Arbuscular Mycorrhizal Fungi Improve the Performance of Tempranillo and Cabernet Sauvignon Facing Water Deficit under Current and Future Climatic Conditions
Abstract
:1. Introduction
2. Results
2.1. Percentage of Mycorrhizal Colonization
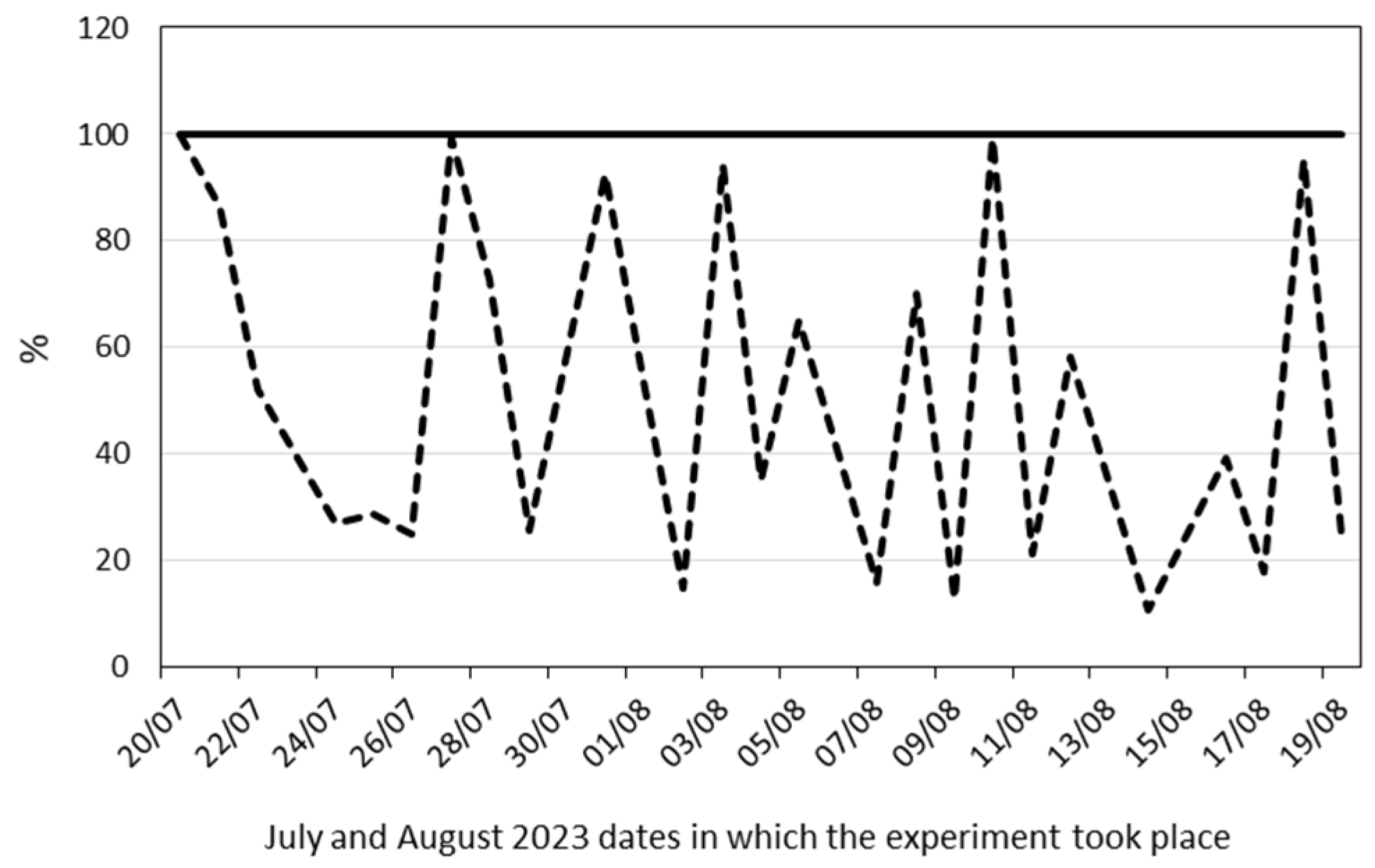
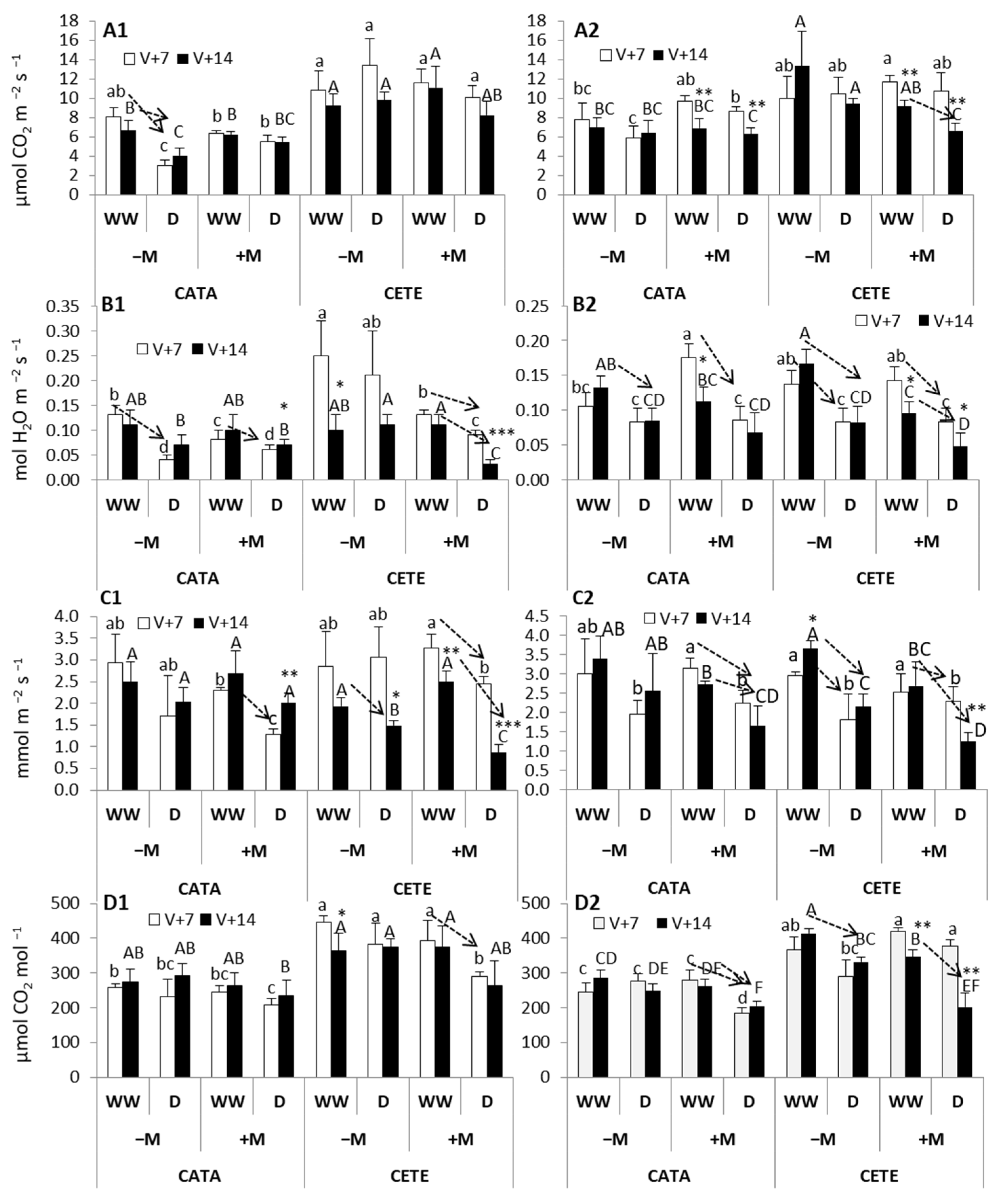
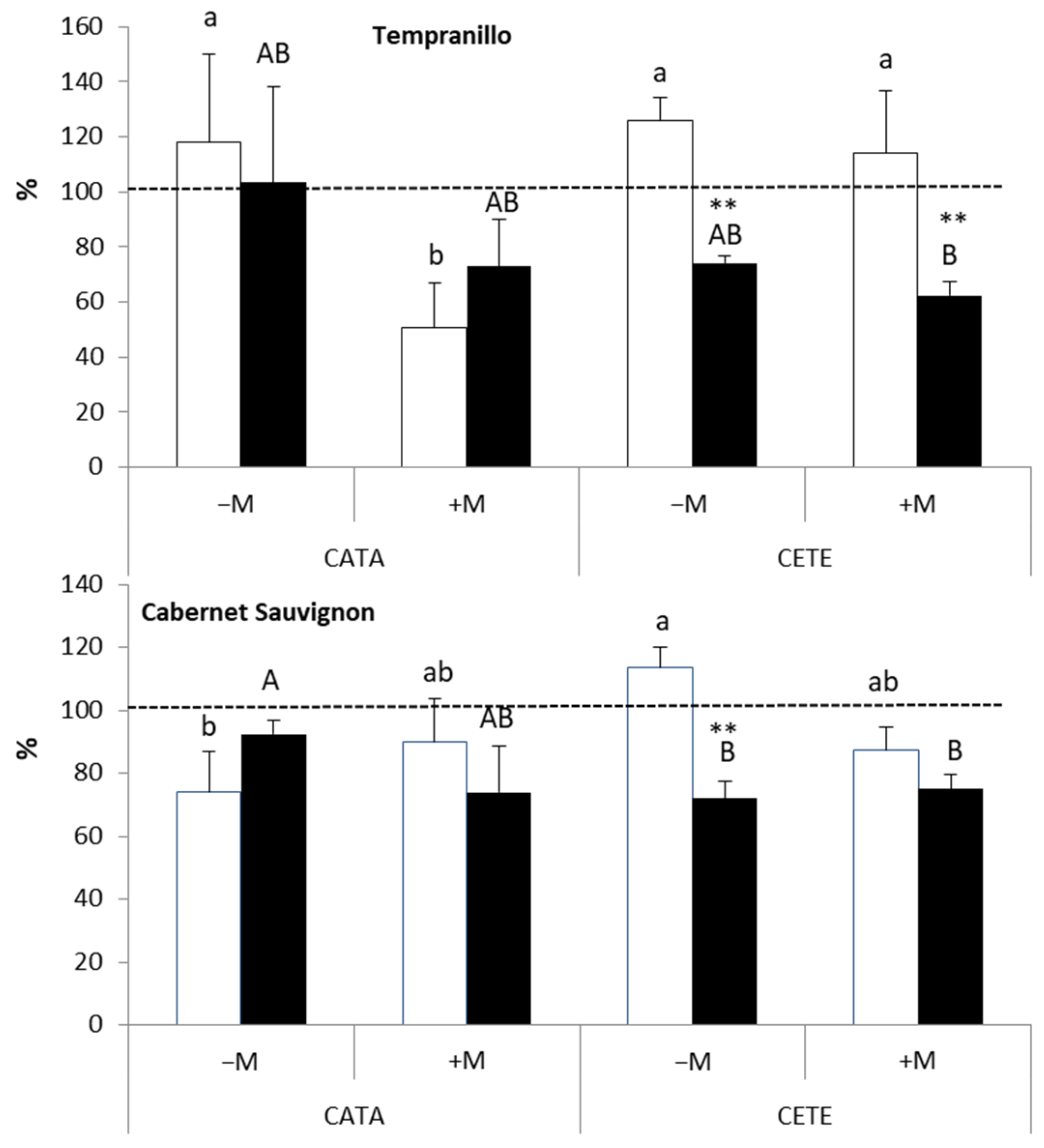
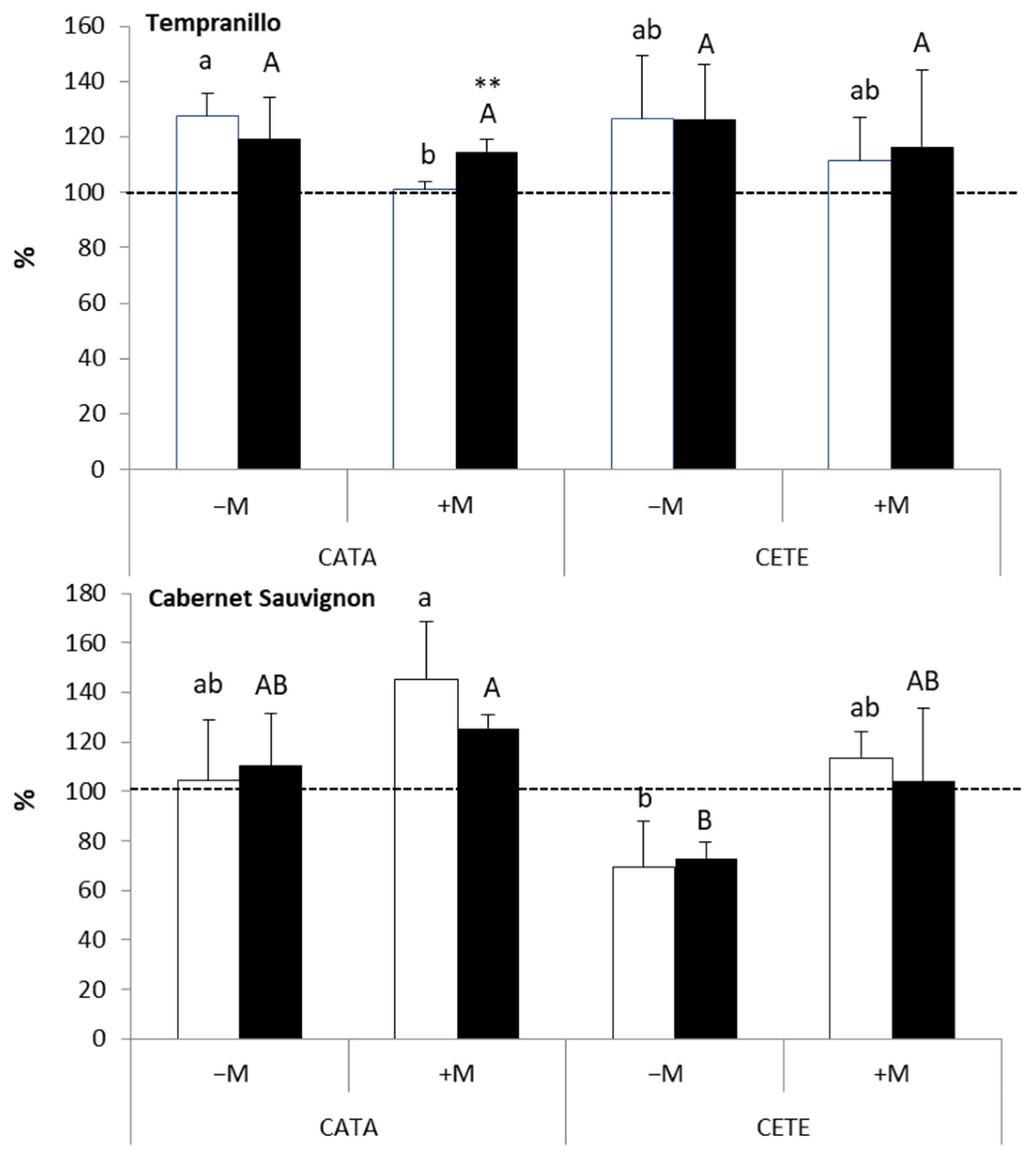
2.2. Physiological and Anatomical Responses to Water Deficit under Different Environmental Conditions
2.2.1. Early Responses
2.2.2. Late Responses
3. Discussion
3.1. Early Responses to Drought
3.2. Late Responses to Drought
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Physiological and Biochemical Determinations
4.3. Stomatal Anatomy
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Intergovernmental Panel on Climate Change. Sixth Assessment Report. Impacts, Adaptation and Vulnerability. Cross-Chapter Paper 4—Mediterranean Region. 2022. Available online: https://www.ipcc.ch/report/ar6/wg2/ (accessed on 23 October 2023).
- OIV. Statistical Report on World Vitiviniculture. International Organisation of Vine and Wine: Paris, France. 2019. Available online: https://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 20 November 2023).
- Fraga, H. Viticulture and winemaking under climate change. Agronomy 2019, 9, 783. [Google Scholar] [CrossRef]
- Droulia, F.; Charalampopoulus, I. Future climate change impacts on European Viticulture: A review on recent scientific advances. Atmosphere 2021, 12, 495. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Manzi, D.; Peruzzi, E.; Mattii, G.B. Effects of Zeowine and compost on leaf functionality and berry composition in Sangiovese grapevines. J. Agric. Sci. 2023, 161, 412–427. [Google Scholar] [CrossRef]
- Ministerio de Agricultura. Pesca y Alimentación. Gobierno de España. 2022. La uva Tempranillo se Convierte en la Principal Variedad de Vinificación Plantada en España. Available online: https://www.mapa.gob.es/fr/prensa/ultimas-noticias/la-uva-tempranillo-se-convierte-en-la--principal-variedad-de-vinificaci%C3%B3n-plantada-en-espa%C3%B1a/tcm:36-614277 (accessed on 13 February 2024).
- Torres, N.; Antolín, M.C.; Garmendia, I.; Goicoechea, N. Nutritional properties of Tempranillo grapevine leaves are affected by clonal diversity, mycorrhizal symbiosis and air temperature regime. Plant Physiol. Biochem. 2018, 130, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, T.; Lebon, E.; Coupel-Ledru, A.; Marguerit, E.; Rossdeutsch, L.; Ollat, N. Adapting plant material to face water stress in vineyards: Which physiological targets for an optimal control of plant water status? OENO One 2017, 51, 167–179. [Google Scholar] [CrossRef]
- Dargie, T.; Dor, A.; Manuel, A.; Molly, C. Responses of grapevine rootstocks to drought stress. Int. J. Plant Physiol. Biochem. 2014, 6, AA681DF42863. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P.; Navarro, J.M. Selecting rootstocks to improve vine performance and vineyard sustainabiligy in deficit irrigated Monastrell grapevines under semiarid conditions. Agric. Water Manag. 2018, 209, 73–93. [Google Scholar] [CrossRef]
- Barrios-Masias, F.H.; Knipfer, T.; McElrone, A.J. Differential responses of grapevine rootstocks to water stress are associated with adjustments in fine root hydraulic physiolohy and suberization. J. Exp. Bot. 2015, 66, 6069–6078. [Google Scholar] [CrossRef]
- De Herralde, F.; Alsina, M.M.; Aranda, X.; Savé, R.; Biel, C. Effects of rootstock and irrigation regime on hydraulic architecture of Vitis vinifera L. cv. Tempranillo. J. Int. Sci. Vigne Vin. 2006, 40, 133–139. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Abdalla, M.; Ahmed, M.A. Arbuscular mycorrhiza symbiosis enhances water status and soil-plant hydraulic conductance under drought. Front. Plant Sci. 2021, 12, 722954. [Google Scholar] [CrossRef]
- Porcel, R.; Aroca, R.; Azcón, R.; Ruiz-Lozano, J.M. PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Mol. Biol. 2006, 60, 389–404. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhang, H.; Zhang, W.; Liu, K.; Liu, M.; Shao, X. Cooperation between arbuscular mycorrhizal fungi and plant growth-promoting bacteria and their effects on plant growth and soil quality. PeerJ 2022, 10, e13080. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Minervini, F.; De Angelis, M.; Papadia, P.; Migoni, D.; Dimaglie, M.; Dinu, D.G.; Quarta, C.; Selleri, F.; Caccioppola, A.; et al. Vineyard establishment under exacerbated summer stress: Effects of mycorrhization on rootstock agronomical parameters, leaf element composition and root-associated bacterial microbiota. Plant Soil 2022, 478, 613–634. [Google Scholar] [CrossRef]
- Darriaut, R.; Lailheugue, V.; Masneuf-Pomarède, I.; Marguerit, E.; Martins, G.; Compant, S.; Ballestra, P.; Upton, S.; Ollat, N.; Lauvergeat, V. Grapevine rootstock and soil microbiome interactions: Keys for a resilient viticulture. Hortic. Res. 2022, 9, uhac019. [Google Scholar] [CrossRef] [PubMed]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S.; Siddique, K.H.M.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in Damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, N.; Antolín, M.C.; Sánchez-Díaz, M. Influence of arbuscular mycorrhizae and Rhizobium on nutrient content and water relations in drought stressed alfalfa. Plant Soil 1997, 192, 261–268. [Google Scholar] [CrossRef]
- Cochelet, N.; Ghan, R.; Toups, H.S.; Degu, A.; Tillett, R.L.; Schlauch, K.A.; Cramer, G.R. Drought tolerance of the grapevine, Vitis champinii cv. Ramsey, is associated with higher photosynthesis and greater transcriptomic responsiveness of abscisic acid biosynthesis and signalling. BMC Plant Biol. 2020, 20, 55. [Google Scholar] [CrossRef]
- Hochberg, U.; Degu, A.; Toubiana, D.; Gendler, T.; Nikoloski, Z.; Rachmilevitch, S.; Fait, A. Metabolite profiling and network analysis reveal coordinated changes in grapevine water stress response. BMC Plant Biol. 2013, 13, 184. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant-Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef]
- Hochberg, U.; Bonel, A.G.; David-Schwartz, R.; Degu, A.; Fait, A.; Cochard, H.; Peterlunger, E.; Herrera, J.C. Grapevine acclimation to water deficit: The adjustment of stomatal and hydraulic conductance differs from petiole embolism vulnerability. Planta 2017, 245, 1091–1104. [Google Scholar] [CrossRef]
- Albuquerque, C.; Scoffoni, C.; Brodersen, C.R.; Buckley, T.N.; Sack, L.; McElrone, A.J. Coordinated decline of leaf hydraulic and stomatal conductances under drought is not linked to leaf xylem embolism for different grapevine cultivars. J. Exp. Bot. 2020, 71, 7286–7300. [Google Scholar] [CrossRef]
- Lakatos, L.; Mitre, Z. Effect of drought on the future sugar content of wine grape varieties till 2100: Possible adaptation in the Hungarian Eger wine region. Biomolecules 2023, 13, 1143. [Google Scholar] [CrossRef]
- Zamorano, D.; Franck, N.; Pastenes, C.; Wallberg, B.; Garrido, M.; Silva, H. Improved physiological performance in grapevine (Vitis vinifera L.) cv. Cabernet Sauvignon facing recurrent drought stress. Aust. J. Grape Wine Res. 2021, 27, 258–268. [Google Scholar] [CrossRef]
- Lehr, P.P.; Hernández-Montes, E.; Ludwig-Müller, J.; Keller, M.; Zörb, C. Abscisic acid and proline are not equivalent markers for heat, drought and combined stress in grapevines. Aust. J. Grape Wine Res. 2021, 28, 119–130. [Google Scholar] [CrossRef]
- Wang, W.-N.; Min, Z.; Wu, J.-R.; Liu, B.-C.; Xu, X.-L.; Fang, Y.-L.; Ju, Y.-L. Physiological and transcriptomic analysis of Cabernet Sauvignon (Vitis vinifera L.) reveals the alleviating effect of exogenous strigolactones on the response of grapevine to drought stress. Plant Physiol. Biochem. 2021, 167, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, A.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal simbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Kelly, J.G.; Duursma, R.A.; Atwell, B.J.; Tissue, D.T.; Medlyn, B.E. Drought × CO2 interactions in trees: A test of the low-intercellular CO2 concentration (Ci) mechanism. New Phytol. 2015, 209, 166–1612. [Google Scholar] [CrossRef]
- Boso, S.; Gago, P.; Alonso-Villaverde, J.L.; Santiago, J.L.; Martínez, M.C. Density and size of stomata in the leaves of different hybrids (Vitis sp.) and Vitis vinifera varieties. Vitis 2016, 55, 17–22. [Google Scholar] [CrossRef]
- Driesen, E.; De Proft, M.; Saeys, W. Drought stress triggers alterations of adaxial and abaxial stomatal development in basil leaves increasing water-use efficiency. Hortic. Res. 2023, 10, uhad075. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, L.T.; Caine, R.S.; Gray, J.E. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front. Plant Sci. 2019, 10, 225. [Google Scholar] [CrossRef]
- Jiao, Z.; Han, S.; Li, Z.; Huang, M.; Niu, M.-X.; Yu, X.; Liu, C.; Wang, H.-L.; Yin, W.; Xia, X. PdEPFL6 reduces stomatal density to improve drought tolerance in poplar. Ind. Crops Prod. 2022, 182, 114873. [Google Scholar] [CrossRef]
- Lawson, T.; Blatt, M.R. Stomatal size, speed, and responsiveness impact on photosynthesis and water use efficiency. Plant Physiol. 2014, 164, 1556–1570. [Google Scholar] [CrossRef] [PubMed]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- IPCC. Intergovernmental Panel on Climate Change. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Ollat, N.; Geny, L.; Soyer, J.-P. Grapevine fruiting cuttings: Validation of an experimental system to study grapevine physiology. I. Main vegetative characteristics. OENO One 1998, 32, 1–9. [Google Scholar] [CrossRef]
- Resúmenes Climatológicos Mensuales. Available online: https://meteo.navarra.es/estaciones/resumenesclimatologicos.cfm (accessed on 15 February 2024).
- Lovisolo, C.; Hartung, W.; Schubert, A. Whole-plant hydraulic conductance and root-to-shoot flow of abscisic acid are independently affected by water stress in grapevines. Funct. Plant Biol. 2002, 29, 1349–1356. [Google Scholar] [CrossRef]
- Goicoechea, N.; Torres, N.; Garmendia, I.; Hilbert, G.; Antolín, M.C. Mycorrhizal symbiosis improve fruit quality in Tempranillo grapevine sensitive to low-moderate warming. Sci. Hortic. 2023, 315, 111993. [Google Scholar] [CrossRef]




| Proline | TSS | ||||||
|---|---|---|---|---|---|---|---|
| 7 Days | 14 Days | 7 Days | 14 Days | ||||
| T | CATA | −M | WW | 2.49 a | 1.29 b | 68.17 b | 89.78 a |
| D | 1.77 a | 1.29 b | 53.31 b | 108.00 a | |||
| +M | WW | 1.29 a | 2.28 a | 104.87 ab | 112.75 a | ||
| D | 1.37 a | 0.96 b | 118.65 a | 104.61 a | |||
| CETE | −M | WW | 0.48 AB | 1.33 A | 85.38 AB | 114.11 A | |
| D | 0.26 B | 0.30 B | 98.52 AB | 129.83 A | |||
| +M | WW | 0.49 AB | 1.57 A | 84.18 B | 109.19 A | ||
| D | 0.56 A | 0.81 A | 101.27 A | 118.34 A | |||
| CS | CATA | −M | WW | 0.43 b | 1.01 a | 100.75 a | 115.47 a |
| D | 0.35 b | 1.07 a | 108.65 a | 132.07 a | |||
| +M | WW | 0.52 b | 1.72 a | 98.38 a | 125.23 a | ||
| D | 1.13 a | 1.30 a | 113.15 a | 127.65 a | |||
| CETE | −M | WW | 0.90 A | 0.52 B | 105.34 A | 118.02 A | |
| D | 0.31 A | 0.74 B | 92.32 A | 106.18 A | |||
| +M | WW | 0.55 A | 1.40 A | 101.76 A | 134.57 A | ||
| D | 0.59 A | 1.13 A | 113.16 A | 101.17 A | |||
| Stomatal Length (µm) | Stomatal Width (µm) | Stomatal Area (µm2) | ||||
|---|---|---|---|---|---|---|
| CATA | CETE | CATA | CETE | CATA | CETE | |
| T | 28.00 a | 28.88 a | 18.25 a | 17.75 a | 404.08 a | 406.97 a |
| CS | 28.75 a | 26.60 a | 19.44 a | 15.20 b | 445.73 a | 319.45 b |
| Average of the Maximum | Average of the Minimum | Absolute Maximum | Absolute Minimum | |
|---|---|---|---|---|
| June | 26.6 | 15.7 | 33.4 | 12.6 |
| July | 28.8 | 16.3 | 36.3 | 13.5 |
| August | 30.7 | 15.5 | 40.4 | 9.4 |
| September | 26.7 | 14.9 | 33.3 | 8.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozikova, D.; Pascual, I.; Goicoechea, N. Arbuscular Mycorrhizal Fungi Improve the Performance of Tempranillo and Cabernet Sauvignon Facing Water Deficit under Current and Future Climatic Conditions. Plants 2024, 13, 1155. https://doi.org/10.3390/plants13081155
Kozikova D, Pascual I, Goicoechea N. Arbuscular Mycorrhizal Fungi Improve the Performance of Tempranillo and Cabernet Sauvignon Facing Water Deficit under Current and Future Climatic Conditions. Plants. 2024; 13(8):1155. https://doi.org/10.3390/plants13081155
Chicago/Turabian StyleKozikova, Daria, Inmaculada Pascual, and Nieves Goicoechea. 2024. "Arbuscular Mycorrhizal Fungi Improve the Performance of Tempranillo and Cabernet Sauvignon Facing Water Deficit under Current and Future Climatic Conditions" Plants 13, no. 8: 1155. https://doi.org/10.3390/plants13081155
APA StyleKozikova, D., Pascual, I., & Goicoechea, N. (2024). Arbuscular Mycorrhizal Fungi Improve the Performance of Tempranillo and Cabernet Sauvignon Facing Water Deficit under Current and Future Climatic Conditions. Plants, 13(8), 1155. https://doi.org/10.3390/plants13081155







