Sequential Defense Strategies: From Ant Recruitment to Leaf Toughness
Abstract
:1. Introduction
| Overview | Prediction | Approach | Resource |
|---|---|---|---|
| H1. There is a turnover of defenses throughout leaf development | |||
| Survey 1—Physical defense | Physical defense peaks when leaves are fully expanded | Evaluation of leaf toughness | Figure 1A |
| Survey 2—Indirect defense (EFNs) | Indirect defense peaks on new flushed leaves | Evaluation of EFN activity | Figure 1B |
| H2. Ants act as an indirect defense | |||
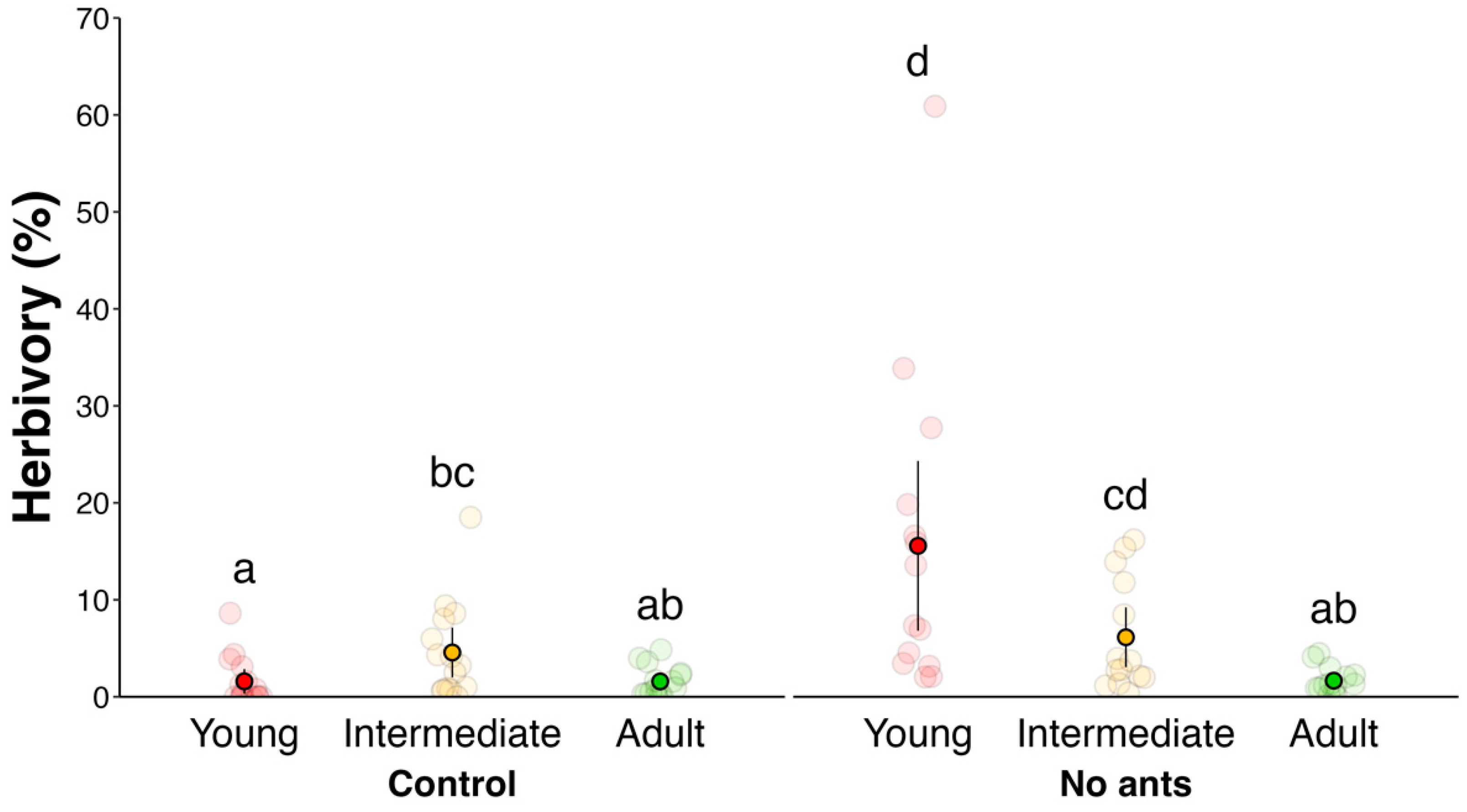
| Experiment 1 | A: Ants decrease total herbivory rate | Evaluation of herbivory rate with and without ants | Figure 2 |
| B: Ants reduce herbivory on earlier foliar stages | Evaluation of herbivory rate throughout leaf development | Figure 2 | |
| H3. Simulated herbivory increases ant visitation, decreasing herbivore numbers | |||
| Experiment 2 | Different intensities of foliar simulated herbivory have different impacts on ant abundance | Assess ant attendance after foliar simulated herbivory | Figure 3A |
| Experiment 3 | Abundance of herbivores vary over time after simulated herbivory | Observation of herbivores along time after simulated herbivory | Figure 3B |
| H4. Different damage intensities impact differently the asymmetry of new flushed leaves | |||
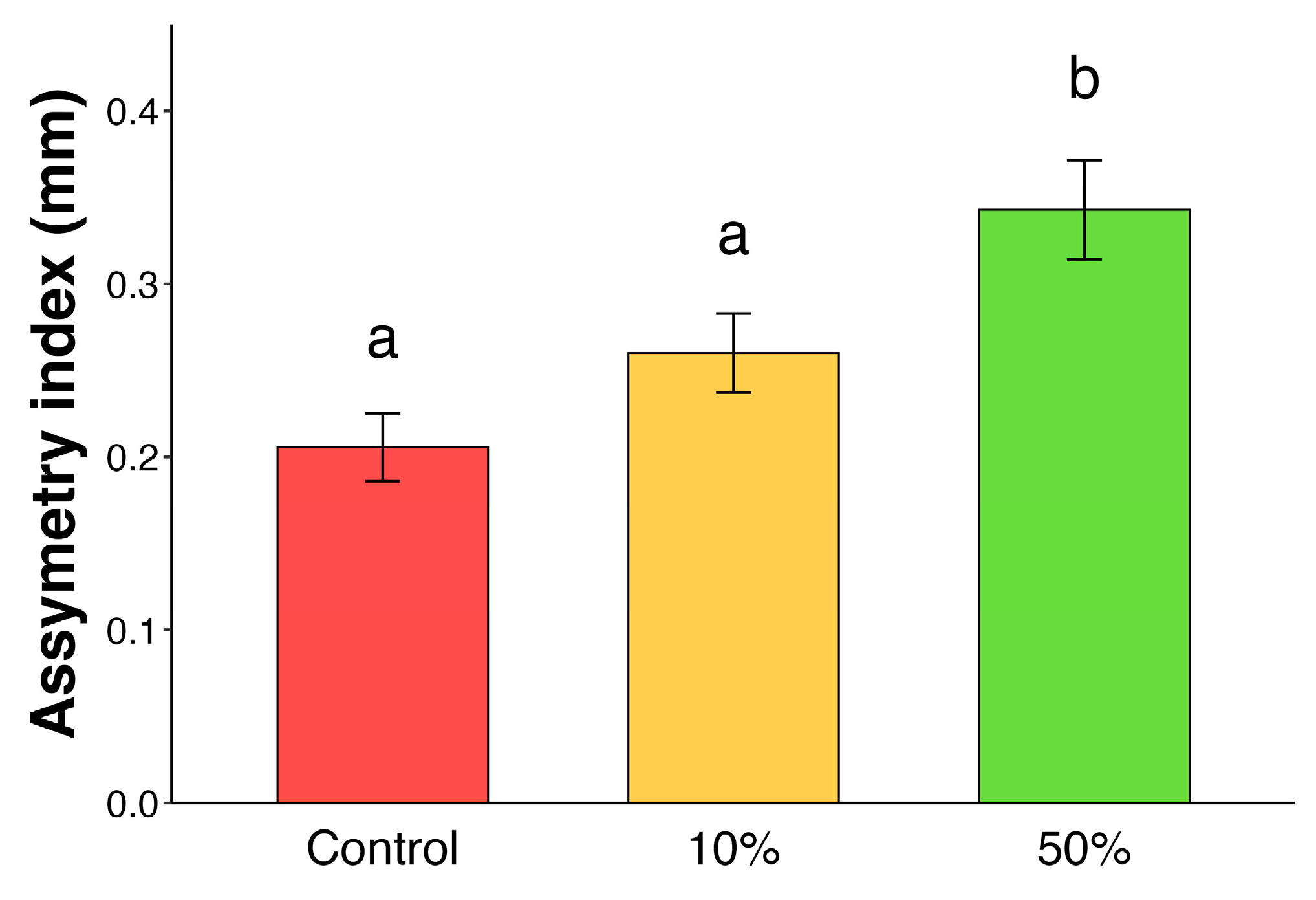
| Experiment 4 | High intensities of simulated herbivory affect asymmetry of new flushing leaves | Measurement of new flushing leaf asymmetry after simulated herbivory | Figure 4 |


2. Results
3. Discussion
3.1. Overview
3.2. Before Damage—Turnover in Defense Strategies
3.3. After damage—Induced indirect defense and tolerance
3.4. Conclusions
4. Materials and Methods
4.1. Study Site and Plant Species
4.2. Experiments and Surveys
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoonhoven, L.M.; Loon, J.J.A.v.; Dicke, M. Insect-Plant Biology; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Boege, K.; Marquis, R.J. Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. 2005, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant Defense against Insect Herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef]
- Mitchell, C.; Brennan, R.M.; Graham, J.; Karley, A.J. Plant Defense against Herbivorous Pests: Exploiting Resistance and Tolerance Traits for Sustainable Crop Protection. Front. Plant Sci. 2016, 7, 1132. [Google Scholar] [CrossRef]
- Pearse, I.S.; LoPresti, E.; Schaeffer, R.N.; Wetzel, W.C.; Mooney, K.A.; Ali, J.G.; Ode, P.J.; Eubanks, M.D.; Bronstein, J.L.; Weber, M.G.; et al. Generalising indirect defence and resistance of plants. Ecol. Lett. 2020, 23, 1137–1152. [Google Scholar] [CrossRef]
- Rico-Gray, V.; Oliveira, P.S. The Ecology and Evolution of Ant-Plant Interactions; University of Chicago Press: Chicago, IL, USA, 2007. [Google Scholar]
- Calixto, E.S.; Lange, D.; Del-Claro, K. Protection mutualism: An overview of ant-plant interactions mediated by extrafloral nectaries. Oecol. Aust. 2018, 22, 410–425. [Google Scholar] [CrossRef]
- González-Teuber, M.; Heil, M. The role of extrafloral nectar amino acids for the preferences of facultative and obligate ant mutualists. J Chem Ecol 2009, 35, 459–468. [Google Scholar] [CrossRef]
- Marazzi, B.; Bronstein, J.L.; Koptur, S. The diversity, ecology and evolution of extrafloral nectaries: Current perspectives and future challenges. Ann. Bot. 2013, 111, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Nahas, L.; Gonzaga, M.O.; Del-Claro, K. Emergent Impacts of Ant and Spider Interactions: Herbivory Reduction in a Tropical Savanna Tree. Biotropica 2012, 44, 498–505. [Google Scholar] [CrossRef]
- Stefani, V.; Pires, T.L.; Torezan-Silingardi, H.M.; Del-Claro, K. Beneficial Effects of Ants and Spiders on the Reproductive Value of Eriotheca gracilipes (Malvaceae) in a Tropical Savanna. PLoS ONE 2015, 10, e0131843. [Google Scholar] [CrossRef] [PubMed]
- Koptur, S.; Jones, I.M.; Peña, J.E. The Influence of Host Plant Extrafloral Nectaries on Multitrophic Interactions: An Experimental Investigation. PLoS ONE 2015, 10, e0138157. [Google Scholar] [CrossRef]
- Calixto, E.S.; Sousa-Lopes, B.; Del-Claro, K. Are rare velvet ants (Hymenoptera: Mutillidae) to feed on extrafloral nectar? J. Environ. Anal. Prog. 2018, 3, 406–409. [Google Scholar] [CrossRef]
- Calixto, E.S.; Lange, D.; Bronstein, J.; Torezan-Silingardi, H.M.; Del-Claro, K. Optimal Defense Theory in an ant–plant mutualism: Extrafloral nectar as an induced defence is maximized in the most valuable plant structures. J. Ecol. 2021, 109, 167–178. [Google Scholar] [CrossRef]
- Karban, R.; Myers, J.H. Induced Plant Responses to Herbivory. Annu. Rev. Ecol. Evol. Syst. 1989, 20, 331–348. [Google Scholar] [CrossRef]
- Karban, R. The ecology and evolution of induced responses to herbivory and how plants perceive risk. Ecol. Entomol. 2020, 45, 1–9. [Google Scholar] [CrossRef]
- Wold, E.N.; Marquis, R.J. Induced defense in white oak: Effects on herbivores and consequences for the plant. Ecology 1997, 78, 1356–1369. [Google Scholar] [CrossRef]
- Zangerl, A.R. Evolution of induced plant responses to herbivores. Basic Appl. Ecol. 2003, 4, 91–103. [Google Scholar] [CrossRef]
- War, A.R.; Taggar, G.K.; Hussain, B.; Taggar, M.S.; Nair, R.M.; Sharma, H.C. Plant defence against herbivory and insect adaptations. AoB Plants 2018, 10, ply037. [Google Scholar] [CrossRef]
- Núñez-Farfán, J.; Fornoni, J.; Valverde, P.L. The Evolution of Resistance and Tolerance to Herbivores. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 541–566. [Google Scholar] [CrossRef]
- Rosenthal, J.P.; Kotanen, P.M. Terrestrial plant tolerance to herbivory. Trends Ecol. Evol. 1994, 9, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Stowe, K.A.; Marquis, R.J.; Hochwender, C.G.; Simms, E.L. The Evolutionary Ecology of Tolerance to Consumer Damage. Annu. Rev. Ecol. Evol. Syst. 2000, 31, 565–595. [Google Scholar] [CrossRef]
- Barton, K.E.; Edwards, K.F.; Koricheva, J. Shifts in woody plant defence syndromes during leaf development. Funct. Ecol. 2019, 33, 2095–2104. [Google Scholar] [CrossRef]
- Calixto, E.S.; Lange, D.; Del-Claro, K. Foliar anti-herbivore defenses in Qualea multiflora Mart. (Vochysiaceae): Changing strategy according to leaf development. Flora-Morphol. Distrib. Funct. Ecol. Plants 2015, 212, 19–23. [Google Scholar] [CrossRef]
- Alves-Silva, E.; Santos, J.C.; Cornelissen, T.G. How many leaves are enough? The influence of sample size on estimates of plant developmental instability and leaf asymmetry. Ecol. Indic. 2018, 89, 912–924. [Google Scholar] [CrossRef]
- Čízek, L. Diet composition and body size in insect herbivores: Why do small species prefer young leaves? Eur. J. Entomol. 2005, 102, 675–681. [Google Scholar] [CrossRef]
- Kursar, T.A.; Coley, P.D. Convergence in defense syndromes of young leaves in tropical rainforests. Biochem. Syst. Ecol. 2003, 31, 929–949. [Google Scholar] [CrossRef]
- Coley, P.D.; Barone, J.A. Herbivory and Plant Defenses in Tropical Forests. Annu. Rev. Ecol. Syst. 1996, 27, 305–335. [Google Scholar] [CrossRef]
- Calixto, E.S.; Novaes, L.R.; dos Santos, D.F.B.; Lange, D.; Moreira, X.; Del-Claro, K. Climate seasonality drives ant–plant–herbivore interactions via plant phenology in an extrafloral nectary-bearing plant community. J. Ecol. 2021, 109, 639–651. [Google Scholar] [CrossRef]
- Del-Claro, K.; Marquis, R.J. Ant Species Identity has a Greater Effect than Fire on the Outcome of an Ant Protection System in Brazilian Cerrado. Biotropica 2015, 47, 459–467. [Google Scholar] [CrossRef]
- do Nascimento, E.A.; Del-Claro, K. Ant visitation to extrafloral nectaries decreases herbivory and increases fruit set in Chamaecrista debilis (Fabaceae) in a Neotropical savanna. Flora-Morphol. Distrib. Funct. Ecol. Plants 2010, 205, 754–756. [Google Scholar] [CrossRef]
- Rosumek, F.B.; Silveira, F.A.; Neves, F.d.S.; Barbosa, N.P.d.U.; Diniz, L.; Oki, Y.; Pezzini, F.; Fernandes, G.W.; Cornelissen, T. Ants on plants: A meta-analysis of the role of ants as plant biotic defenses. Oecologia 2009, 160, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Price, P.W.; Lewinsohn, T.; Fernandes, G.W.; Benson, W.W. Plant-Animal Interactions: Evolutionary Ecology in Tropical and Temperate Regions; Price, P.W., Lewinsohn, T.M., Fernandes, G.W., Benson, W.W., Eds.; Wiley-Interscience: Hoboken, NJ, USA, 1991. [Google Scholar]
- Heil, M.; Hilpert, A.; Fiala, B.; Linsenmair, K.E. Nutrient Availability and Indirect (Biotic) Defence in a Malaysian Ant-Plant. Oecologia 2001, 126, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Ness, J.H. Catalpa bignonioides alters extrafloral nectar production after herbivory and attracts ant bodyguards. Oecologia 2003, 134, 210–218. [Google Scholar] [CrossRef]
- Heil, M. Indirect defence via tritrophic interactions. New Phytol. 2008, 178, 41–61. [Google Scholar] [CrossRef]
- Silva, H.V.; Alves-Silva, E.; Santos, J.C. On the relationship between fluctuating asymmetry, sunlight exposure, leaf damage and flower set in Miconia fallax (Melastomataceae). Trop. Ecol. 2016, 57, 419–427. [Google Scholar]
- Gangestad, S.W.; Thornhill, R. Individual differences in developmental precision and fluctuating asymmetry: A model and its implications. J. Evol. Biol. 1999, 12, 402–416. [Google Scholar] [CrossRef]
- Carl Freeman, D.; Brown, M.L.; Duda, J.J.; Graraham, J.H.; Emlen, J.M.; Krzysik, A.J.; Balbach, H.; Kovacic, D.A.; Zak, J.C. Leaf fluctuating asymmetry, soil disturbance and plant stress: A multiple year comparison using two herbs, Ipomoea pandurata and Cnidoscolus stimulosus. Ecol. Indic. 2005, 5, 85–95. [Google Scholar] [CrossRef]
- Bentley, B.L. Extrafloral Nectaries and Protection by Pugnacious Bodyguards. Annu. Rev. Ecol. Syst. 1977, 8, 407–427. [Google Scholar] [CrossRef]
- Marquis, R.J. Leaf herbivores decrease fitness of a tropical plant. Science 1984, 226, 537–539. [Google Scholar] [CrossRef]
- Møller, A.P.; Thornhill, R. Bilateral symmetry and sexual selection: A meta-analysis. Am. Nat. 1998, 151, 174–192. [Google Scholar] [CrossRef] [PubMed]
- Lehtilä, K.; Strauss, S.Y. Effects of foliar herbivory on male and female reproductive traits of wild radish, Raphanus raphanistrum. Ecology 1999, 80, 116–124. [Google Scholar] [CrossRef]
- Leamy, L.J.; Klingenberg, C.P. The Genetics and Evolution of Fluctuating Asymmetry. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 1–21. [Google Scholar] [CrossRef]
- Del-Claro, K.; Rodriguez-Morales, D.; Calixto, E.S.; Martins, A.S.; Torezan-Silingardi, H.M. Ant pollination of Paepalanthus lundii (Eriocaulaceae) in Brazilian savanna. Ann. Bot. 2019, 123, 1159–1165. [Google Scholar] [CrossRef]
- Vilela, A.A.; Torezan-Silingardi, H.M.; Del-Claro, K. Conditional outcomes in ant–plant–herbivore interactions influenced by sequential flowering. Flora-Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 359–366. [Google Scholar] [CrossRef]
- Mariana, V.; Kleber, D.-C. Host plant phenology may determine the abundance of an ecosystem engineering herbivore in a tropical savanna. Ecol. Entomol. 2016, 41, 421–430. [Google Scholar] [CrossRef]
- Novaes, L.R.; Calixto, E.S.; Oliveira, M.L.; Alves-de-Lima, L.; Almeida, O.; Torezan-Silingardi, H.M. Environmental variables drive phenological events of anemocoric plants and enhance diaspore dispersal potential: A new wind-based approach. Sci. Total Environ. 2020, 730, 139039. [Google Scholar] [CrossRef]
- Souza, J.P.; Preado, C.H.B.A.; Albino, A.L.S.; Damascos, M.A. Shoot-foliage relationships in deciduous, semideciduous, and evergreen cerrado tree species. Braz. J. Plant Physiol. 2009, 21, 75–86. [Google Scholar] [CrossRef]
- Oliveira, P.S.; Leitão-Filho, H.F. Extrafloral Nectaries: Their Taxonomic Distribution and Abundance in the Woody Flora of Cerrado Vegetation in Southeast Brazil. Biotropica 1987, 19, 140–148. [Google Scholar] [CrossRef]
- Machado, S.R.; Morellato, L.P.C.; Sajo, M.G.; Oliveira, P.S. Morphological patterns of extrafloral nectaries in woody plant species of the Braziliancerrado. Plant Biol. 2008, 10, 660–673. [Google Scholar] [CrossRef]
- Alves-Silva, E.; Del-Claro, K. Herbivory-induced stress: Leaf developmental instability is caused by herbivore damage in early stages of leaf development. Ecol. Indic. 2016, 61, 359–365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, D.F.B.; Calixto, E.S.; Torezan-Silingardi, H.M.; Del-Claro, K. Sequential Defense Strategies: From Ant Recruitment to Leaf Toughness. Plants 2025, 14, 49. https://doi.org/10.3390/plants14010049
dos Santos DFB, Calixto ES, Torezan-Silingardi HM, Del-Claro K. Sequential Defense Strategies: From Ant Recruitment to Leaf Toughness. Plants. 2025; 14(1):49. https://doi.org/10.3390/plants14010049
Chicago/Turabian Styledos Santos, Danilo F. B., Eduardo S. Calixto, Helena M. Torezan-Silingardi, and Kleber Del-Claro. 2025. "Sequential Defense Strategies: From Ant Recruitment to Leaf Toughness" Plants 14, no. 1: 49. https://doi.org/10.3390/plants14010049
APA Styledos Santos, D. F. B., Calixto, E. S., Torezan-Silingardi, H. M., & Del-Claro, K. (2025). Sequential Defense Strategies: From Ant Recruitment to Leaf Toughness. Plants, 14(1), 49. https://doi.org/10.3390/plants14010049








