Rosarugosides A and D from Rosa rugosa Flower Buds: Their Potential Anti-Skin-Aging Effects in TNF-α-Induced Human Dermal Fibroblasts
Abstract
:1. Introduction
2. Results
2.1. Identification of Compounds 1–9 Isolated from R. rugosa Flower Buds
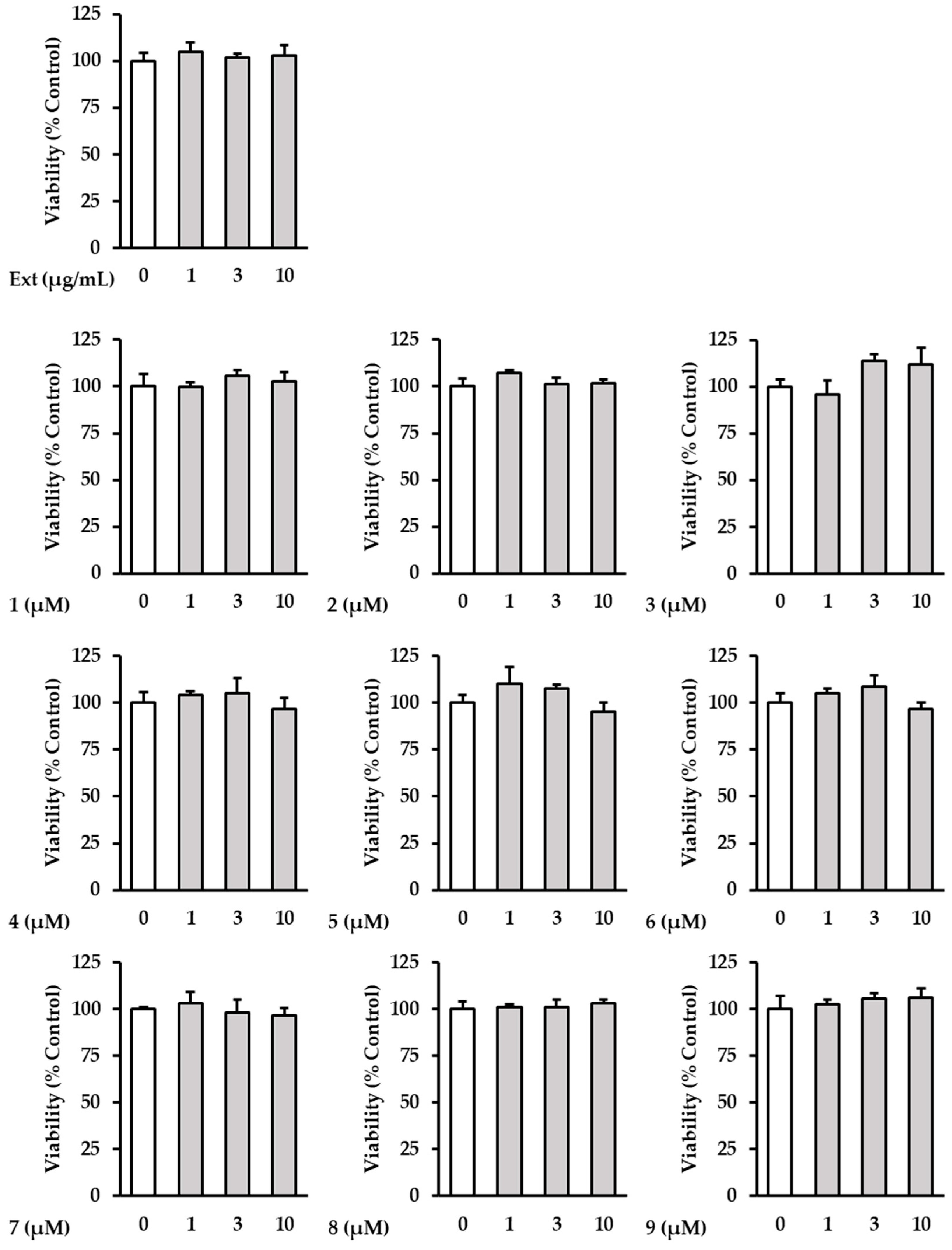
2.2. The Effects of Hot Water Extracts of R. rugosa Flower Buds and Compounds 1–9 on NHDF Cell Viability
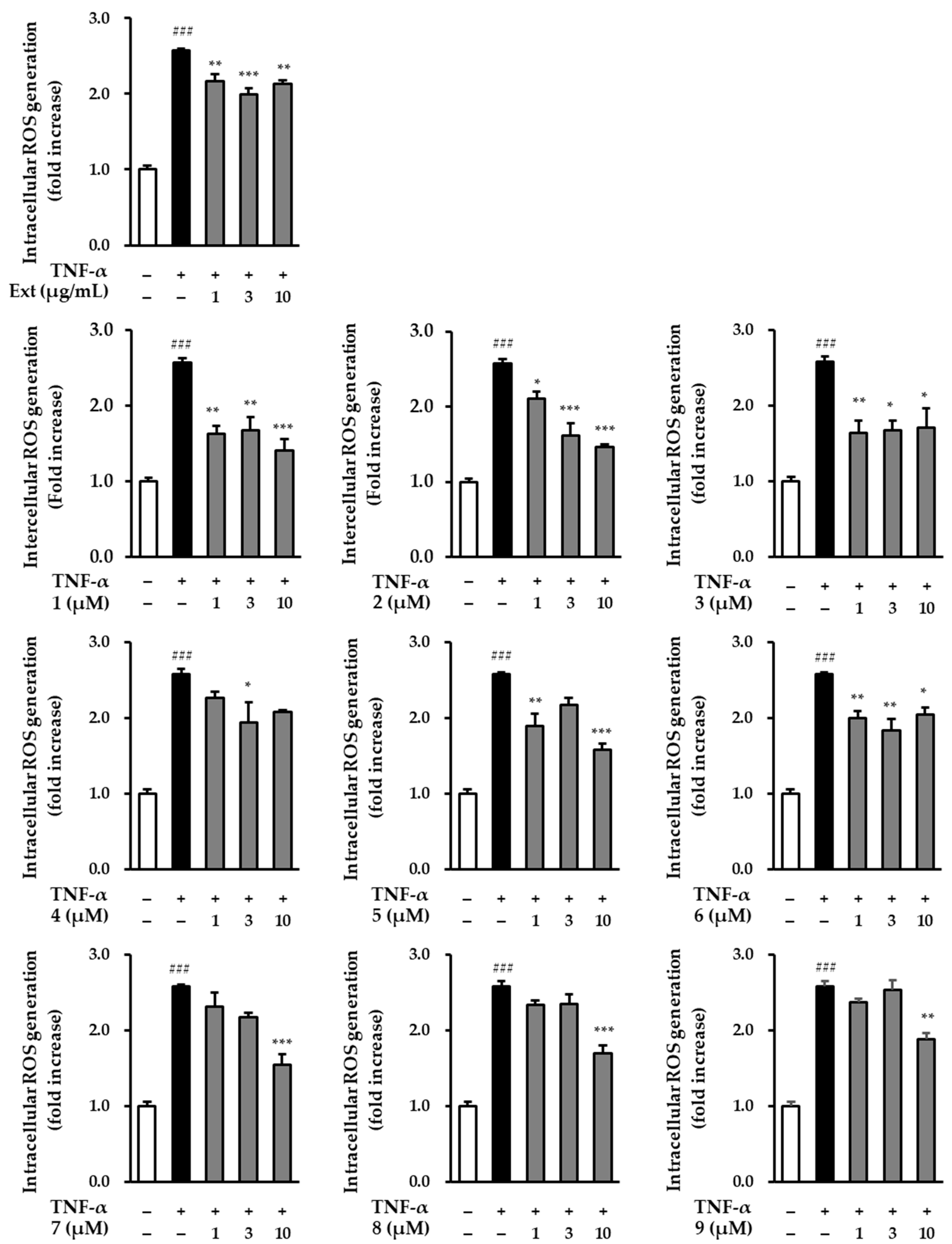
2.3. The Effect of Hot Water Extracts of R. rugosa Flower Buds and Compounds 1–9 on TNF-α-Induced Intracellular ROS Production in NHDFs
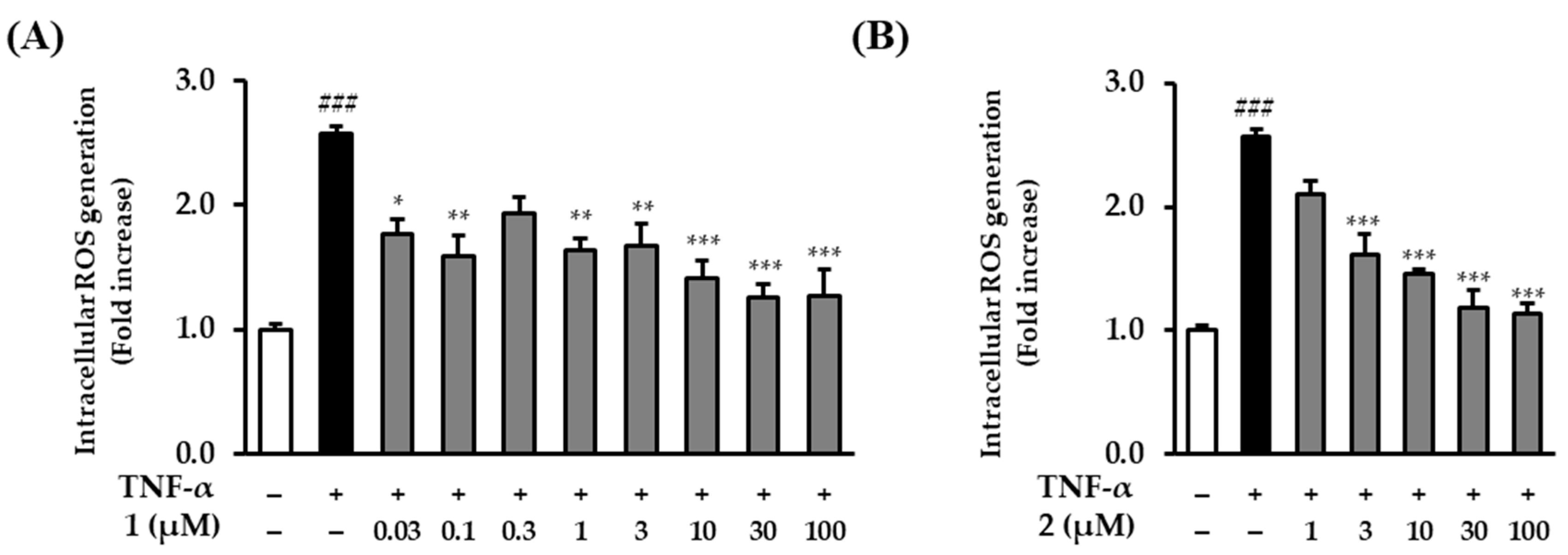
2.4. The Effects of Rosarugosides D (1) and A (2) on TNF-α-Induced Intracellular ROS Production in NHDFs
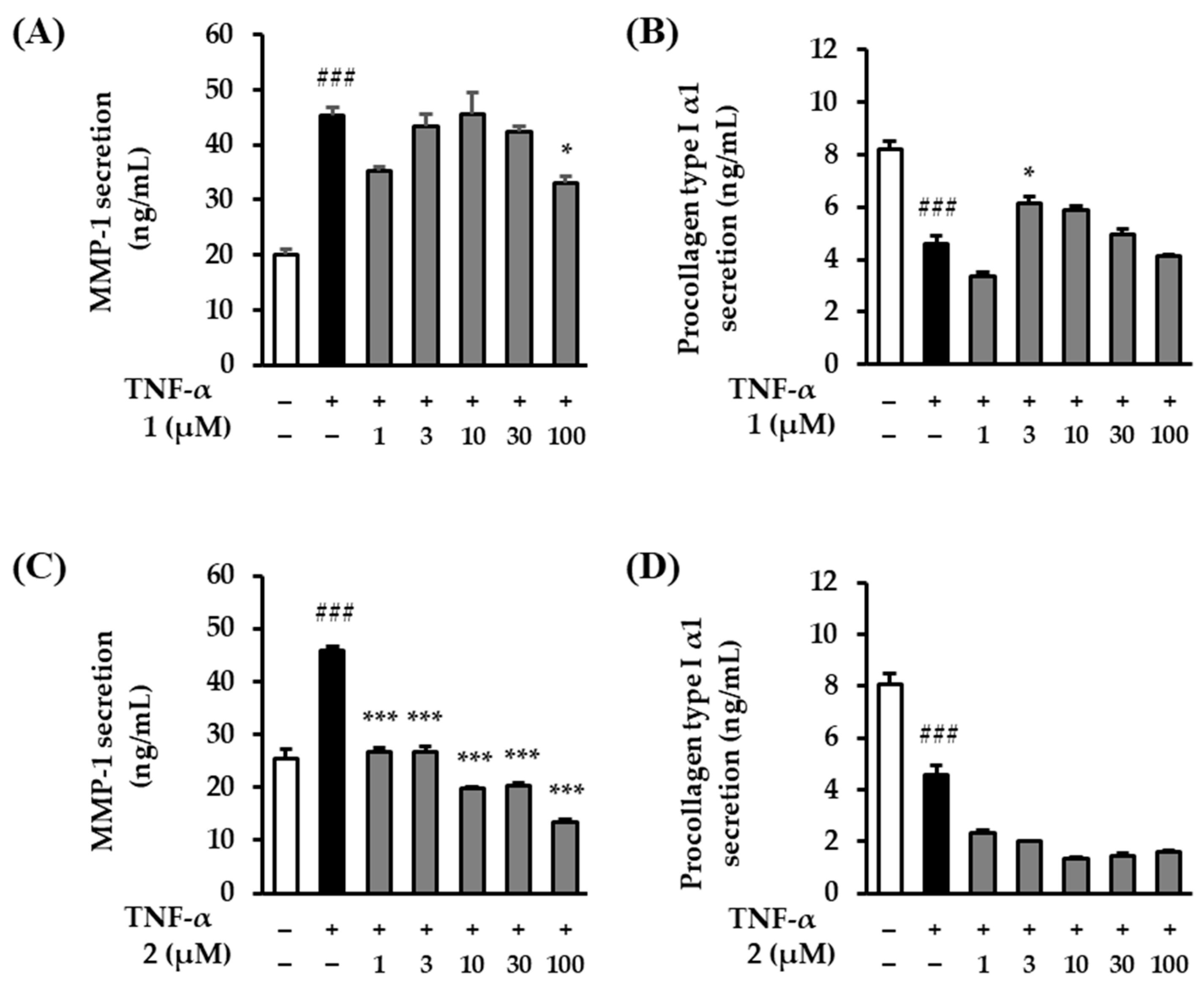
2.5. The Effects of Rosarugosides D (1) and A (2) on TNF-α-Induced MMP-1 and Procollagen Type Ι α1 Expressions in NHDFs
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Identification of Sugar
4.4. Cell Culture
4.5. Cell Viability
4.6. Intracellular Reactive Oxygen Species (ROS)
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kelager, A.; Pedersen, J.S.; Bruun, H.H. Multiple introductions and no loss of genetic diversity: Invasion history of Japanese Rose, Rosa rugosa, in Europe. Biol. Invasions 2013, 15, 1125–1141. [Google Scholar] [CrossRef]
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci.Technol. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Olech, M.; Nowak, R.; Los, R.; Rzymowska, J.; Malm, A.; Chrusciel, K. Biological activity and composition of teas and tinctures prepared from Rosa rugosa Thunb. Open Life Sci. 2012, 7, 172–182. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, W. Antihypertensive activity of Rosa rugosa Thunb. flowers: Angiotensin I converting enzyme inhibitor. J. Ethnopharmacol. 2012, 144, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Hashidoko, Y. The phytochemistry of Rosa rugosa. Phytochemistry 1996, 43, 535–549. [Google Scholar] [CrossRef]
- Okuda, T.; Hatano, T.; Yazaki, K.; Ogawa, N. Rugosin A, B, C and praecoxin A, tannins having a valoneoyl group. Chem. Pharm. Bull. 1982, 30, 4230–4233. [Google Scholar] [CrossRef]
- Okuda, T.; Hatano, T.; Ogawa, N. Rugosin D, E, F and G, dimeric and trimeric hydrolyzable tannins. Chem. Pharm. Bull. 1982, 30, 4234–4237. [Google Scholar] [CrossRef]
- Kuo, P.-L.; Hsu, Y.-L.; Lin, T.-C.; Tzeng, W.-S.; Chen, Y.-Y.; Lin, C.-C. Rugosin E, an ellagitannin, inhibits MDA-MB-231 human breast cancer cell proliferation and induces apoptosis by inhibiting nuclear factor-κB signaling pathway. Cancer Lett. 2007, 248, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Uenishi, Y.; Inagaki, Y.; Kurokawa, M.; Kawabata, J. Isolation of rugosin A, B and related compounds as dipeptidyl peptidase-IV inhibitors from rose bud extract powder. Biosci. Biotechnol. Biochem. 2016, 80, 2087–2092. [Google Scholar] [CrossRef]
- Nitta, Y.; Kikuzaki, H.; Ueno, H. Novel inhibitors for histidine decarboxylase from plant components. Int. Biol. Rev. 2017, 1. [Google Scholar] [CrossRef]
- Chang, S.W.; Du, Y.E.; Qi, Y.; Lee, J.S.; Goo, N.; Koo, B.K.; Bae, H.J.; Ryu, J.H.; Jang, D.S. New depsides and neuroactive phenolic glucosides from the flower buds of rugosa rose (Rosa rugosa). J. Agric. Food Chem. 2019, 67, 7289–7296. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-S.; Choi, J.-E.; Woo, W.-H.; Mun, Y.-J. The Study on Pharmacological Activation as Cosmetic Material of Rosa rugosa Thunb. Flowers Extract. Korean J. Acupunct. 2014, 31, 188–194. [Google Scholar] [CrossRef]
- Ng, T.; Gao, W.; Li, L.; Niu, S.; Zhao, L.; Liu, J.; Shi, L.; Fu, M.; Liu, F. Rose (Rosa rugosa)-flower extract increases the activities of antioxidant enzymes and their gene expression and reduces lipid peroxidation. Biochem. Cell Biol. 2005, 83, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Mun, Y.; Woo, W.; Song, J. Anti-melanogenic effects of ethanol extracts from Rosa rugosa Thunb. J. Korean Soc. Cosmetol. 2014, 20, 39–41. [Google Scholar]
- Ya, W.; Chun-Meng, Z.; Tao, G.; Yi-Lin, Z.; Ping, Z. Preliminary screening of 44 plant extracts for anti-tyrosinase and antioxidant activities. Pak. J. Pharm. Sci. 2015, 28, 1737–1744. [Google Scholar] [PubMed]
- Zhang, C.; Zhao, F.; Li, R.; Wu, Y.; Liu, S.; Liang, Q. Purification, characterization, antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals. Int. J. Biol. Macromol. 2019, 124, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Makrantonaki, E.; Bekou, V.; Zouboulis, C.C. Genetics and skin aging. Dermato-Endocrinol. 2012, 4, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef]
- Papaccio, F.; D′Arino, A.; Caputo, S.; Bellei, B. Focus on the contribution of oxidative stress in skin aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Kim, J.-S. Cherry fruit anthocyanins cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside protect against blue light-induced cytotoxicity in HaCaT cells. Appl. Biol. Chem. 2023, 66, 3. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Oh, S.-R.; Park, S.-K.; Lee, P.; Kim, Y.-M. The ginsenoside Rg2 downregulates MMP-1 expression in keratinocyte (HaCaT)-conditioned medium-treated human fibroblasts (Hs68). Appl. Biol. Chem. 2023, 66, 85. [Google Scholar] [CrossRef]
- Tu, Y.; Quan, T. Oxidative stress and human skin connective tissue aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, E.-H.; Cho, E.-B.; Kim, D.-H.; Kim, B.-O.; Kang, I.-k.; Jung, H.-Y.; Cho, Y.-J. Protective effects of galangin against UVB irradiation-induced photo-aging in CCD-986sk human skin fibroblasts. Appl. Biol. Chem. 2019, 62, 1–8. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Lee, E.-H.; Yoo, J.; Kwon, S.-I.; Choi, H.W.; Kang, I.-K. Analysis of functional properties in the new Korean apple cultivar Arisoo. Hortic. Environ. Biotechnol. 2019, 60, 787–795. [Google Scholar] [CrossRef]
- Shekhter, A.B.; Balakireva, A.V.; Kuznetsova, N.V.; Vukolova, M.N.; Litvitsky, P.F.; Zamyatnin, A.A., Jr. Collagenolytic enzymes and their applications in biomedicine. Curr. Med. Chem. 2019, 26, 487–505. [Google Scholar] [CrossRef]
- Min, D.-H.; Yu, Y.-B.; Kim, T.-H.; Kim, H.; Lee, S. Pharmacological effects of pentacyclic triterpenoids isolated from Centella asiatica. Hortic. Environ. Biotechnol. 2024, 65, 189–197. [Google Scholar] [CrossRef]
- Phung, H.M.; Lee, S.; Hong, S.; Lee, S.; Jung, K.; Kang, K.S. Protective effect of polymethoxyflavones isolated from Kaempferia parviflora against TNF-α-induced human dermal fibroblast damage. Antioxidants 2021, 10, 1609. [Google Scholar] [CrossRef]
- Xie, J.; Li, M.-X.; Du, Z.-Z. Chemical compounds, anti-aging and antibacterial properties of Rosa rugosa purple branch. Ind. Crops Prod. 2022, 181, 114814. [Google Scholar] [CrossRef]
- Krammer, G.E.; Buttery, R.G.; Takeoka, G.R. Studies on Tomato Glycosides; ACS Publications: Washington, DC, USA, 1995. [Google Scholar]
- Kim, K.H.; Lee, K.H.; Choi, S.U.; Kim, Y.H.; Lee, K.R. Terpene and phenolic constituents of Lactuca indica L. Arch. Pharm. Res. 2008, 31, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, C.; Pan, L.; Benatrehina, P.A.; Chai, H.; Keller, W.J.; Naman, C.B.; Kinghorn, A.D. Bioassay-guided isolation of antioxidant and cytoprotective constituents from a maqui berry (Aristotelia chilensis) dietary supplement ingredient as markers for qualitative and quantitative analysis. J. Agric. Food Chem. 2017, 65, 8634–8642. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, S.; Wang, Y.; Huang, K. Target-guided isolation and purification of antioxidants from Selaginella sinensis by offline coupling of DPPH-HPLC and HSCCC experiments. J. Chromatogr. B 2011, 879, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; He, K.; Roller, M.; Zheng, B.; Chen, X.; Shao, Z.; Peng, T.; Zheng, Q. Active compounds from Lagerstroemia speciosa, insulin-like glucose uptake-stimulatory/inhibitory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J. Agric. Food Chem. 2008, 56, 11668–11674. [Google Scholar] [CrossRef] [PubMed]
- Hirokane, T.; Hirata, Y.; Ishimoto, T.; Nishii, K.; Yamada, H. A unified strategy for the synthesis of highly oxygenated diaryl ethers featured in ellagitannins. Nat. Commun. 2014, 5, 3478. [Google Scholar] [CrossRef] [PubMed]
- Borg, M.; Brincat, S.; Camilleri, G.; Schembri-Wismayer, P.; Brincat, M.; Calleja-Agius, J. The role of cytokines in skin aging. Climacteric 2013, 16, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Langton, A.K.; Halai, P.; Griffiths, C.E.; Sherratt, M.J.; Watson, R.E. The impact of intrinsic ageing on the protein composition of the dermal-epidermal junction. Mech. Ageing Dev. 2016, 156, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and function of the epidermis related to barrier properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef]
- Nielsen, K.P.; Zhao, L.; Stamnes, J.J.; Stamnes, K.; Moan, J. The importance of the depth distribution of melanin in skin for DNA protection and other photobiological processes. J. Photochem. Photobiol. B 2006, 82, 194–198. [Google Scholar] [CrossRef]
- Supp, D.M.; Boyce, S.T. Engineered skin substitutes: Practices and potentials. Dermatol. Clin. 2005, 23, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, B.; Ali, J.; Baboota, S. Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int. J. Dermatol. 2018, 57, 646–660. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, D.; Koxholt, I.; Thiemann, K.; Nischt, R. Skin basement membrane: The foundation of epidermal integrity—BM functions and diverse roles of bridging molecules nidogen and perlecan. BioMed Res. Int. 2013, 2013, 179784. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol. 2020, 8, 100041. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharm. Pharm. 2012, 3, 118–126. [Google Scholar]
- Budden, T.; Gaudy-Marqueste, C.; Porter, A.; Kay, E.; Gurung, S.; Earnshaw, C.H.; Roeck, K.; Craig, S.; Traves, V.; Krutmann, J. Ultraviolet light-induced collagen degradation inhibits melanoma invasion. Nat. Commun. 2021, 12, 2742. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.; Sharma, M.; Werth, V. TNF-α production in the skin. Arch. Dermatol. Res. 2009, 301, 87–91. [Google Scholar] [CrossRef]
- Marcos-Garcés, V.; Molina Aguilar, P.; Bea Serrano, C.; García Bustos, V.; Benavent Seguí, J.; Ferrández Izquierdo, A.; Ruiz-Saurí, A. Age-related dermal collagen changes during development, maturation and ageing–a morphometric and comparative study. J. Anat. 2014, 225, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Rodriguez, S.; Folgueras, A.R.; Lopez-Otin, C. The role of matrix metalloproteinases in aging: Tissue remodeling and beyond. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2015–2025. [Google Scholar] [CrossRef]
- Glasauer, A.; Chandel, N.S. Targeting antioxidants for cancer therapy. Biochem. Pharmacol. 2014, 92, 90–101. [Google Scholar] [CrossRef]
- Mellone, M.; Hanley, C.J.; Thirdborough, S.; Mellows, T.; Garcia, E.; Woo, J.; Tod, J.; Frampton, S.; Jenei, V.; Moutasim, K.A. Induction of fibroblast senescence generates a non-fibrogenic myofibroblast phenotype that differentially impacts on cancer prognosis. Aging 2017, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.W.; Kwon, H.-Y.; Park, Y.; Turk, A.; Lee, S.; Ryu, S.H.; Han, Y.K.; Lee, K.Y.; Ela, M.A.; Hwang, B.Y. Antioxidant and α-glucosidase inhibitory potential of the pollen of Hibiscus spp. Hortic. Environ. Biotechnol. 2024, 1–9. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant polyphenols, more than just simple natural antioxidants: Oxidative stress, aging and age-related diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Chintha, P.; Sarkar, D.; Pecota, K.; Dogramaci, M.; Hatterman-Valenti, H.; Shetty, K. Phenolic bioactive-linked antioxidant, anti-hyperglycemic, and antihypertensive properties of sweet potato cultivars with different flesh color. Hortic. Environ. Biotechnol. 2023, 64, 877–893. [Google Scholar] [CrossRef]
- Liu, J.-K. Natural products in cosmetics. Nat. Rev. Immunol. 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Parks, W.C.; Wilson, C.L.; López-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Mirastschijski, U.; Lupše, B.; Maedler, K.; Sarma, B.; Radtke, A.; Belge, G.; Dorsch, M.; Wedekind, D.; McCawley, L.J.; Boehm, G. Matrix metalloproteinase-3 is key effector of TNF-α-induced collagen degradation in skin. Int. J. Mol. Sci. 2019, 20, 5234. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Varani, J.; Perone, P.; Fligiel, S.E.; Fisher, G.J.; Voorhees, J.J. Inhibition of type I procollagen production in photodamage: Correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J. Investig. Dermatol. 2002, 119, 122–129. [Google Scholar] [CrossRef]
- Naomi, R.; Ridzuan, P.M.; Bahari, H. Current insights into collagen type I. Polymers 2021, 13, 2642. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.-Y.; Kim, J.-H.; Baek, D.-S.; Kim, S.-J.; Kang, S.; Yang, W.S.; Song, J.-A.; Lee, M.-S.; Kim, S.; Kim, Y.-S. Production of recombinant human procollagen type I C-terminal propeptide and establishment of a sandwich ELISA for quantification. Sci. Rep. 2017, 7, 15946. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiratchayamaethasakul, C.; Lee, S.-H. Protocatechuic aldehyde attenuates UVA-induced photoaging in human dermal fibroblast cells by suppressing MAPKs/AP-1 and NF-κB signaling pathways. Int. J. Mol. Sci. 2020, 21, 4619. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, anti-inflammatory, and anti-aging properties of mycosporine-like amino acids: Molecular and cellular mechanisms in the protection of skin-aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [PubMed]

 ) and NOESY (B,
) and NOESY (B,  ) correlations of compound 1.
) correlations of compound 1.
| Position | δH Multi (J in Hz) | δC | Position | δH Multi (J in Hz) | δC |
|---|---|---|---|---|---|
| 1 | 109.4 | 4′ | 150.5 | ||
| 2 | 156.4 | 5′ | 6.94 d (8.5) | 115.7 | |
| 3 | 6.67 d (2.5) | 101.5 | 6′ | 7.59 dd (8.5, 2.0) | 124.4 |
| 4 | 156.4 | 7′ | 166.4 | ||
| 5 | 6.48 d (2.0) | 104.6 | Glc-1″ | 5.04 d (7.0) | 100.9 |
| 6 | 150.2 | Glc-2″ | 3.55–3.61 m a | 72.8 | |
| 7 | 3.61 m a | 29.0 | Glc-3″ | 3.55–3.61 m a | 75.6 |
| 8 | 176.0 | Glc-4″ | 3.48 t (9.5) | 69.3 | |
| 1′ | 119.9 | Glc-5″ | 3.55–3.61 m a | 76.2 | |
| 2′ | 7.55 d (2.5) | 117.3 | Glc-6″ | 3.74 dd (12.5, 5.5) 3.92 dd (12.5, 2.0) | 60.5 |
| 3′ | 143.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.S.; Son, S.-R.; Choi, Y.J.; Kim, Y.; Ahn, S.-Y.; Jang, D.S.; Lee, S. Rosarugosides A and D from Rosa rugosa Flower Buds: Their Potential Anti-Skin-Aging Effects in TNF-α-Induced Human Dermal Fibroblasts. Plants 2024, 13, 1266. https://doi.org/10.3390/plants13091266
Kim KS, Son S-R, Choi YJ, Kim Y, Ahn S-Y, Jang DS, Lee S. Rosarugosides A and D from Rosa rugosa Flower Buds: Their Potential Anti-Skin-Aging Effects in TNF-α-Induced Human Dermal Fibroblasts. Plants. 2024; 13(9):1266. https://doi.org/10.3390/plants13091266
Chicago/Turabian StyleKim, Kang Sub, So-Ri Son, Yea Jung Choi, Yejin Kim, Si-Young Ahn, Dae Sik Jang, and Sullim Lee. 2024. "Rosarugosides A and D from Rosa rugosa Flower Buds: Their Potential Anti-Skin-Aging Effects in TNF-α-Induced Human Dermal Fibroblasts" Plants 13, no. 9: 1266. https://doi.org/10.3390/plants13091266
APA StyleKim, K. S., Son, S.-R., Choi, Y. J., Kim, Y., Ahn, S.-Y., Jang, D. S., & Lee, S. (2024). Rosarugosides A and D from Rosa rugosa Flower Buds: Their Potential Anti-Skin-Aging Effects in TNF-α-Induced Human Dermal Fibroblasts. Plants, 13(9), 1266. https://doi.org/10.3390/plants13091266







