar] [CrossRef]
- Pérombelon, M.C.M. Soft Rot Erwinia Diseases of Potato Potato Diseases Caused by Soft Rot Erwinias: An Overview of Pathogenesis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; Volume 51. [Google Scholar]
- Agyemang, P.A.; Kabir, M.N.; Kersey, C.M.; Dumenyo, C.K. The Bacterial Soft Rot Pathogens, Pectobacterium carotovorum and P. Atrosepticum, Respond to Different Classes of Virulence-Inducing Host Chemical Signals. Horticulturae 2020, 6, 13. [Google Scholar] [CrossRef]
- Brown, E.W.; Davis, R.M.; Gouk, C.; Van der Zwet, T. Phylogenetic Relationships of Necrogenic Erwinia and Brenneria Species as Revealed by Glyceraldehyde-3-Phosphate Dehydrogenase Gene Sequences. Int. J. Syst. Evol. Microbiol. 2000, 50, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Amir, R.; Sani, Q.-A.; Maqsood, W.; Munir, F.; Fatima, N.; Siddiqa, A.; Ahmad, J. Pan-Genomics of Plant Pathogens and Its Applications. In Pan-Genomics: Applications, Challenges, and Future Prospects; Elsevier: Amsterdam, The Netherlands, 2020; pp. 121–145. [Google Scholar]
- Toth, I.K.; Bell, K.S.; Holeva, M.C.; Birch, P.R.J. Soft Rot Erwiniae: From Genes to Genomes. Mol. Plant Pathol. 2003, 4, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.; Choi, J.-G.; Lee, Y.-G.; Kwon, M.; Hwang, I.; Heu, S. The Plant Pathology Journal Distribution of Pectobacterium Species Isolated in South Korea and Comparison of Temperature Effects on Pathogenicity. Plant Pathol. J 2020, 36, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Naas, H.; Sebaihia, M.; Orfei, B.; Rezzonico, F.; Buonaurio, R.; Moretti, C. Pectobacterium carotovorum subsp. brasiliense and Pectobacterium carotovorum subsp. Carotovorum as Causal Agents of Potato Soft Rot in Algeria. Eur. J. Plant Pathol. 2018, 151, 1027–1034. [Google Scholar] [CrossRef]
- Ozturk, M.; Aksoy, H.M.; Potrykus, M.; Lojkowska, E. Genotypic and Phenotypic Variability of Pectobacterium Strains Causing Blackleg and Soft Rot on Potato in Turkey. Eur. J. Plant Pathol. 2018, 152, 143–155. [Google Scholar] [CrossRef]
- Zaczek-Moczydłowska, M.A.; Fleming, C.C.; Young, G.K.; Campbell, K.; O’Hanlon, R. Pectobacterium and Dickeya Species Detected in Vegetables in Northern Ireland. Eur. J. Plant Pathol. 2019, 154, 635–647. [Google Scholar] [CrossRef]
- Dees, M.W.; Lebecka, R.; Perminow, J.I.S.; Czajkowski, R.; Grupa, A.; Motyka, A.; Zoledowska, S.; Śliwka, J.; Lojkowska, E.; Brurberg, M.B. Characterization of Dickeya and Pectobacterium Strains Obtained from Diseased Potato Plants in Different Climatic Conditions of Norway and Poland. Eur. J. Plant Pathol. 2017, 148, 839–851. [Google Scholar] [CrossRef]
- Waleron, M.; Waleron, K.; Lojkowska, E. Occurrence of Pectobacterium Wasabiae in Potato Field Samples. Eur. J. Plant Pathol. 2013, 137, 149–158. [Google Scholar] [CrossRef]
- Li, L.; Yuan, L.; Shi, Y.; Xie, X.; Chai, A.; Wang, Q.; Li, B. Comparative Genomic Analysis of Pectobacterium carotovorum subsp. brasiliense SX309 Provides Novel Insights into Its Genetic and Phenotypic Features. BMC Genom. 2019, 20, 486. [Google Scholar] [CrossRef] [PubMed]
- Portier, P.; Pédron, J.; Taghouti, G.; Dutrieux, C.; Barny, M.A. Updated Taxonomy of Pectobacterium Genus in the Cirm-Cfbp Bacterial Collection: When Newly Described Species Reveal “Old” Endemic Population. Microorganisms 2020, 8, 1441. [Google Scholar] [CrossRef] [PubMed]
- Jonkheer, E.M.; Brankovics, B.; Houwers, I.M.; van der Wolf, J.M.; Bonants, P.J.M.; Vreeburg, R.A.M.; Bollema, R.; de Haan, J.R.; Berke, L.; Smit, S.; et al. The Pectobacterium Pangenome, with a Focus on Pectobacterium brasiliense, Shows a Robust Core and Extensive Exchange of Genes from a Shared Gene Pool. BMC Genom. 2021, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Zoledowska, S.; Motyka-Pomagruk, A.; Sledz, W.; Mengoni, A.; Lojkowska, E. High Genomic Variability in the Plant Pathogenic Bacterium Pectobacterium Parmentieri Deciphered from de Novo Assembled Complete Genomes. BMC Genom. 2018, 19, 751. [Google Scholar] [CrossRef] [PubMed]
- Logan, C.; O’Neill, R.; McGRANE, P.; Little, G. Methods for Detection of the Blackleg, Ring Rot and Gangrene Pathogens in Potato Nuclear Stock Mother Tubers and Plantlets. EPPO Bull. 1987, 17, 17–23. [Google Scholar] [CrossRef]
- Dupuis, B.; Nkuriyingoma, P.; Gijsegem, F. Economic Impact of Pectobacterium and Dickeya Species on Potato Crops: A Review and Case Study. Plant Dis. Caused by Dickeya Pectobact. Species 2021, 9, 263–282. [Google Scholar] [CrossRef]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic Use in Plant Agriculture. Annu. Rev. Phytopathol. 2003, 40, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.; Reeder, R. Antibiotic Use on Crops in Low and Middle-Income Countries Based on Recommendations Made by Agricultural Advisors. CABI Agric. Biosci. 2020, 1, 1. [Google Scholar] [CrossRef]
- Verhaegen, M.; Mahillon, J.; Caulier, S.; Mingeot-Leclercq, M.-P.; Bragard, C. Data Collection on Antibiotics for Control of Plant Pathogenic Bacteria. EFSA Support. Publ. 2024, 21, 8522E. [Google Scholar] [CrossRef]
- Debnath, T.; Bhowmik, S.; Islam, T.; Chowdhury, M.M.H. Presence of Multidrug-Resistant Bacteria on Mobile Phones of Healthcare Workers Accelerates the Spread of Nosocomial Infection and Regarded as a Threat to Public Health in Bangladesh. J. Microsc. Ultrastruct. 2018, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Kubra, K.; Chowdhury, M.M.H. Prevalence of Methicillin-Resistant Staphylococcus Aureus in Hospitals in Chittagong, Bangladesh: A Threat of Nosocomial Infection. J. Microsc. Ultrastruct. 2018, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Sagor, M.S.; Hossain, M.S.; Islam, T.; Mahmud, M.A.; Miah, M.S.; Karim, M.R.; Giasuddin, M.; Samad, M.A. Phenotypic and Genotypic Antibiotic Resistance and Virulence Profiling of Enterococcus Faecalis Isolated from Poultry at Two Major Districts in Bangladesh. Pak. Vet. J. 2022, 42, 153–160. [Google Scholar] [CrossRef]
- Sultana, A.; Saha, O.; Rahman Sid, A.; Saha, A.; Hussain, M.S.; Islam, T. Molecular Detection of Multidrug Resistance Pathogenic Bacteria from Protective Materials Used by Healthcare Workers (HCW); Bangladesh Scenario. J. Appl. Sci. 2018, 18, 48–55. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic Resistance in the Environment. Nat. Rev. Microbiol. 2021, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Bai, K.; Kan, Y.; Jiang, N.; Thapa, S.P.; Coaker, G.; Li, J.; Luo, L. Variation in Streptomycin Resistance Mechanisms in Clavibacter mchiganensis. Phytopathology 2019, 109, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.W.; Kim, H.J.; Yi, P.H.; Hwang, R.Y.; Park, E.W. Mode of Action of Streptomycin Resistance in the Citrus Canker Pathogen (Xanthomonas smithii subsp. citri) in Jeju Island. Plant Pathol. J. 2012, 28, 207–211. [Google Scholar] [CrossRef]
- Gutiérrez-Barranquero, J.A.; Carrión, V.J.; Murillo, J.; Arrebola, E.; Arnold, D.L.; Cazorla, F.M.; De Vicente, A. A Pseudomonas syringae Diversity Survey Reveals a Differentiated Phylotype of the Pathovar Syringae Associated with the Mango Host and Mangotoxin Production. Phytopathology 2013, 103, 1115–1129. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, Q.; Zhou, M. Identification and Characterization of Integron-Mediated Antibiotic Resistance in the Phytopathogen Xanthomonas oryzae pv. Oryzae. PLoS ONE 2013, 8, e55962. [Google Scholar] [CrossRef] [PubMed]
- Amirul Islam, M.; Mohammad Mazumdar, R.; Islam, S.; Jahangir Alam, M.; Ahmed Urmee, S. Isolation, Identification and in-Vitro Antibiotic Sensitivity Pattern of Citrus Canker Causing Organism Xanthomonas axonopodis. Adv. Life Sci. 2014, 1, 215–222. [Google Scholar]
- Valenzuela, M.; Méndez, V.; Montenegro, I.; Besoain, X.; Seeger, M. Streptomycin Resistance in Clavibacter mchiganensis subsp. Michiganensis Strains from Chile Is Related to an RpsL Gene Mutation. Plant Pathol. 2019, 68, 426–433. [Google Scholar] [CrossRef]
- Islam, M.; Alam, J.; Urmee, S.A.; Rahaman, M.; Razu, M.H.; Mazumdar, R. Isolation, Identification, In Vitro Antibiotic Resistance and Plant Extract Sensitivity of Fire Blight Causing Erwinia Amylovora. J. Plant Pathol. Microbiol. 2014, 5, 233. [Google Scholar] [CrossRef]
- Herbert, A.; Hancock, C.N.; Cox, B.; Schnabel, G.; Moreno, D.; Carvalho, R.; Jones, J.; Paret, M.; Geng, X.; Wang, H. Oxytetracycline and Streptomycin Resistance Genes in Xanthomonas Arboricola Pv. Pruni, the Causal Agent of Bacterial Spot in Peach. Front. Microbiol. 2022, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Tancos, K.A.; Villani, S.M.; Borejsza-Wysocka, E.; Kuehne, S.; Breth, D.; Aldwinckle, H.S.; Carol, J.; Cox, K.D. Prevalence of Streptomycin-Resistant Erwinia Amylovora in New York Apple Orchards. Plant Dis. 2016, 100, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Ahmed, K.; Hussain, A.; Imran. Incidence and Severity of Crown Gall Disease of Cherry, Apple and Apricot Plants Caused by Agrobacterium Tumefaciens in Nagar Valley of Gilgit-Baltistan, Pakistan. Pak. J. Nutr. 2010, 9, 577–581. [Google Scholar] [CrossRef]
- Qayum, A.; Mondol, P.C.; Islam, S.; Firoz, M. Identification of Virulent Agrobacterium Tumefaciens Strains from Some Dicotyledonous Plants in Bangladesh. Agric. Conspec. Sci. Cus 2011, 76, 147–152. [Google Scholar]
- Terta, M.; Azelmat, S.; M’Hand, R.A.; Achbani, E.H.; Barakate, M.; Bouteau, F.; Ennaji, M.M. Molecular Typing of Pectobacterium carotovorum Isolated from Potato Tuber Soft Rot in Morocco. Ann. Microbiol. 2011, 62, 1411–1417. [Google Scholar] [CrossRef]
- Ferro, P.; Vaz-Moreira, I.; Manaia, C.M. Evolution of Gentamicin and Arsenite Resistance Acquisition in Ralstonia Pickettii Water Isolates. Res. Microbiol. 2021, 172, 103790. [Google Scholar] [CrossRef] [PubMed]
- Rafique Rind, M.; Yasmin, A.; Raza, S.; Jatoi, W.A. Isolation, Characterization and Evolution of Wild Virulent Strains of Agrobacterium for Their Potential Transformation through Use Potato Doscs. Pak. J. Bot. 2020, 52, 2237–2244. [Google Scholar] [CrossRef]
- Santamaría-Hernando, S.; Senovilla, M.; González-Mula, A.; Martínez-García, P.M.; Nebreda, S.; Rodríguez-Palenzuela, P.; López-Solanilla, E.; Rodríguez-Herva, J.J. The Pseudomonas syringae Pv. Tomato DC3000 PSPTO_0820 Multidrug Transporter Is Involved in Resistance to Plant Antimicrobials and Bacterial Survival during Tomato Plant Infection. PLoS ONE 2019, 14, e0218815. [Google Scholar] [CrossRef] [PubMed]
- Islam, T.; Saha, O.; Sultana, S.; Hridoy, M.; Hasan, M.; Marzan, S.; Musfiqur Rahman, M. Comparison between Reduced Susceptibility to Disinfectants and Multidrug Resistance among Hospital Isolates of Pseudomonas Aeruginosa and Staphylococcus Aureus in Bangladesh. Bagcilar Med. Bull. 2017, 2, 88–97. [Google Scholar] [CrossRef]
- Wilson, D.N. Ribosome-Targeting Antibiotics and Mechanisms of Bacterial Resistance. Nat. Rev. Microbiol. 2013, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Islam, T. Heavy Metal Tolerance Pattern of Textile Dye Degrading Native Bacteria: A Bioremediation Viewpoint. Ann. Med. Health Sci. Res. 2017, 7, 67–73. [Google Scholar]
- Miller, S.A.; Ferreira, J.P.; Lejeune, J.T. Antimicrobial Use and Resistance in Plant Agriculture: A One Health Perspective. Agriculture 2022, 12, 289. [Google Scholar] [CrossRef]
- Evaggelopoulou, E.N.; Samanidou, V.F. Development and Validation of an HPLC Method for the Determination of Six Penicillin and Three Amphenicol Antibiotics in Gilthead Seabream (Sparus aurata) Tissue According to the European Union Decision 2002/657/EC. Food Chem. 2013, 136, 1322–1329. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, S.H.; Francisco, J.G.; Campion, T.F.; Pimpinato, R.F.; Moura Andrade, G.C.R.; Garcia, F.; Tornisielo, V.L. Multiresidue Antimicrobial Determination in Nile Tilapia (Oreochromis niloticus) Cage Farming by Liquid Chromatography Tandem Mass Spectrometry. Aquaculture 2015, 447, 37–43. [Google Scholar] [CrossRef]
- Rezk, M.R.; Riad, S.M.; Khattab, F.I.; Marzouk, H.M. Multi-Residues Determination of Antimicrobials in Fish Tissues by HPLC-ESI-MS/MS Method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 978–979, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.; Ramos, F. Analytical Strategies for the Detection and Quantification of Antibiotic Residues in Aquaculture Fishes: A Review. Trends Food Sci. Technol. 2016, 52, 16–30. [Google Scholar] [CrossRef]
- Zhang, L.; Du, S.; Liu, D.; Dong, D.; Zhang, W.; Guo, Z. Antibiotics in Fish Caught from Ice-Sealed Waters: Spatial and Species Variations, Tissue Distribution, Bioaccumulation, and Human Health Risk. Sci. Total Environ. 2022, 821, 153354. [Google Scholar] [CrossRef] [PubMed]
- Modupe, S.L.; Yaa, N.-B.; Henaku, O.E.; Ohya, K.; Masato, S.; Opare, O.J.; Baboreka, K.B. Protected but Not from Contamination: Antimicrobial Resistance Profiles of Bacteria from Birds in a Ghanaian Forest Protected Area. Environ. Health Insights 2021, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Elsohaby, I.; Samy, A.; Elmoslemany, A.; Alorabi, M.; Alkafafy, M.; Aldoweriej, A.; Al-Marri, T.; Elbehiry, A.; Fayez, M. Migratory Wild Birds as a Potential Disseminator of Antimicrobial-Resistant Bacteria around Al-Asfar Lake, Eastern Saudi Arabia. Antibiotics 2021, 10, 260. [Google Scholar] [CrossRef] [PubMed]
- Saiful Islam, M.; Paul, A.; Talukder, M.; Roy, K.; Abdus Sobur, M.; Ievy, S.; Mehedi Hasan Nayeem, M.; Rahman, S.; Nazmul Hussain Nazir, K.H.M.; Tofazzal Hossain, M.; et al. Migratory Birds Travelling to Bangladesh Are Potential Carriers of Multi-Drug Resistant Enterococcus spp., Salmonella spp., and Vibrio spp. Saudi J. Biol. Sci. 2021, 28, 5963–5970. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, I.; Fahsi, N.; Hijri, M.; Sobeh, M. Antibiotic Resistance in Plant Growth Promoting Bacteria: A Comprehensive Review and Future Perspectives to Mitigate Potential Gene Invasion Risks. Front. Microbiol. 2022, 13, 999988. [Google Scholar] [CrossRef] [PubMed]
- Marti, R.; Scott, A.; Tien, Y.C.; Murray, R.; Sabourin, L.; Zhang, Y.; Topp, E. Impact of Manure Fertilization on the Abundance of Antibiotic-Resistant Bacteria and Frequency of Detection of Antibiotic Resistance Genes in Soil and on Vegetables at Harvest. Appl. Environ. Microbiol. 2013, 79, 5701–5709. [Google Scholar] [CrossRef]
- Kang, D.H.; Gupta, S.; Rosen, C.; Fritz, V.; Singh, A.; Chander, Y.; Murray, H.; Rohwer, C. Antibiotic Uptake by Vegetable Crops from Manure-Applied Soils. J. Agric. Food Chem. 2013, 61, 9992–10001. [Google Scholar] [CrossRef] [PubMed]
- Bassil, R.J.; Bashour, I.I.; Sleiman, F.T.; Abou-Jawdeh, Y.A. Antibiotic Uptake by Plants from Manure-Amended Soils. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2013, 48, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Q.; Luo, Y. Occurrence and Source Analysis of Typical Veterinary Antibiotics in Manure, Soil, Vegetables and Groundwater from Organic Vegetable Bases, Northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar] [CrossRef] [PubMed]
- Amelin, V.G.; Avdeeva, N.M. Determination of Penicillins G and V in Vegetables and Fruits by Exact Masses of Ions of Protonated Adducts with Methanol by Ultra-High-Performance Liquid Chromatography—Time-of-Flight High Resolution Mass Spectrometry. J. Anal. Chem. 2018, 73, 922–928. [Google Scholar] [CrossRef]
- Pan, M.; Chu, L.M. Transfer of Antibiotics from Wastewater or Animal Manure to Soil and Edible Crops. Environ. Pollut. 2017, 231, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.M.; Rajapaksha, A.U.; Lim, J.E.; Vu, N.T.; Kim, I.S.; Kang, H.M.; Lee, S.S.; Ok, Y.S. Distribution and Accumulative Pattern of Tetracyclines and Sulfonamides in Edible Vegetables of Cucumber, Tomato, and Lettuce. J. Agric. Food Chem. 2015, 63, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Alechaga, É.; Moyano, E.; Galceran, M.T. Simultaneous Analysis of Kasugamycin and Streptomycin in Vegetables by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Methods 2015, 7, 3600–3607. [Google Scholar] [CrossRef]
- Sidhu, H.; O’Connor, G.; Kruse, J. Plant Toxicity and Accumulation of Biosolids-Borne Ciprofloxacin and Azithromycin. Sci. Total Environ. 2019, 648, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Gbylik-Sikorska, M.; Gajda, A.; Nowacka-Kozak, E.; Posyniak, A. Simultaneous Determination of 45 Antibacterial Compounds in Mushrooms—Agaricus Bisporus by Ultra-High Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2019, 1587, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Aldeek, F.; Canzani, D.; Standland, M.; Hammack, W.; Cook, J.M.; Crosswhite, M.R.; Gerard, G. LC-MS/MS Validation of a Residue Analysis Method for Penicillin G and Its Metabolites in Commercial Orange Juice. J. AOAC Int. 2017, 100, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Aldeek, F.; Rosana, M.R.; Hamilton, Z.K.; Crosswhite, M.R.; Burrows, C.W.; Singh, S.; Gerard, G.; Hammack, W.; Cook, J.M. LC-MS/MS Method for the Determination and Quantitation of Penicillin G and Its Metabolites in Citrus Fruits Affected by Huanglongbing. J. Agric. Food Chem. 2015, 63, 5993–6000. [Google Scholar] [CrossRef] [PubMed]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Antimicrobial Growth Promoters Used in Animal Feed: Effects of Less Well Known Antibiotics on Gram-Positive Bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.C.; Radl, V.; Schloter-Hai, B.; Jechalke, S.; Heuer, H.; Smalla, K.; Schloter, M. Dynamics of Soil Bacterial Communities in Response to Repeated Application of Manure Containing Sulfadiazine. PLoS ONE 2014, 9, e92958. [Google Scholar] [CrossRef] [PubMed]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic Use in Aquaculture, Policies and Regulation, Health and Environmental Risks: A Review of the Top 15 Major Producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food Animals and Antimicrobials: Impacts on Human Health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Deloy, A.; Volkert, A.M.; Leonhardt, S.D.; Pufal, G. Antibiotics Impact Plant Traits, Even at Small Concentrations. AoB Plants 2017, 9, plx010. [Google Scholar] [CrossRef]
- Timmerer, U.; Lehmann, L.; Schnug, E.; Bloem, E. Toxic Effects of Single Antibiotics and Antibiotics in Combination on Germination and Growth of Sinapis alba L. Plants 2020, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Krupka, M.; Piotrowicz-Cieślak, A.I.; Michalczyk, D.J. Effects of Antibiotics on the Photosynthetic Apparatus of Plants. J. Plant Interact. 2022, 17, 96–104. [Google Scholar] [CrossRef]
- Opris, O.; Copaciu, F.; Loredana Soran, M.; Ristoiu, D.; Niinemets, Ü.; Copolovici, L. Influence of Nine Antibiotics on Key Secondary Metabolites and Physiological Characteristics in Triticum Aestivum: Leaf Volatiles as a Promising New Tool to Assess Toxicity. Ecotoxicol. Environ. Saf. 2013, 87, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Minden, V.; Schnetger, B.; Pufal, G.; Leonhardt, S.D. Antibiotic-Induced Effects on Scaling Relationships and on Plant Element Contents in Herbs and Grasses. Ecol. Evol. 2018, 8, 6699–6713. [Google Scholar] [CrossRef] [PubMed]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of Antibiotics on the Human Microbiome and Consequences for Host Health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef]
- Wong, W.S.W.; Sabu, P.; Deopujari, V.; Levy, S.; Shah, A.A.; Clemency, N.; Provenzano, M.; Saadoon, R.; Munagala, A.; Baker, R.; et al. Prenatal and Peripartum Exposure to Antibiotics and Cesarean Section Delivery Are Associated with Differences in Diversity and Composition of the Infant Meconium Microbiome. Microorganisms 2020, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, J.; Bobe, A.M.; Miyoshi, S.; Huang, Y.; Hubert, N.; Delmont, T.O.; Eren, A.M.; Leone, V.; Chang, E.B. Peripartum Antibiotics Promote Gut Dysbiosis, Loss of Immune Tolerance, and Inflammatory Bowel Disease in Genetically Prone Offspring. Cell Rep. 2017, 20, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Konya, T.; Persaud, R.R.; Guttman, D.S.; Chari, R.S.; Field, C.J.; Sears, M.R.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; et al. Impact of Maternal Intrapartum Antibiotics, Method of Birth and Breastfeeding on Gut Microbiota during the First Year of Life: A Prospective Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Coker, M.O.; Hoen, A.G.; Dade, E.; Lundgren, S.; Li, Z.; Wong, A.D.; Zens, M.S.; Palys, T.J.; Morrison, H.G.; Sogin, M.L.; et al. Specific Class of Intrapartum Antibiotics Relates to Maturation of the Infant Gut Microbiota: A Prospective Cohort Study. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, S.; Pike, K.; Jones, D.R.; Brocklehurst, P.; Marlow, N.; Salt, A.; Taylor, D.J. Childhood Outcomes after Prescription of Antibiotics to Pregnant Women with Spontaneous Preterm Labour: 7-Year Follow-up of the ORACLE II Trial. Lancet 2008, 372, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.T.; Whyatt, R.; Hoepner, L.; Oberfield, S.; Dominguez-Bello, M.G.; Widen, E.M.; Hassoun, A.; Perera, F.; Rundle, A. Prenatal Exposure to Antibiotics, Cesarean Section and Risk of Childhood Obesity. Int. J. Obes. 2014, 39, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Stokholm, J.; Sevelsted, A.; Bønnelykke, K.; Bisgaard, H. Maternal Propensity for Infections and Risk of Childhood Asthma: A Registry-Based Cohort Study. Lancet Respir. Med. 2014, 2, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Tormo-Badia, N.; Håkansson, Å.; Vasudevan, K.; Molin, G.; Ahrné, S.; Cilio, C.M. Antibiotic Treatment of Pregnant Non-Obese Diabetic Mice Leads to Altered Gut Microbiota and Intestinal Immunological Changes in the Offspring. Scand. J. Immunol. 2014, 80, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.U.; Zaura, E.; Buijs, M.J.; Keijser, B.J.F.; Crielaard, W.; Nord, C.E.; Weintraub, A. Determining the Long-Term Effect of Antibiotic Administration on the Human Normal Intestinal Microbiota Using Culture and Pyrosequencing Methods. Clin. Infect. Dis. 2015, 60, S77–S84. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, G.; Duar, R.M.; Vance, D.P.; Mitchell, R.; Contreras, L.; Frese, S.A.; Smilowitz, J.T.; Underwood, M.A. Early-Life Gut Microbiome Modulation Reduces the Abundance of Antibiotic-Resistant Bacteria. Antimicrob. Resist. Infect. Control 2019, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.M.; Patel, S.; Forsberg, K.J.; Wang, B.; Bentley, G.; Razia, Y.; Qin, X.; Tarr, P.I.; Dantas, G. Pediatric Fecal Microbiota Harbor Diverse and Novel Antibiotic Resistance Genes. PLoS ONE 2013, 8, e78822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kinkelaar, D.; Huang, Y.; Li, Y.; Li, X.; Wang, H.H. Acquired Antibiotic Resistance: Are We Born with It? Appl. Environ. Microbiol. 2011, 77, 7134–7141. [Google Scholar] [CrossRef]
- Pärnänen, K.; Karkman, A.; Hultman, J.; Lyra, C.; Bengtsson-Palme, J.; Larsson, D.G.J.; Rautava, S.; Isolauri, E.; Salminen, S.; Kumar, H.; et al. Maternal Gut and Breast Milk Microbiota Affect Infant Gut Antibiotic Resistome and Mobile Genetic Elements. Nat. Commun. 2018, 9, 3891. [Google Scholar] [CrossRef] [PubMed]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Goh, E.B.; Yim, G.; Tsui, W.; McClure, J.A.; Surette, M.G.; Davies, J. Transcriptional Modulation of Bacterial Gene Expression by Subinhibitory Concentrations of Antibiotics. Proc. Natl. Acad. Sci. USA 2002, 99, 17025–17030. [Google Scholar] [CrossRef] [PubMed]
- Varkey, J.; Nagaraj, R. Antibacterial Activity of Human Neutrophil Defensin HNP-1 Analogs without Cysteines. Antimicrob. Agents Chemother. 2005, 49, 4561–4566. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, M.; Méndez-García, C.; Rojo, D.; Barbas, C.; Moya, A. Antibiotic Use and Microbiome Function. Biochem. Pharmacol. 2017, 134, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Murci, L.; Clark, A.G. Population Genetic Tools for Dissecting Innate Immunity in Humans. Nat. Rev. Immunol. 2013, 13, 280. [Google Scholar] [CrossRef] [PubMed]
- Bersch, K.L.; Demeester, K.E.; Zagani, R.; Chen, S.; Wodzanowski, K.A.; Liu, S.; Mashayekh, S.; Reinecker, H.C.; Grimes, C.L. Bacterial Peptidoglycan Fragments Differentially Regulate Innate Immune Signaling. ACS Cent. Sci. 2021, 7, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Willing, B.P.; Russell, S.L.; Finlay, B.B. Shifting the Balance: Antibiotic Effects on Host–Microbiota Mutualism. Nat. Rev. Microbiol. 2011, 9, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Kalghatgi, S.; Spina, C.S.; Costello, J.C.; Liesa, M.; Morones-Ramirez, J.R.; Slomovic, S.; Molina, A.; Shirihai, O.S.; Collins, J.J. Bactericidal Antibiotics Induce Mitochondrial Dysfunction and Oxidative Damage in Mammalian Cells. Sci. Transl. Med. 2013, 5, 192ra85. [Google Scholar] [CrossRef] [PubMed]
- Morgun, A.; Dzutsev, A.; Dong, X.; Greer, R.L.; Sexton, D.J.; Ravel, J.; Schuster, M.; Hsiao, W.; Matzinger, P.; Shulzhenko, N. Uncovering Effects of Antibiotics on the Host and Microbiota Using Transkingdom Gene Networks. Gut 2015, 64, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Li, L.; Wijlaars, L.; Gilbert, R.E. Associations between Use of Macrolide Antibiotics during Pregnancy and Adverse Child Outcomes: A Systematic Review and Meta-Analysis. PLoS ONE 2019, 14, e0212212. [Google Scholar] [CrossRef] [PubMed]
- Tamim, H.M.; Hanley, J.A.; Hajeer, A.H.; Boivin, J.F.; Collet, J.P. Risk of Breast Cancer in Relation to Antibiotic Use. Pharmacoepidemiol. Drug Saf. 2008, 17, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Velicer, C.M.; Heckbert, S.R.; Lampe, J.W.; Potter, J.D.; Robertson, C.A.; Taplin, S.H. Antibiotic Use in Relation to the Risk of Breast Cancer. JAMA 2004, 291, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Bhargava, P.; McCloskey, D.; Mao, N.; Palsson, B.O.; Collins, J.J. Antibiotic-Induced Changes to the Host Metabolic Environment Inhibit Drug Efficacy and Alter Immune Function. Cell Host Microbe 2017, 22, 757–765.e3. [Google Scholar] [CrossRef] [PubMed]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Georgia Ștefan, M.; Loghin, F. Antibiotics in the Environment: Causes and Consequences. Med. Pharm. Rep. 2020, 93, 231. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-H.; Jiang, W.-T.; Li, Z.; Jean, J.-S.; Kuo, C.-Y. Antibiotic Tetracycline in the Environments—A Review. J. Pharm. Anal. 2016, 4, 86–111. [Google Scholar]
- Cheng, W.; Li, J.; Wu, Y.; Xu, L.; Su, C.; Qian, Y.; Zhu, Y.G.; Chen, H. Behavior of Antibiotics and Antibiotic Resistance Genes in Eco-Agricultural System: A Case Study. J. Hazard. Mater. 2016, 304, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bloor, M.C.; Kiryushina, A.; Kydralieva, K.; Bondarenko, L.; Pozdnyakov, L.; Manucharova, N.; Terekhova, V. Divergent Effects of Antibiotics on Plants and Microbiota in Soils with Contrasting Humus Content. Water. Air. Soil Pollut. 2021, 232, 518. [Google Scholar] [CrossRef]
- Böger, B.; Surek, M.; de Vilhena, R.O.; Fachi, M.M.; Junkert, A.M.; Santos, J.M.; Domingos, E.L.; Alexandre de Cobre, F.; Momade, D.R.; Pontarolo, R. Occurrence of Antibiotics and Antibiotic Resistant Bacteria in Subtropical Urban Rivers in Brazil. J. Hazard. Mater. 2021, 402, 123448. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.; Yang, X.; Ji, X.; Zou, H.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of Antibiotic Residues in Various Environmental Compartments of Shandong Province in Eastern China: Its Potential for Resistance Development and Ecological and Human Risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Felis, E.; Kalka, J.; Sochacki, A.; Kowalska, K.; Bajkacz, S.; Harnisz, M.; Korzeniewska, E. Antimicrobial Pharmaceuticals in the Aquatic Environment—Occurrence and Environmental Implications. Eur. J. Pharmacol. 2020, 866, 172813. [Google Scholar] [CrossRef] [PubMed]
- Kairigo, P.; Ngumba, E.; Sundberg, L.R.; Gachanja, A.; Tuhkanen, T. Occurrence of Antibiotics and Risk of Antibiotic Resistance Evolution in Selected Kenyan Wastewaters, Surface Waters and Sediments. Sci. Total Environ. 2020, 720, 137580. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Resistance in the Environment. J. Antimicrob. Chemother. 2004, 54, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Moreau-Guigon, E.; Labadie, P.; Alliot, F.; Teil, M.J.; Blanchard, M.; Chevreuil, M. Occurrence of Antibiotics in Rural Catchments. Chemosphere 2017, 168, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.J.; Paíga, P.; Silva, A.; Llaguno, C.P.; Carvalho, M.; Vázquez, F.M.; Delerue-Matos, C. Antibiotics and Antidepressants Occurrence in Surface Waters and Sediments Collected in the North of Portugal. Chemosphere 2020, 239, 12472. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yan, X.; Shen, Y.; Di, M.; Wang, J. Antibiotics in Surface Water and Sediments from Hanjiang River, Central China: Occurrence, Behavior and Risk Assessment. Ecotoxicol. Environ. Saf. 2018, 157, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Achmon, Y.; Cao, Y.; Liang, X.; Chen, L.; Wang, H.; Siame, B.A.; Leung, K.Y. Distribution of Antibiotic Resistance Genes in the Environment. Environ. Pollut. 2021, 285, 117402. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P. Antimicrobial Resistance in Rivers: A Review of the Genes Detected and New Challenges. Environ. Toxicol. Chem. 2022, 41, 687–714. [Google Scholar] [CrossRef] [PubMed]
- Heß, S.; Berendonk, T.U.; Kneis, D. Antibiotic Resistant Bacteria and Resistance Genes in the Bottom Sediment of a Small Stream and the Potential Impact of Remobilization. FEMS Microbiol. Ecol. 2018, 94, fiy128. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, G.; Xu, W.; Jian, S.; Peng, L.; Jia, D.; Sun, J. New Estimation of Antibiotic Resistance Genes in Sediment Along the Haihe River and Bohai Bay in China: A Comparison Between Single and Successive DNA Extraction Methods. Front. Microbiol. 2021, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Armalytė, J.; Skerniškytė, J.; Bakienė, E.; Krasauskas, R.; Šiugždinienė, R.; Kareivienė, V.; Kerzienė, S.; Klimienė, I.; Sužiedėlienė, E.; Ružauskas, M. Microbial Diversity and Antimicrobial Resistance Profile in Microbiota from Soils of Conventional and Organic Farming Systems. Front. Microbiol. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Bougnom, B.P.; Thiele-Bruhn, S.; Ricci, V.; Zongo, C.; Piddock, L.J.V. Raw Wastewater Irrigation for Urban Agriculture in Three African Cities Increases the Abundance of Transferable Antibiotic Resistance Genes in Soil, Including Those Encoding Extended Spectrum β-Lactamases (ESBLs). Sci. Total Environ. 2020, 698, 134201. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, W.; Xie, S.; Zeng, M.; Liu, H.; Yang, J.; Liu, X.; Yang, F. Increasing Prevalence of Antibiotic Resistance Genes in Manured Agricultural Soils in Northern China. Front. Environ. Sci. Eng. 2019, 14, 1. [Google Scholar] [CrossRef]
- Pauter, K.; Szultka-Młýnska, M.; Buszewski, B. Determination and Identification of Antibiotic Drugs and Bacterial Strains in Biological Samples. Molecules 2020, 25, 2556. [Google Scholar] [CrossRef] [PubMed]
- Legrand, T.; Vodovar, D.; Tournier, N.; Khoudour, N.; Hulin, A. Simultaneous Determination of Eight β-Lactam Antibiotics, Amoxicillin, Cefazolin, Cefepime, Cefotaxime, Ceftazidime, Cloxacillin, Oxacillin, and Piperacillin, in Human Plasma by Using Ultra-High-Performance Liquid Chromatography with Ultraviolet Detection. Antimicrob. Agents Chemother. 2016, 60, 4734–4742. [Google Scholar] [CrossRef] [PubMed]
- Borner, K.; Borner, E.; Lode, H. Determination of Linezolid in Human Serum and Urine by High-Performance Liquid Chromatography. Int. J. Antimicrob. Agents 2001, 18, 253–258. [Google Scholar] [CrossRef]
- He, G.; Guo, B.; Zhang, J.; Li, Y.; Wu, X.; Fan, Y.; Chen, Y.; Cao, G.; Yu, J. Determination of the Sulfate and Glucuronide Conjugates of Levornidazole in Human Plasma and Urine, and Levornidazole and Its Five Metabolites in Human Feces by High Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. B 2018, 1081–1082, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Szultka-Mlynska, M.; Buszewski, B. Chromatographic Behavior of Selected Antibiotic Drugs Supported by Quantitative Structure-Retention Relationships. J. Chromatogr. A 2016, 1478, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Wongchang, T.; Winterberg, M.; Tarning, J.; Sriboonvorakul, N.; Muangnoicharoen, S.; Blessborn, D. Determination of Ceftriaxone in Human Plasma Using Liquid Chromatography–Tandem Mass Spectrometry. Wellcome Open Res. 2019, 4, 47. [Google Scholar] [CrossRef] [PubMed]
- Ongas, M.; Standing, J.; Ogutu, B.; Waichungo, J.; Berkley, J.A.; Kipper, K. Liquid Chromatography–Tandem Mass Spectrometry for the Simultaneous Quantitation of Ceftriaxone, Metronidazole and Hydroxymetronidazole in Plasma from Seriously Ill, Severely Malnourished Children. Wellcome Open Res. 2018, 2, 43. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, B.; Jiang, X.; Yang, Y.; Wells, G.F.; Zhang, T.; Li, X. Antibiotic Resistome in a Large-Scale Healthy Human Gut Microbiota Deciphered by Metagenomic and Network Analyses. Environ. Microbiol. 2018, 20, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Berendes, D.; Knee, J.; Sumner, T.; Capone, D.; Lai, A.; Wood, A.; Patel, S.; Nalá, R.; Cumming, O.; Brown, J. Gut Carriage of Antimicrobial Resistance Genes among Young Children in Urban Maputo, Mozambique: Associations with Enteric Pathogen Carriage and Environmental Risk Factors. PLoS ONE 2019, 14, e0225464. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Chen, Z.; Tan, L.; Wang, X.; Xue, Y.; Wang, S.; Wang, Q.; Das, R.; Lin, H.; Hou, J.; et al. Gut Resistomes, Microbiota and Antibiotic Residues in Chinese Patients Undergoing Antibiotic Administration and Healthy Individuals. Sci. Total Environ. 2020, 705, 135674. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dyall-Smith, M.; Marenda, M.; Hu, H.W.; Browning, G.; Billman-Jacobe, H. Antibiotic Resistance Genes in Antibiotic-Free Chicken Farms. Antibiotics 2020, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Abdelgader, S.A.; Shi, D.; Chen, M.; Zhang, L.; Hejair, H.M.A.; Muhammad, U.; Yao, H.; Zhang, W. Antibiotics Resistance Genes Screening and Comparative Genomics Analysis of Commensal Escherichia coli Isolated from Poultry Farms between China and Sudan. Biomed Res. Int. 2018, 2018, 5327450. [Google Scholar] [CrossRef] [PubMed]
- Eckstrom, K.; Barlow, J.W. Resistome Metagenomics from Plate to Farm: The Resistome and Microbial Composition during Food Waste Feeding and Composting on a Vermont Poultry Farm. PLoS ONE 2019, 14, e0219807. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Ruzauskas, M.; Bartkevics, V.; Pugajeva, I.; Zavistanaviciute, P.; Starkute, V.; Zokaityte, E.; Lele, V.; Dauksiene, A.; Grashorn, M.; et al. Study of the Antibiotic Residues in Poultry Meat in Some of the EU Countries and Selection of the Best Compositions of Lactic Acid Bacteria and Essential Oils against Salmonella Enterica. Poult. Sci. 2020, 99, 4065–4076. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, M.; Xu, J.; Wang, J.; Tong, J.; Sun, N.; Qian, M. Determining a Wide Range of Antibiotics and Pesticides in Poultry Feathers Using Selective Accelerated Solvent Extraction-Liquid Chromatography-Mass Spectrometry. Anal. Methods 2022, 14, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Oyedeji, A.O.; Msagati, T.A.M.; Williams, A.B.; Benson, N.U. Detection and Quantification of Multiclass Antibiotic Residues in Poultry Products Using Solid-Phase Extraction and High-Performance Liquid Chromatography with Diode Array Detection. Heliyon 2021, 7, e08469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, X.-x.; Zhao, Z.; Duan, C.; Chen, H.; Wang, M.; Ren, H.; Yin, Y.; Ye, L. Metagenomic Analysis Revealed the Prevalence of Antibiotic Resistance Genes in the Gut and Living Environment of Freshwater Shrimp. J. Hazard. Mater. 2018, 350, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Muziasari, W.I.; Pitkänen, L.K.; Sørum, H.; Stedtfeld, R.D.; Tiedje, J.M.; Virta, M. The Resistome of Farmed Fish Feces Contributes to the Enrichment of Antibiotic Resistance Genes in Sediments below Baltic Sea Fish Farms. Front. Microbiol. 2017, 7, 2137. [Google Scholar] [CrossRef] [PubMed]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current Knowledge and Perspectives of Paenibacillus: A Review. Microb. Cell Fact. 2016, 15, 203. [Google Scholar] [CrossRef] [PubMed]
- Kasselaki, A.M.; Goumas, D.; Tamm, L.; Fuchs, J.; Cooper, J.; Leifert, C. Effect of Alternative Strategies for the Disinfection of Tomato Seed Infected with Bacterial Canker (Clavibacter mchiganensis subzsp. Michiganensis). NJAS—Wagening. J. Life Sci. 2011, 58, 145–147. [Google Scholar] [CrossRef]
- Nandi, M.; Macdonald, J.; Liu, P.; Weselowski, B.; Yuan, Z.C. Clavibacter mchiganensis ssp. Michiganensis: Bacterial Canker of Tomato, Molecular Interactions and Disease Management. Mol. Plant Pathol. 2018, 19, 2036. [Google Scholar] [CrossRef] [PubMed]
- Utkhede, R.; Koch, C. Biological Treatments to Control Bacterial Canker of Greenhouse Tomatoes. Biocontrol 2004, 49, 305–313. [Google Scholar] [CrossRef]
- Yuan, H.P.; Min, H.; Lv, Z.M.; Li, Z.M. Antimicrobial Activity of Isolate HL-12 against Clavibacter mchiganensis subsp. Michiganensis in the Presence of Cadmium. Ecotoxicology 2009, 18, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, Y.A.; Suharjono, S.; Widyaningsih, S. Biological Control of Citrus Canker Pathogen Xanthomonas citri subsp. Citri Using Rangpur Lime Endophytic Bacteria. Egypt. J. Biol. Pest Control 2022, 32, 63. [Google Scholar] [CrossRef]
- Mácha, H.; Marešová, H.; Juříková, T.; Švecová, M.; Benada, O.; Škríba, A.; Baránek, M.; Novotný, Č.; Palyzová, A. Killing Effect of Bacillus velezensis Fzb42 on a Xanthomonas campestris pv. Campestris (Xcc) Strain Newly Isolated from Cabbage Brassica Oleracea Convar. Capitata (L.): A Metabolomic Study. Microorganisms 2021, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Pajčin, I.; Vlajkov, V.; Frohme, M.; Grebinyk, S.; Grahovac, M.; Mojićević, M.; Grahovac, J. Pepper Bacterial Spot Control by Bacillus velezensis: Bioprocess Solution. Microorganisms 2020, 8, 1463. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, L.; Lin, Y.; Shen, D.; Shao, X.; Zhong, C.; Qian, G. Targeted Isolation of Biocontrol Agents from Plants through Phytopathogen Co-Culture and Pathogen Enrichment. Phytopathol. Res. 2022, 4, 1–14. [Google Scholar] [CrossRef]
- Elsayed, T.R.; Jacquiod, S.; Nour, E.H.; Sørensen, S.J.; Smalla, K. Biocontrol of Bacterial Wilt Disease Through Complex Interaction Between Tomato Plant, Antagonists, the Indigenous Rhizosphere Microbiota, and Ralstonia solanacearum. Front. Microbiol. 2020, 10, 2835. [Google Scholar] [CrossRef]
- Abd-El-Khair, H.; Abdel-Gaied, T.G.; Mikhail, M.S.; Abdel-Alim, A.I.; El-Nasr, H.I.S. Biological Control of Pectobacterium carotovorum subsp. carotovorum, the Causal Agent of Bacterial Soft Rot in Vegetables, in Vitro and in Vivo Tests. Bull. Natl. Res. Cent. 2021, 45, 37. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Wang, C.W.; Lu, B.H. First Report of Bacterial Root Rot of Ginseng Caused by Pseudomonas Aeruginosa in China. Plant Dis. 2014, 98, 1577. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Déziel, E.; Schweizer, H.P.; Rahme, L.G.; Fall, R.; Vivanco, J.M. Pseudomonas Aeruginosa-Plant Root Interactions. Pathogenicity, Biofilm Formation, and Root Exudation. Plant Physiol. 2004, 134, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Arora, D.S. Prospecting the Antimicrobial and Antibiofilm Potential of Chaetomium globosum an Endophytic Fungus from Moringa oleifera. AMB Express 2020, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Yonuis Abdulhadi, S.; Qasim Hasan, G.; Nawaf Gergees, R. Detection and Activity of Endophytic Fungi. Ann. Trop. Med. Public Health 2020, 23, 231–384. [Google Scholar] [CrossRef]
- Soltani, J.; Moghaddam, M.S.H. Diverse and Bioactive Endophytic Aspergilli Inhabit Cupressaceae Plant Family. Arch. Microbiol. 2014, 196, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.B.; Jackson, L.E.; Balogh, B.; Obradovic, A.; Iriarte, F.B.; Momol, M.T. Bacteriophages for Plant Disease Control. Annu. Rev. Phytopathol. 2007, 45, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Neuman, B.W.; Ramírez, C.A. Bacteriophages as Promising Agents for the Biological Control of Moko Disease (Ralstonia solanacearum) of Banana. Biol. Control 2020, 149, 104238. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; van Vaerenbergh, J.; Vandenheuvel, D.; Dunon, V.; Ceyssens, P.J.; de Proft, M.; Kropinski, A.M.; Noben, J.P.; Maes, M.; Lavigne, R. T4-Related Bacteriophage LIMEstone Isolates for the Control of Soft Rot on Potato Caused by ‘Dickeya Solani’. PLoS ONE 2012, 7, e33227. [Google Scholar] [CrossRef]
- Czajkowski, R.; Ozymko, Z.; Lojkowska, E. Isolation and Characterization of Novel Soilborne Lytic Bacteriophages Infecting Dickeya Spp. Biovar 3 (‘D. Solani’). Plant Pathol. 2014, 63, 758–772. [Google Scholar] [CrossRef]
- Lim, J.A.; Jee, S.; Lee, D.H.; Roh, E.; Jung, K.; Oh, C.; Heu, S. Biocontrol of Pectobacterium carotovorum subsp. carotovorum Using Bacteriophage PP1. J. Microbiol. Biotechnol. 2013, 23, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Zaczek-Moczydłowska, M.A.; Young, G.K.; Trudgett, J.; Plahe, C.; Fleming, C.C.; Campbell, K.; O’Hanlon, R. Phage Cocktail Containing Podoviridae and Myoviridae Bacteriophages Inhibits the Growth of Pectobacterium Spp. under in Vitro and in Vivo Conditions. PLoS ONE 2020, 15, e0230842. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Vu, N.T.; Oh, E.J.; Rahimi-Midani, A.; Thi, T.N.; Song, Y.R.; Hwang, I.S.; Choi, T.J.; Oh, C.S. Biocontrol of Soft Rot Caused by Pectobacterium Odoriferum with Bacteriophage PhiPccP-1 in Kimchi Cabbage. Microorganisms 2021, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, J.; Gartemann, K.-H.; Eichenlaub, R.; Dreiseikelmann, B. Genomic and Molecular Analysis of Phage CMP1 from Clavibacter mchiganensis subspecies michiganensis. Bacteriophage 2011, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, J.; Eichenlaub, R.; Dreiseikelmann, B. The Endolysins of Bacteriophages CMP1 and CN77 Are Specific for the Lysis of Clavibacter mchiganensis Strains. Microbiology 2010, 156, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Nazir, A.; Dong, Z.; Liu, J.; Zhang, X.; Tahir, R.A.; Ashraf, N.; Qing, H.; Peng, D.; Tong, Y. Sequence Analysis of a Jumbo Bacteriophage, Xoo-Sp14, That Infects Xanthomonas oryzae pv. Oryzae. Microbiol. Resour. Announc. 2020, 9, 10-1128. [Google Scholar] [CrossRef]
- Nazir, A.; Dong, Z.; Liu, J.; Tahir, R.A.; Rasheed, M.; Qing, H.; Peng, D.; Tong, Y. Genomic Analysis of Bacteriophage Xoo-Sp13 Infecting Xanthomonas oryzae pv. Oryzae. Arch. Virol. 2021, 166, 1263–1265. [Google Scholar] [CrossRef] [PubMed]
- Solís-Sánchez, G.A.; Quiñones-Aguilar, E.E.; Fraire-Velázquez, S.; Vega-Arreguín, J.; Rincón-Enríquez, G. Complete Genome Sequence of XaF13, a Novel Bacteriophage of Xanthomonas Vesicatoria from Mexico. Microbiol. Resour. Announc. 2020, 9, e01371-19. [Google Scholar] [CrossRef] [PubMed]
- Papaianni, M.; Paris, D.; Woo, S.L.; Fulgione, A.; Rigano, M.M.; Parrilli, E.; Tutino, M.L.; Marra, R.; Manganiello, G.; Casillo, A.; et al. Plant Dynamic Metabolic Response to Bacteriophage Treatment After Xanthomonas campestris pv. Campestris Infection. Front. Microbiol. 2020, 11, 732. [Google Scholar] [CrossRef] [PubMed]
- Stefani, E.; Obradović, A.; Gašić, K.; Altin, I.; Nagy, I.K.; Kovács, T. Bacteriophage-Mediated Control of Phytopathogenic Xanthomonads: A Promising Green Solution for the Future. Microorganisms 2021, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Carstens, A.B.; Djurhuus, A.M.; Kot, W.; Hansen, L.H. A Novel Six-Phage Cocktail Reduces Pectobacterium atrosepticum Soft Rot Infection in Potato Tubers under Simulated Storage Conditions. FEMS Microbiol. Lett. 2019, 366, 101. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.T.; Roh, E.; Thi, T.N.; Oh, C.S. Antibiotic Resistance of Pectobacterium Korean Strains Susceptible to the Bacteriophage PhiPccP-1. Res. Plant Dis. 2022, 28, 166–171. [Google Scholar] [CrossRef]
- Song, Y.R.; Vu, N.T.; Park, J.; Hwang, I.S.; Jeong, H.J.; Cho, Y.S.; Oh, C.S. Phage PPPL-1, A New Biological Agent to Control Bacterial Canker Caused by Pseudomonas syringae Pv. Actinidiae in Kiwifruit. Antibiotics 2021, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.A.M.; Pereira, C.; Barreal, M.E.; Gallego, P.P.; Balcão, V.M.; Almeida, A. Use of Phage Φ6 to Inactivate Pseudomonas syringae Pv. Actinidiae in Kiwifruit Plants: In Vitro and Ex Vivo Experiments. Appl. Microbiol. Biotechnol. 2019, 104, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, M.; Ozaktan, H. Evaluation of Bacteriophages in the Biocontrol of Pseudomonas syringae Pv. syringae Isolated from Cankers on Sweet Cherry (Prunus avium L.) in Turkey. Egypt. J. Biol. Pest Control 2021, 31, 35. [Google Scholar] [CrossRef]
- Tsuda, K.; Somssich, I.E. Transcriptional Networks in Plant Immunity. N. Phytol. 2015, 206, 932–947. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, J.; Brancato, C.; Berendzen, K.W.; Dreiseikelmann, B. Development of a Tomato Plant Resistant to Clavibacter mchiganensis Using the Endolysin Gene of Bacteriophage CMP1 as a Transgene. Plant Pathol. 2016, 65, 496–502. [Google Scholar] [CrossRef]
- Someya, N.; Akutsu, K. Biocontrol of Plant Diseases by Genetically Modified Microorganisms: Current Status and Future Prospects. In PGPR: Biocontrol and Biofertilization; Springer: Dordrecht, The Netherlands, 2006; pp. 297–312. [Google Scholar] [CrossRef]
- Horvath, D.M.; Stall, R.E.; Jones, J.B.; Pauly, M.H.; Vallad, G.E.; Dahlbeck, D.; Staskawicz, B.J.; Scott, J.W. Transgenic Resistance Confers Effective Field Level Control of Bacterial Spot Disease in Tomato. PLoS ONE 2012, 7, e42036. [Google Scholar] [CrossRef] [PubMed]
- Ger, M.J.; Louh, G.Y.; Lin, Y.H.; Feng, T.Y.; Huang, H.E. Ectopically Expressed Sweet Pepper Ferredoxin PFLP Enhances Disease Resistance to Pectobacterium carotovorum subsp. carotovorum Affected by Harpin and Protease-Mediated Hypersensitive Response in Arabidopsis. Mol. Plant Pathol. 2014, 15, 892–906. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, A.M.; Lim, G.T.T.; Jones, D.A. The Tomato I-3 Gene: A Novel Gene for Resistance to Fusarium Wilt Disease. N. Phytol. 2015, 207, 106–118. [Google Scholar] [CrossRef] [PubMed]



| Crop | Diseases | Causative Agent | Recommended Antibiotic |
|---|---|---|---|
| Rice | Bacterial panicle blight | Burkholderia glumae | Oxolinic acid, Streptocycline |
| Bacterial leaf blight | X. oryzae pv. oryzae | ||
| Tobacco | Wildfire | P. syringae pv. tabaci | Streptomycin |
| Tomato | Bacterial canker | Clavibacter michiganensis pv. michiganensis | Oxytetracycline, Gentamycin, Streptocycline |
| Bacterial wilt | R. solanacearum | ||
| Bacterial speck | P. syringae pv. tomato | ||
| Citrus | Citrus canker | Xanthomonas axonopodis pv. citri | Streptomycin |
| Paprika | Bacterial canker | C. michiganensis subsp. capsici | Streptomycin |
| Maize | Wilt and blight | C. michiganensis subsp. nebraskensis | Streptomycin |
| Potato | Blackleg | P. atrosepticum | Oxytetracycline, Gentamycin, Streptocycline |
| Bacterial wilt | R. solanacearum | ||
| Soft rot | Pectobacterium carotovorum | ||
| Ring rot | C. michiganensis subsp. sepedonicus | ||
| Eggplant | Bacterial wilt or southern wilt | R. solanacearum | Oxytetracycline, Gentamycin, Streptocycline |
| Cabbage | Bacterial black rot | X. campestris pv. campestris | Oxytetracycline, Gentamycin, Streptocycline |
| Soft rot | P. carotovorum | ||
| Watermelon | Black rot | Xanthomonas spp. | Gentamycin |
| Onion | Brown rot | Pseudomonas aeruginosa | Streptocycline |
| Antibiotic | Bacterial % of Resistance (Resistance/Total Samples) | Resistance Mechanism | Reference |
|---|---|---|---|
| Streptomycin | P. syringae 8.42% (8/95) | Probable chromosomal mutation | [31] |
| P. syringae 5.26 (3/57) | Probable chromosomal mutation | [118] | |
| X. smithii subsp. citri 44.1%-88.7% (49/111-219/247) | Presence of the strB gene, chromosomal mutation | [117] | |
| Xanthomonas oryzae pv. Oryzae 26.67% (4/15) | Presence of the aadA1 gene | [119] | |
| X. axonopodis 55.5% (11/20) | Not mentioned | [120] | |
| C. michiganensis 1.68% (3/179) | Mutation in the rpsL gene | [116] | |
| C. michiganensis subsp. michiganensis 84% (21/25) | Mutation in the rpsL gene | [121] | |
| Erwinia amylovora 18.1% (20) | Not mentioned | [122] | |
| X. arboricola pv. Pruni 7.1% (7/99) | Presence of strAB genes | [123] | |
| E. amylovora 2.66% (34/1280) | Presence of the strA/strB gene | [124] | |
| Tetracycline | P. syringae 1.01% (1/95) | Genetic modification | [31] |
| P. syringae 3.5% (2/57) | Chromosomal mutation | [118] | |
| Agrobacterium tumefaciens 6.67% (02/30) | Not mentioned | [125] | |
| A. tumefaciens 100% (4/4) | Not mentioned | [126] | |
| Oxytetracycline | X. arboricola pv. Pruni 7.1% (7/99) | Presence of tetC, tetR genes | [123] |
| Ampicillin | P. syringae 57.9% (55/95) | Not mentioned | [31] |
| P. syringae 61.4% (35/57) | Chromosomal mutation | [118] | |
| P. carotovorum 22.73% (5/22) | Not mentioned | [127] | |
| A. tumefaciens 100% (30/30) | Not mentioned | [125] | |
| Amoxicillin | A. tumefaciens 100% (30/30) | Not mentioned | [125] |
| Doxycycline | A. tumefaciens 13.34% (04/30) | Not mentioned | [125] |
| Copper | P. syringae 75% (69/92) | Not mentioned | [31] |
| Chloramphenicol | P. syringae 37.9% (36/95) | Not mentioned | [31] |
| P. syringae 10.53% (6/57) | Chromosomal mutation | [118] | |
| E. amylovora 0% (20) | Not mentioned | [122] | |
| Rifampicin | P. syringae 16.8% (16/95) | Not mentioned | [31] |
| A. tumefaciens 100% (4/4) | Not mentioned | [126] | |
| Kanamycin | P. syringae 1.01% (1/95) | Genetic modification | [31] |
| P. syringae 0% (0/57) | Chromosomal mutation | [118] | |
| A. tumefaciens 0% (0/4) | Not mentioned | [126] | |
| Gentamicin | Ralstonia pickettii 96.97% (32/33) | Presence of ICE- and ars-operon-related genes | [128] |
| X. axonopodis 33.3% (20) | Not mentioned | [120] | |
| E. amylovora 9.99% (20) | Not mentioned | [122] | |
| P. syringae 1.75% (1/57) | Chromosomal mutation | [118] | |
| Bacitracin | X. axonopodis 77.7% (20) | Not mentioned | [120] |
| E. amylovora 81.89% (20) | Not mentioned | [122] | |
| Cefotaxime | X. axonopodis 100% (20) | Not mentioned | [120] |
| E. amylovora 100% (20) | Not mentioned | [122] | |
| Cephalothin | P. carotovorum 22.73% (5/22) | Not mentioned | [127] |
| Cephradine | A. tumefaciens 26.66% (08/30) | Not mentioned | [125] |
| Cefuroxime | A. tumefaciens 10% (2/19) | Not mentioned | [129] |
| A. tumefaciens 0% (0/4) | Not mentioned | [126] | |
| Spectinomycin | P. syringae 3.5% (2/57) | Chromosomal mutation | [118] |
| Antibiotic | PPB | MIC50/MIC90 (µg/mL) | Lowest Observed MIC (µg/mL) | Highest Observed MIC (µg/mL) | Reference |
|---|---|---|---|---|---|
| Streptomycin | X. oryzae pv. Oryzae | ≤100/300 | 1 | 300 | [119] |
| C. michiganensis | 4/128 | 4 | 128 | [116] | |
| C. michiganensis subsp. michiganensis | 250/500 | 2 | 500 | [9] | |
| Ampicillin | P. syringae | 6/6 | 6 | 6 | [130] |
| Chloramphenicol | P. syringae | 4/4 | 4 | 4 | [130] |
| Colistin (polymyxin E) | P. syringae | 0.094/0.094 | 0.094 | 0.094 | [130] |
| Erythromycin | P. syringae | 4/4 | 2 | 4 | [130] |
| Kanamycin | X. oryzae pv. Oryzae | 100/>100 | 1 | >100 | [119] |
| Netilmicin | X. oryzae pv. Oryzae | 100/100 | 1 | 100 | [119] |
| Sulfamethoxazole | P. syringae | 192/192 | 192 | 192 | [130] |
| Tetracycline | P. syringae | 0.25/0.25 | 0.19 | 0.25 | [130] |
| Gentamicin | X. oryzae pv. Oryzae | 50/100 | 1 | 100 | [119] |
| R. pickettii | >256/>256 | 4 | >256 | [128] | |
| Rifampicin | X. oryzae pv. Oryzae | 5/10 | 0.1 | 10 | [119] |
| Tobramycin | X. oryzae pv. Oryzae | 10/50 | 1 | 50 | [119] |
| Spectinomycin | X. oryzae pv. Oryzae | >500/>500 | 1 | >500 | [119] |
| Sample Plant | Antibiotic Compounds | Amount Detected (µg/kg) | Antibiotic-Resistant Genes Detected | References on ACs Detection |
|---|---|---|---|---|
| Radish root | Gentamicin | 0.051 | incP oriT, incQ oriV, int3, aad(A), str(A), str(B), sul1, erm(B), blaOXAIl, int2, tet(A), erm(E), blaCTX-M, blaVIM, blaTEM, erm(F) | [145,146,147] |
| Streptomycin | 0.015 | |||
| Oxytetracycline | 8.3 | |||
| Sulfadoxine | 0.1–0.4 | |||
| Lincomycin | 0.9–3.1 | |||
| Sulfamethazine | 1.1 | |||
| Carrot | Sulfamethazine | <0.98 | incP, oriT, incQ, oriV, aad(A), str(A), str(B), sul1, erm(C), int1, tet(A), tet(S), sul1, erm(B), erm(E), blaVIM, blaTEM, qnr(B), tet(B), tet(T), blaOXA-20 | [145,148,149] |
| Monensin | <3.44–4 | |||
| Erythromycin | 0–0.52 | |||
| Chloramphenicol | 0.96–3.99 | |||
| Norfloxacin | 2.52–6.54 | |||
| Tetracycline | 0–1.33 | |||
| Sulfamethazine | 0–0.37 | |||
| Penicillins G & V | 0.05–0.3 | |||
| Lettuce leaf | Tetracycline | 1.35–1.85 | incP, oriT, incQ, repB, incW, int3, tet(A), tet(Q), tet(S), aad(A), str(A), sul1, erm(B), blaOXA1, blaVIM, blaTEM | [149,150,151,152] |
| Chloramphenicol | 0.86–2.72 | |||
| Norfloxacin | 2.88–7.43 | |||
| Azithromycin | 0.8–4 | |||
| Ciprofloxacin | 3.8–4 | |||
| Kasugamycin | 5–10 | |||
| Streptomycin | 5–10 | |||
| Tetracycline | 77–211 | |||
| Oxytetracycline | 35–318 | |||
| Chlortetracycline | 346–1364 | |||
| Sulfamethazine | 7813–25,993 | |||
| Sulfamethoxazole | 8582–30,589 | |||
| Sulfadimethoxine | 1773–7876 | |||
| Tomato | Tetracycline | 199–1009 | tet(T), str(A), incP oriT, incY, int2, int3, tet(A), tet(S), aad(A), str(A), str(B), erm(B), erm(E), blaCTX-M, blaVIM, blaTEM, tet(T), erm(F), blaPSE, blaOXA-20 | [148,150,151] |
| Oxytetracycline | 590–3231 | |||
| Chlortetracycline | 231–864 | |||
| Sulfamethazine | 9573–42,445 | |||
| Sulfamethoxazole | 17,193–38,467 | |||
| Sulfadimethoxine | 6113–20,887 | |||
| Kasugamycin | 5–10 | |||
| Streptomycin | 5–10 | |||
| Penicillins G & V | 0.05–0.3 | |||
| Cucumber | Tetracycline | 89–496 | incP, oriT, incP, trfA1, str(B), sul1, erm(B), blaOXAII | [148,150] |
| Oxytetracycline | 175–1603 | |||
| Chlortetracycline | 310–1320 | |||
| Sulfamethazine | 5359–16,319 | |||
| Sulfamethoxazole | 5633–11,330 | |||
| Sulfadimethoxine | 4924–12,692 | |||
| Penicillins G & V | 0.05–0.3 | |||
| Pepper | Chlortetracycline | <10 | int3, tet(T), str(B), sul1, vat(B), blaOXAII | [145,153] |
| Sulfamethazine | <10 | |||
| virginiamycin | <10 | |||
| Mushroom | Tetracyclines | 0.3–1.5 | ||
| Sulfonamides | 0.3–1.5 | |||
| Penicyllins | 0.3–3 | |||
| Macrolides | 0.3–3 | |||
| Fluoroquinolones | 0.3–1.5 | |||
| cephalosporins | 0.3–1.5 | |||
| Orange | Penicillin G | 0.1–0.25 | [148,154] | |
| Penicillins G & V | 0.05–0.3 | |||
| Lemon | Penicillin G | 0.1–0.25 | [148,154,155] | |
| Penicillins G & V | 0.05–0.3 | |||
| Grapefruit | Penicillin G | 0.1–0.25 | [154,155] |
| Samples | Antibiotic Compounds | Amount Detected (ng/kg) | Antibiotic-Resistant Genes Detected | References on ACs Detection | References on ARGs Detection |
|---|---|---|---|---|---|
| Drinking water or groundwater | Sulfapyridine | 0.052 | tetM, tetO, tetQ, ermF, sul1 | [197,198,199,200] | [198,200] |
| Sulfamethoxazole | 0.3–18.6 | ||||
| Ciprofloxacin | 0.4–224.4 | ||||
| Enrofloxacin | 0.2–11.2 | ||||
| Norfloxacin | 0.4–3.6 | ||||
| Florfenicol | 3.3–26.1 | ||||
| Erythromycin | 0.05 | ||||
| River water or surface water | Sulfapyridine | 0.2–3.1 | blaCTX, blaTEM, tetA, tetB, tetM, tetW, tetO, tetQ, tetX, ermB, ermC, ermF, aac(6′)-Ib-cr, qepA, qnrS, sul1, sul2, vanA, mecA, ampC | [196,197,198,199,200,201,202,203] | [198,200] |
| Sulfamethoxazole | 0.3–13.0 | ||||
| Ciprofloxacin | 0.2–18.8 | ||||
| Enrofloxacin | 0.2–52.2 | ||||
| Levofloxacin | 0.3–6.0 | ||||
| Norfloxacin | 0.2–78.1 | ||||
| Florfenicol | 1.6–15.3 | ||||
| Doxycycline | 1.9–3.5 | ||||
| Metronidazole | 0.4–1.6 | ||||
| Erythromycin | 0.1–1.7 | ||||
| Clarithromycin | 0.35 | ||||
| Roxithromycin | 1–913 | ||||
| Ofloxacin | 2.2–2.9 | ||||
| Azithromycin | 0.14–30.27 | ||||
| Norfloxacin | 0.11–2200 | ||||
| Wastewater | Sulfapyridine | 0.4–2.2 | blaCTX, blaTEM, blaOXA, blaSHV, tetA, tetB, tetM, tetW, tetO, tetQ, tetX, ermB, ermC, ermF, aac(6′)-Ib-cr, qepA, qnrS, sul1, sul2, vanA, mecA, ampC | [197,198,199,200,201,203] | [198,200,204] |
| Sulfamethoxazole | 0.6–20.9 | ||||
| Ciprofloxacin | 0.91–99.3 | ||||
| Enrofloxacin | 0.86–3579.6 | ||||
| Levofloxacin | 0.5–19,981.6 | ||||
| Norfloxacin | 0.6–24.6 | ||||
| Chloramphenicol | 0.99 | ||||
| Florfenicol | 2.4–6.8 | ||||
| Doxycycline | 1.8–264.4 | ||||
| Metronidazole | 0.64–1.45 | ||||
| Ampicillin | 900–1600 | ||||
| Erythromycin | up to 6.0 | ||||
| Clarithromycin | 18–1800 | ||||
| Roxithromycin | 32–1492 | ||||
| Ofloxacin | 1210 | ||||
| Azithromycin | 329.55 | ||||
| Norfloxacin | 44.04–2900 | ||||
| River sediment | Ciprofloxacin | 0.16–21.74 | intI1, sul2, blaTEM, floR, ermB, sul1, ereA, tetW, tetM, tetC, mecA, blaOXA-58, blaKPC-3, | [94,97,98,99,100,101,104]. | [205,206,207] |
| Enrofloxacin | 0.16–24.42 | ||||
| Levofloxacin | 0.82–2.89 | ||||
| Norfloxacin | 0.14–2.20 | ||||
| Chloramphenicol | 0.98–1.53 | ||||
| Ciprofloxacin | 0.68–112.7 | ||||
| Enrofloxacin | 0.4–112.69 | ||||
| Levofloxacin | 0.77–100.91 | ||||
| Norfloxacin | 0.04–6600 | ||||
| Chloramphenicol | 0.62–16.10 | ||||
| Doxycycline | 1.44–57.32 | ||||
| Ampicillin | 4600–43,800 | ||||
| Clarithromycin | 330–9930 | ||||
| Azithromycin | 43,000 | ||||
| Soil | Ciprofloxacin | 0.3–18.2 | aac(6′)-IB-CR, sul2, tetA, tetX, tetW, sul1, ermF, blaTEM, aadA15, aadA13, aadA, blaLCR-1, aac(3)-Ia, blaOXA-347, tetC, mefC, aph(6)-Ib, aadA16, dfrA1, aph(3′)-Ib, tetM, shv, ermC, | [197] | [208,209,210] |
| Enrofloxacin | 0.4–5.5 | ||||
| Levofloxacin | 0.2–6.5 | ||||
| Norfloxacin | 0.2–4.6 | ||||
| Chloramphenicol | 1.0–10.5 | ||||
| Doxycycline | 1.1–5.5 | ||||
| Human plasma, serum, urine, and feces | Ceftriaxone | 1.01–200 | cfxA, aacA, ermB, ermD, tetQ, tetW, tetO, sul2, tet32, tolC, aadA1, blaSHV, blaSHV(156G), acrB, blaSHV(238G240E), tetM, ompF, ermA, mefA, tetA, tetB, bacA, vanR, aadE, vanS, tet32, macB, bcrA, | [211,212,213,214,215,216,217] | [204,218,219,220] |
| Metronidazole | 0.05–50 | ||||
| Amoxicillin | 0.0015–0.015/50 | ||||
| Ampicillin | 0.0015–0.015/50 | ||||
| Levornidazole | 0.005–2.0 | ||||
| Linezolid | 0.07/4.7 × 106 | ||||
| Oxacillin | 2–100 × 106 | ||||
| Ceftazidime | 2–100 × 106 | ||||
| Piperacillin | 2–100 × 106 | ||||
| Pig manure and fecal samples | Ciprofloxacin | 0.33–13.71 | etQ, lnuC, tet40, aadE, ermF, tet44, ermB, cfxA, aph(3′)-IIIa, cfxA6, tetX, tetL, tetW, tetO, mefA, | [197,202] | [120] |
| Enrofloxacin | 0.45–3.86 | ||||
| Levofloxacin | 0.51–5.66 | ||||
| Norfloxacin | 0.42–1057.60 | ||||
| Doxycycline | 1.04–36.46 | ||||
| Metronidazole | 0.33–0.42 | ||||
| Poultry meat, manure, and fecal samples | Enrofloxacin | 5.37–55.4 | aadA, aadA2, aadA3, strB, ermB, sul2, tetK, tetM, tetW, tetX, aac3-1, tetA, acrA, ampC, pKD13, tetB, ermA, aadA1, ermC, strA, mphA, aadA14, blaCARB8, blaCARB10, aph(3′)-Ic, aadA24, aph(6′)-ld, tet39, aph(3′)-IIa, lsaE, tetL, lnuB, vanA, vanB, vanC2 | [204,221,222,223] | [113,224,225,226] |
| Sulfadimethoxine | 5.37–55.4 | ||||
| Sulfamerazine | 5.37–55.4 | ||||
| Tylosin | 5.37–55.4 | ||||
| Metronidazole | 1.0 | ||||
| Chloramphenicol | 0.4 | ||||
| Carbendazim | 0.2 | ||||
| Diethofencarb | 2.5 | ||||
| Sulfabenzamide | 2.0 | ||||
| Erythromycin | 1.0 | ||||
| Enrofloxacin | 460 | ||||
| Doxycycline | 50 | ||||
| Fish farms | Chloramphenicol | 0.0548 ± 0.0099 | mexB, mexF, mexW, mexD, acrB, oprN, adeB, mexE, emrD, mdtG, mdtF, tolC, bcR, acrA, mdtH, sul1, tet32, tetM, tetO, tetT, tetW, aadA1, aadA2, catA1, emrB, matA, mefA, msrA | [204,227,228] | [135,136,137,138,139] |
| Quinolones | 1000–11,600 | ||||
| Sulfonamide | 100–7000 | ||||
| Penicillin | 11,000–20,400 | ||||
| Tetracyclines | 300–1300 | ||||
| Ciprofloxacin | 1000 |
| Bacterial Pathogens | Experimental Plant | Bacteriophage Used | Phage Family | Reference |
|---|---|---|---|---|
| P. carotovorum subsp. carotovorum, P. wasabiae | Solanum tuberosum (potato) | ΦPD10.3 and PD23.1 | Myoviridae | [14] |
| P. carotovorum subsp. carotovorum | Lactuca sativa (lettuce) | PP1 | Podoviridae | [249] |
| P. atrosepticum | Solanum tuberosum (potato) | Peat1 and phiM1 | Autographiviridae | [259] |
| Pectobacteriun | Brassica rapa (Kimchi cabbage) | phiPccP-1 | Autographiviridae | [251,260] |
| Dickeya solani | Solanum tuberosum (potato) | LIMEstone1 and LIMEstone2 | Myoviridae | [247] |
| ΦD1, ΦD10 and ΦD11 | [248] | |||
| ΦPD10.3 and PD23.1 | [14] | |||
| P. syringae pv. actinidiae | Actinidia deliciosa (kiwifruit) | PPPL-1 and KHUφ38 | Podoviridae | [261] |
| KHUφ34 | Myoviridae | [261] | ||
| Φ6 | [262] | |||
| P. syringae pv. syringae | Prunus avium (cherry) | Φ1215, Φ1226, 137, Φ358, Φ369) | [263] | |
| R. solanacearum | Musa acuminate (banana) | M5 and M8 | Podoviridae | [246] |
| Solanum lycopersicum (tomato) and Solanum tuberosum (potato) | ɸsp1 | Myoviridae | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, T.; Haque, M.A.; Barai, H.R.; Istiaq, A.; Kim, J.-J. Antibiotic Resistance in Plant Pathogenic Bacteria: Recent Data and Environmental Impact of Unchecked Use and the Potential of Biocontrol Agents as an Eco-Friendly Alternative. Plants 2024, 13, 1135. https://doi.org/10.3390/plants13081135
Islam T, Haque MA, Barai HR, Istiaq A, Kim J-J. Antibiotic Resistance in Plant Pathogenic Bacteria: Recent Data and Environmental Impact of Unchecked Use and the Potential of Biocontrol Agents as an Eco-Friendly Alternative. Plants. 2024; 13(8):1135. https://doi.org/10.3390/plants13081135
Chicago/Turabian StyleIslam, Tarequl, Md Azizul Haque, Hasi Rani Barai, Arif Istiaq, and Jong-Joo Kim. 2024. "Antibiotic Resistance in Plant Pathogenic Bacteria: Recent Data and Environmental Impact of Unchecked Use and the Potential of Biocontrol Agents as an Eco-Friendly Alternative" Plants 13, no. 8: 1135. https://doi.org/10.3390/plants13081135
APA StyleIslam, T., Haque, M. A., Barai, H. R., Istiaq, A., & Kim, J.-J. (2024). Antibiotic Resistance in Plant Pathogenic Bacteria: Recent Data and Environmental Impact of Unchecked Use and the Potential of Biocontrol Agents as an Eco-Friendly Alternative. Plants, 13(8), 1135. https://doi.org/10.3390/plants13081135








