Investigating the Allelopathic and Bioherbicidal Potential of Solidago altissima with a Focus on Chemical Signaling in Trifolium repens
Abstract
1. Introduction
2. Results
2.1. Allelopathic Effects of Solidago altissima on Trifolium repens
2.1.1. Effect of S. altissima Extracts on Seedling Fresh Weight
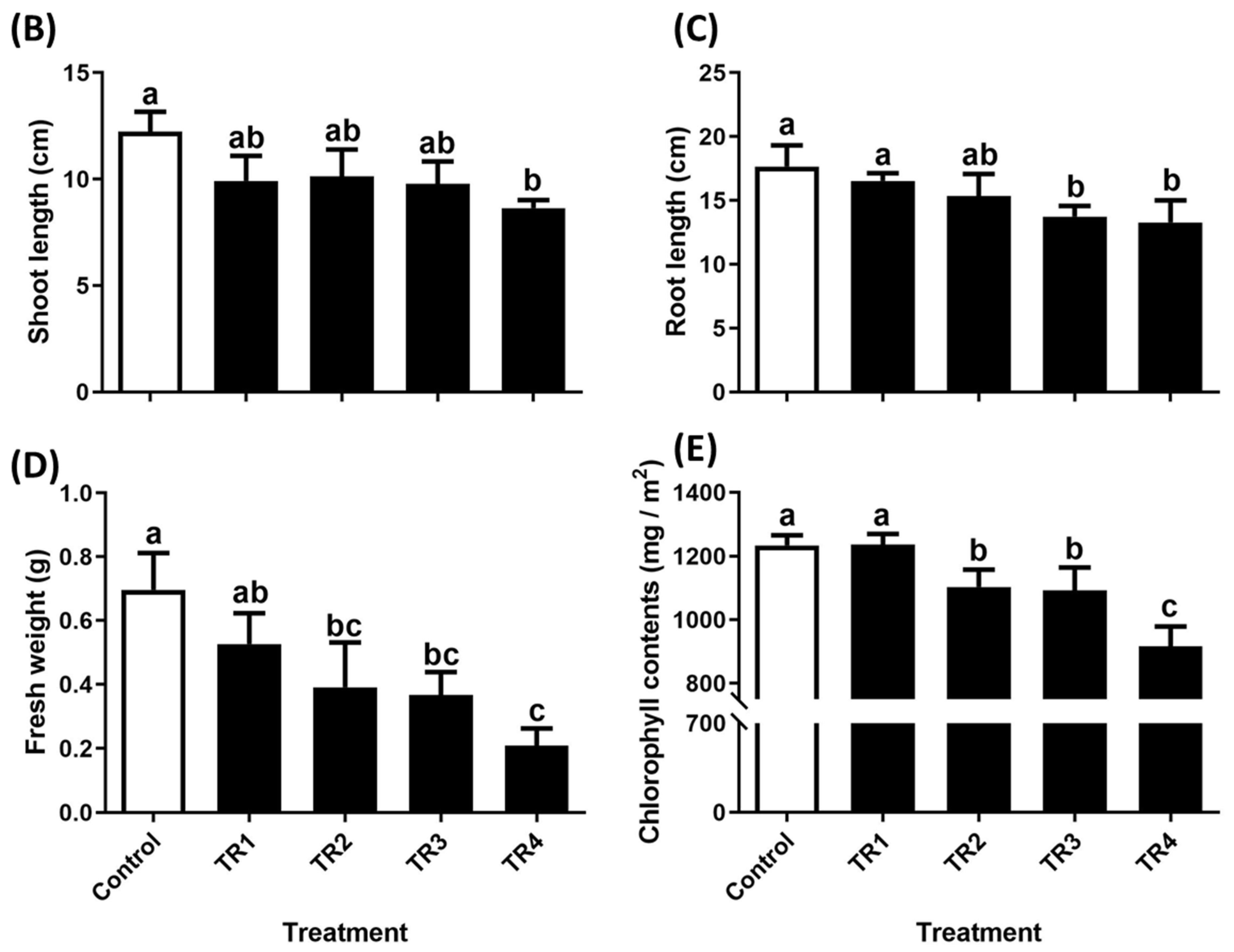
2.1.2. Effect of Solidago altissima Root Extract Foliar Treatment on the Growth and Chlorophyll Content of Trifolium repens
2.2. Effects of Solidago altissima Extract Foliar Treatment on Reactive Oxygen Species Content and Antioxidant Enzyme Activity
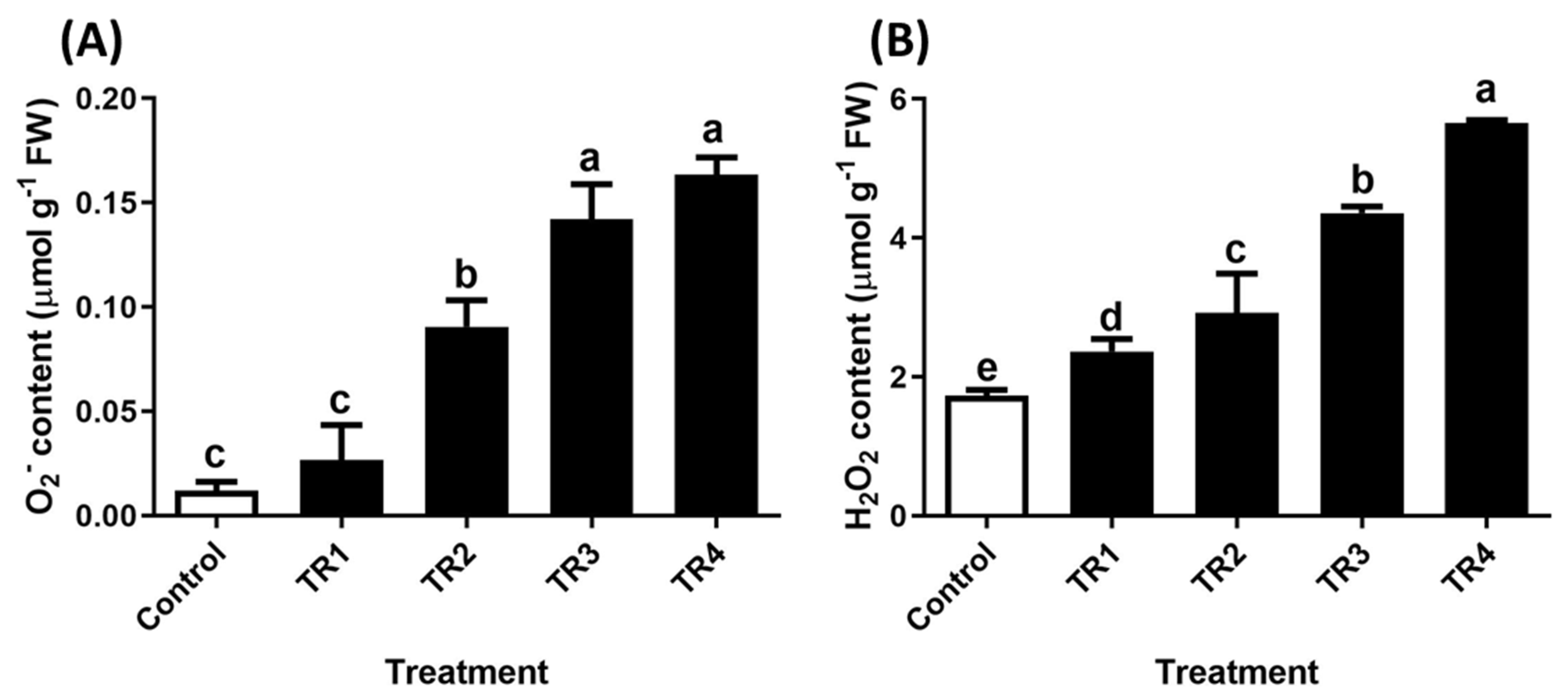
2.2.1. Reactive Oxygen Species Content
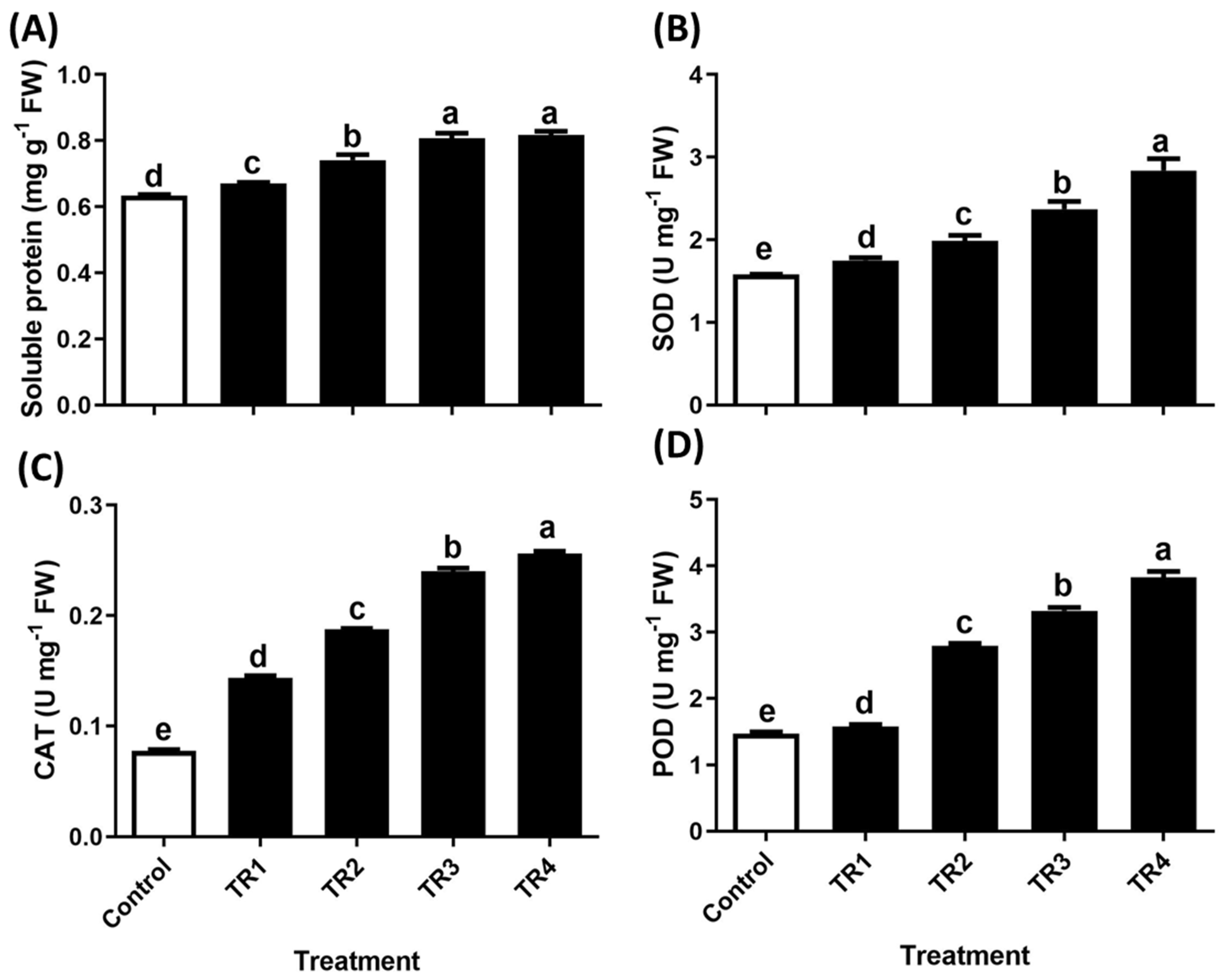
2.2.2. Effects of Solidago altissima Root Extract Foliar Application on Antioxidant Enzyme Activity
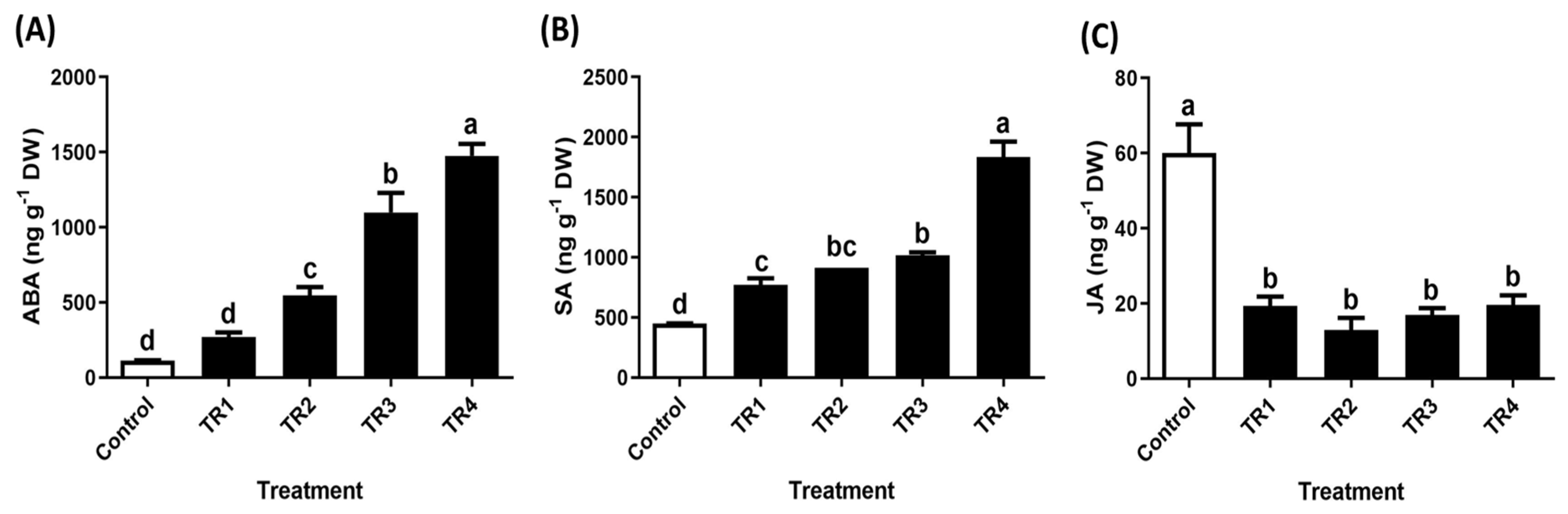
2.3. Quantification of Endogenous Phytohormones in Trifolium repens Leaves
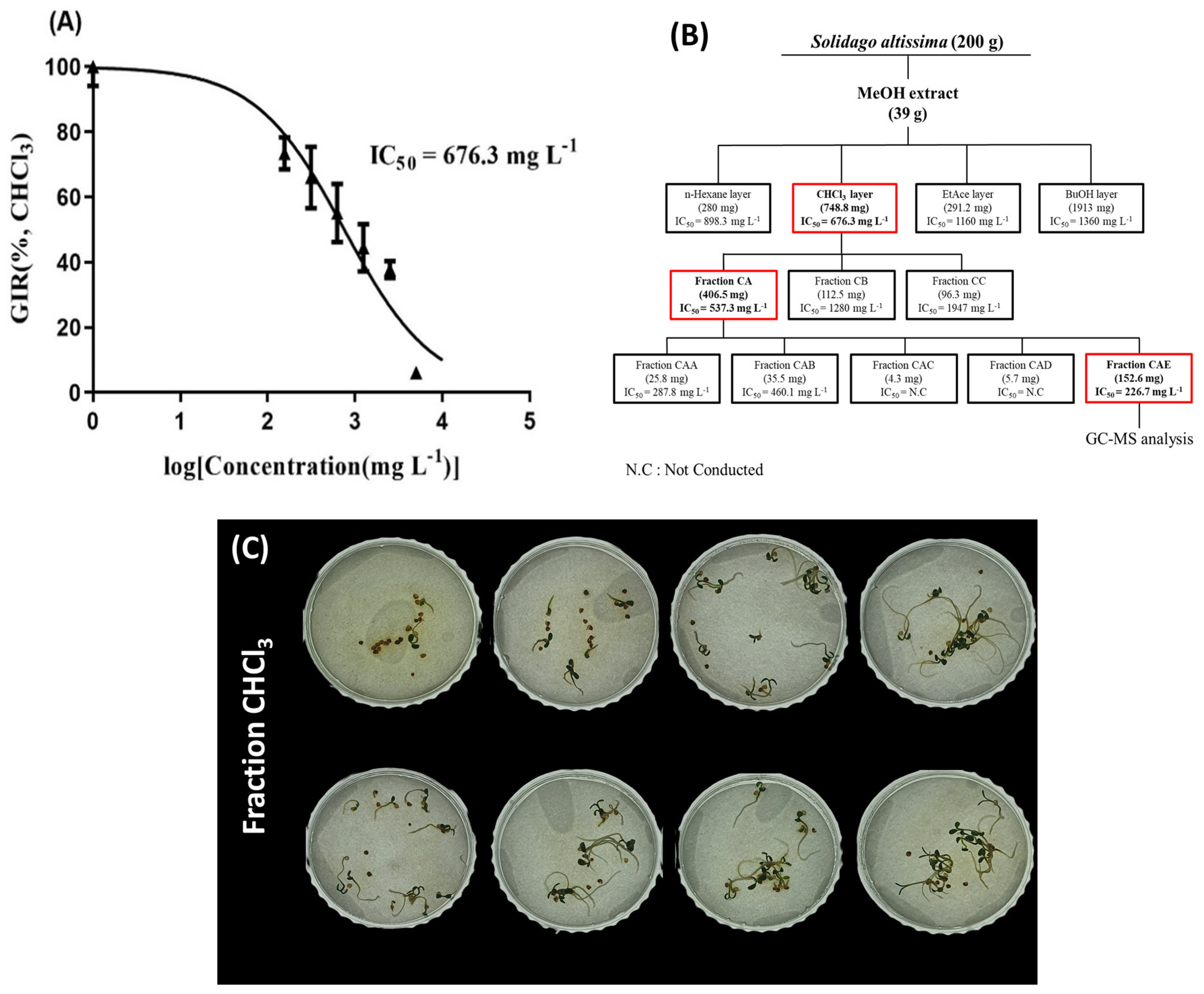
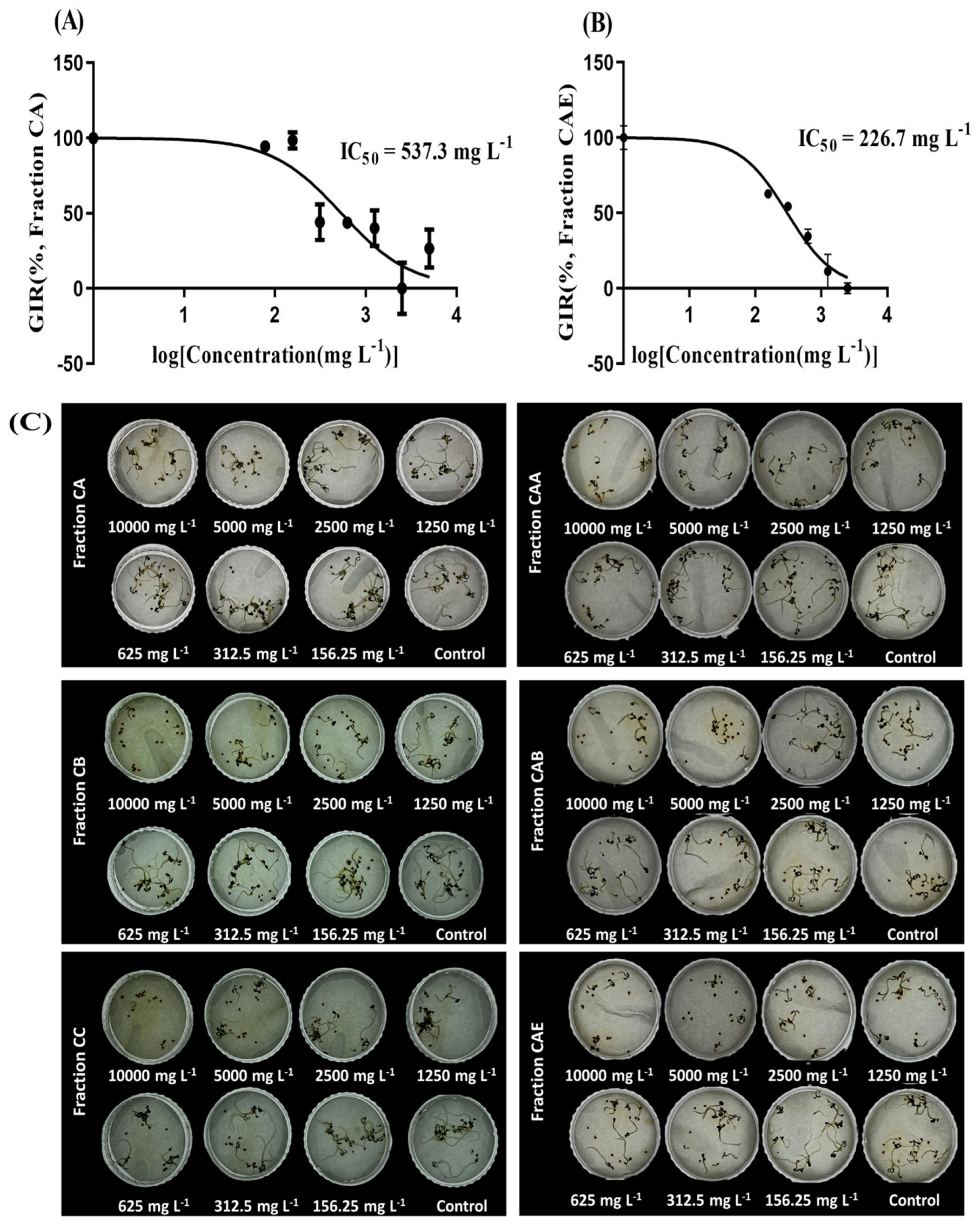
2.4. Identification of Allelochemicals in Solidago altissima Roots
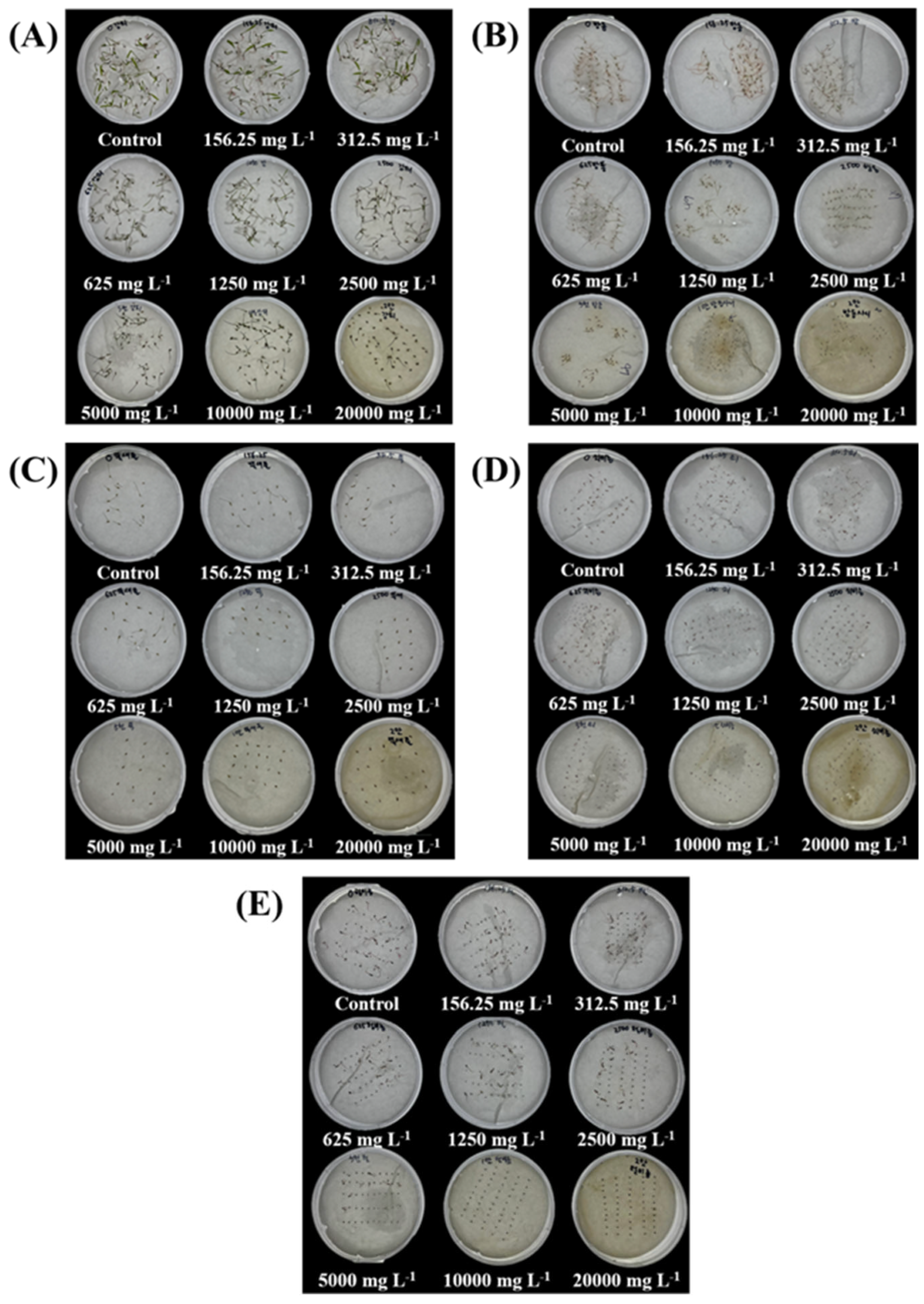
2.5. Effect of SRE Treatment on Germination Inhibition of Weed Species
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Plant Experiment
4.2.1. Preparation of Solidago altissima Extract
4.2.2. In Vitro Seed Bioassay for T. repens and Weed Species
4.2.3. Solidago altissima Root Treatment on Trifolium repens Seedlings
4.3. Reactive Oxygen Species Analysis in Trifolium repens
DAB Test and Analysis of O2− and H2O2 Levels
4.4. Antioxidant Analysis
4.4.1. Soluble Protein Content
4.4.2. Measurement of Superoxide Dismutase, Catalase, and Peroxidase Activities
4.5. Phytohormone Analysis
4.5.1. Quantification of Abscisic Acid and Jasmonic Acid
4.5.2. Quantification of Salicylic Acid
4.6. Identification of Allelochemicals in Solidago altissima
4.6.1. Liquid–Liquid Extraction
4.6.2. Column Chromatography and Instrumental Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SSE | S. altissima shoot extract |
| SRE | S. altissima root extract |
| WS | Weed species |
| ABA | Abscisic acid |
| JA | Jasmonic acid |
| SA | Salicylic acid |
| ROS | Reactive oxygen species |
References
- Hussain, M.I.; Abideen, Z.; Danish, S.; Asghar, M.A.; Iqbal, K. Integrated weed management for sustainable agriculture. Sustain. Agric. Rev. 2021, 52, 367–393. [Google Scholar]
- Etterson, J.R.; Delf, D.E.; Craig, T.P.; Ando, Y.; Ohgushi, T. Parallel patterns of clinal variation in Solidago altissima in its native range in central USA and its invasive range in Japan. Botany 2008, 86, 91–97. [Google Scholar] [CrossRef]
- Maddox, G.D.; Cook, R.E.; Wimberger, P.H.; Gardescu, S. Clone structure in four Solidago altissima (Asteraceae) populations: Rhizome connections within genotypes. Am. J. Bot. 1989, 76, 318–326. [Google Scholar] [CrossRef]
- Meyer, A.H.; Schmid, B. Seed dynamics and seedling establishment in the invading perennial Solidago altissima under different experimental treatments. J. Ecol. 1999, 87, 28–41. [Google Scholar] [CrossRef]
- Cain, M.L. Models of clonal growth in Solidago altissima. J. Ecol. 1990, 78, 27–46. [Google Scholar] [CrossRef]
- Weber, E. Biological flora of central Europe: Solidago altissima L. Flora 2000, 195, 123–134. [Google Scholar] [CrossRef]
- Jung, J.S.; Kim, J.G.; Park, H.S.; Lee, S.H.; Kim, H.S.; Kim, W.H.; Kim, Y.J.; Choi, G.J. The effects of improvement of botanical composition technology application on botanical composition and dry matter productivity in Rumex acetosella dominated hilly pasture. J. Korean Soc. Grassl. Forage Sci. 2016, 36, 81–88. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, C.; Choi, Y.; Lim, Y.; Han, S.; Na, K. Effect of herbicide application on weed control and forage production in alpine grassland predominated with red sorrel (Rumex acetosella L.). J. Anim. Sci. Technol. 2003, 45, 865–874. [Google Scholar]
- Wang, H.; Wu, Y.; He, Y.; Li, G.; Ma, L.; Li, S.; Huang, J.; Yang, G. High-quality chromosome-level de novo assembly of the Trifolium repens. BMC Genom. 2023, 24, 326. [Google Scholar] [CrossRef]
- Ahmad, S.; Zeb, A. Phytochemical profile and pharmacological properties of Trifolium repens. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 20200015. [Google Scholar] [CrossRef]
- Cho, H.-S.; Seo, M.-C.; Kim, J.-H.; Sang, W.-G.; Shin, P.; Lee, G.H. Effects of spring seeding on growth and carbon uptake of clover species in upland soil. Korean J. Soil Sci. Fertil. 2017, 50, 644–652. [Google Scholar] [CrossRef]
- Lin, H.; Liu, C.; Li, B.; Dong, Y. Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 2021, 402, 123829. [Google Scholar] [CrossRef]
- Vivanco, J.M.; Bais, H.P.; Stermitz, F.R.; Thelen, G.C.; Callaway, R.M. Biogeographical variation in community response to root allelochemistry: Novel weapons and exotic invasion. Ecol. Lett. 2004, 7, 285–292. [Google Scholar] [CrossRef]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia Eng. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Kobayashi, A.; Morimoto, S.; Shibata, Y.; Yamashita, K.; Numata, M. C10-polyacetylenes as allelopathic substances in dominants in early stages of secondary succession. J. Chem. Ecol. 1980, 6, 119–131. [Google Scholar] [CrossRef]
- Nishino, C.; Manabe, S.; Kazui, M.; Matsuzaki, T. Piscicidal cis-clerodane diterpenes from Solidago altissima L: Absolute configurations of 5α, 10α-cis-clerodanes. Tetrahedron Lett. 1984, 25, 2809–2812. [Google Scholar] [CrossRef]
- Tori, M.; Katto, A.; Sono, M. Nine new clerodane diterpenoids from rhizomes of Solidago altissima. Phytochemistry 1999, 52, 487–493. [Google Scholar] [CrossRef]
- Wu, B.; Takahashi, T.; Kashiwagi, T.; Tebayashi, S.-i.; Kim, C.-S. New flavonoid glycosides from the leaves of Solidago altissima. Chem. Pharm. Bull. 2007, 55, 815–816. [Google Scholar] [CrossRef][Green Version]
- Jin, H.; Tanaka, T.; Kouno, I.; Ishimaru, K. A new kaempferol trioside from Solidago altissima L. J. Nat. Med. 2007, 61, 351–354. [Google Scholar] [CrossRef]
- Nishidono, Y.; Tanaka, K. Comprehensive characterization of polyacetylenes and diterpenes from the underground parts of Solidago altissima L. and their contribution to the overall allelopathic activity. Phytochemistry 2022, 193, 112986. [Google Scholar] [CrossRef]
- Souza, L.A.; Monteiro, C.C.; Carvalho, R.F.; Gratão, P.L.; Azevedo, R.A. Dealing with abiotic stresses: An integrative view of how phytohormones control abiotic stress-induced oxidative stress. Theor. Exp. Plant Physiol. 2017, 29, 109–127. [Google Scholar] [CrossRef]
- Złotek, U.; Szymanowska, U.; Jakubczyk, A.; Sikora, M.; Świeca, M. Effect of arachidonic and jasmonic acid elicitation on the content of phenolic compounds and antioxidant and anti-inflammatory properties of wheatgrass (Triticum aestivum L.). Food Chem. 2019, 288, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Chiapusio, G.; Weston, L. Allelopathy and the role of allelochemicals in plant defence. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2017; Volume 82, pp. 19–54. [Google Scholar]
- Torun, H.; Novák, O.; Mikulík, J.; Pěnčík, A.; Strnad, M.; Ayaz, F.A. Timing-dependent effects of salicylic acid treatment on phytohormonal changes, ROS regulation, and antioxidant defense in salinized barley (Hordeum vulgare L.). Sci. Rep. 2020, 10, 13886. [Google Scholar] [CrossRef]
- Pardo, G.; Marí, A.I.; Aibar, J.; Cirujeda, A. Do crop rotations in rice reduce weed and Echinochloa spp. infestations? Recomm. Integr. Weed Control. Agron. 2021, 11, 454. [Google Scholar]
- Zhao, N.; Li, Q.; Guo, W.; Zhang, L.; Wang, J. Effect of environmental factors on germination and emergence of shortawn foxtail (Alopecurus aequalis). Weed Sci. 2018, 66, 47–56. [Google Scholar] [CrossRef]
- Elbalola, A.A. Evidence for increased competitive ability (EICA) in Prosopis juliflora (Sw.) Dc (mesquite) under P. juliflora-Portulaca oleracea L. (purslane) field competition. Afr. J. Ecol. 2021, 59, 1075–1079. [Google Scholar] [CrossRef]
- Khan, A.M.; Mobli, A.; Werth, J.A.; Chauhan, B.S. Germination and seed persistence of Amaranthus retroflexus and Amaranthus viridis: Two emerging weeds in Australian cotton and other summer crops. PLoS ONE 2022, 17, e0263798. [Google Scholar] [CrossRef]
- Roberts, J.; Florentine, S.; Fernando, W.D.; Tennakoon, K.U. Achievements, developments and future challenges in the field of bioherbicides for weed control: A global review. Plants 2022, 11, 2242. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.-R.; Adhikari, A.; Kang, Y.; Gam, H.-J.; Kang, S.-M.; Kim, K.-Y.; Lee, I.-J. Investigation of Solanum carolinense Dominance and Phytotoxic Effect in Festuca arundinacea with Special Reference to Allelochemical Identification, Analysis of Phytohormones and Antioxidant Mechanisms. Agronomy 2022, 12, 1954. [Google Scholar] [CrossRef]
- Morimoto, M. Chemical defense against insects in Heterotheca subaxillaris and three Orobanchaceae species using exudates from trichomes. Pest Manag. Sci. 2019, 75, 2474–2481. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.-C.; Li, W.-Y.; Guo, K.; Liu, Y.; Li, S.-H. Antifeedant, cytotoxic, and anti-inflammatory neo-clerodane diterpenoids in the peltate glandular trichomes and fresh leaves of Ajuga forrestii. Phytochemistry 2021, 186, 112731. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.V.; Han, J.W.; Le Dang, Q.; Ryu, S.M.; Lee, D.; Kim, H.; Choi, G.J. Clerodane Diterpenoids Identified from Polyalthia longifolia Showing Antifungal Activity against Plant Pathogens. J. Agric. Food Chem. 2021, 69, 10527–10535. [Google Scholar] [CrossRef]
- Murthy, M.M.; Subramanyam, M.; Bindu, M.H.; Annapurna, J. Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds. Fitoterapia 2005, 76, 336–339. [Google Scholar] [CrossRef]
- Zhao, G.X.; Jung, J.H.; Smith, D.L.; Wood, K.V.; McLaughlin, J.L. Cytotoxic clerodane diterpenes from Polyalthia longifolia. Planta Med. 1991, 57, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, R.; Akbar, N.; Khatoon, B.; Kawish, M.; Ali, M.S.; Shah, M.R.; Khan, N.A. Novel Plant-Based Metabolites as Disinfectants against Acanthamoeba castellanii. Antibiotics 2022, 11, 248. [Google Scholar] [CrossRef]
- Barbosa, A.L.P.; Wenzel-Storjohann, A.; Barbosa, J.D.; Zidorn, C.; Peifer, C.; Tasdemir, D.; Çiçek, S.S. Antimicrobial and cytotoxic effects of the Copaifera reticulata oleoresin and its main diterpene acids. J. Ethnopharmacol. 2019, 233, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Faizi, S.; Khan, R.A.; Mughal, N.R.; Malik, M.S.; Sajjadi, K.e.S.; Ahmad, A. Antimicrobial activity of various parts of Polyalthia longifolia var. pendula: Isolation of active principles from the leaves and the berries. Phytother. Res. 2008, 22, 907–912. [Google Scholar]
- Rudrappa, T.; Bonsall, J.; Gallagher, J.L.; Seliskar, D.M.; Bais, H.P. Root-secreted allelochemical in the noxious weed Phragmites australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J. Chem. Ecol. 2007, 33, 1898–1918. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Rama Shanker, D.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Szőllősi, R. Superoxide dismutase (SOD) and abiotic stress tolerance in plants: An overview. In Oxidative Damage Plants; Academic Press: Cambridge, MA, USA, 2014; pp. 89–129. [Google Scholar] [CrossRef]
- Corpas, F.J.; González-Gordo, S.; Palma, J.M. Plant Peroxisomes: A Factory of Reactive Species. Front. Plant Sci. 2020, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Chaitanya, K.V.; Sundar, D.; Masilamani, S.; Ramachandra Reddy, A. Variation in heat stress-induced antioxidant enzyme activities among three mulberry cultivars. Plant Growth Regul. 2002, 36, 175–180. [Google Scholar] [CrossRef]
- Rajput, V.D.; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; Mandzhieva, S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Duan, X.; Jiang, Y.; Zhang, P. Increased expression of native cytosolic Cu/Zn superoxide dismutase and ascorbate peroxidase improves tolerance to oxidative and chilling stresses in cassava (Manihot esculenta Crantz). BMC Plant Biol. 2014, 14, 208. [Google Scholar] [CrossRef]
- Krumsri, R.; Kato-Noguchi, H.; Poonpaiboonpipat, T. Allelopathic effect of sphenoclea zeylanica gaertn. On rice (‘Oryza sativa’ L.) germination and seedling growth. Aust. J. Crop Sci. 2020, 14, 1450–1455. [Google Scholar] [CrossRef]
- Poonpaiboonpipat, T.; Krumsri, R.; Kato-Noguchi, H. Allelopathic and Herbicidal Effects of Crude Extract from Chromolaena odorata (L.) RM King and H. Rob. on Echinochloa crus-galli and Amaranthus viridis. Plants 2021, 10, 1609. [Google Scholar] [CrossRef]
- Pospíšil, P. Production of reactive oxygen species by photosystem II. Biochim. Biophys. Acta (BBA)-Bioenerg. 2009, 1787, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63. [Google Scholar] [CrossRef]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.-M.; Lee, I.-J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef]
- Ciura, J.; Kruk, J. Phytohormones as targets for improving plant productivity and stress tolerance. J. Plant Physiol. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of phytohormone signaling: A primary function of flavonoids in plant–environment interactions. Front. Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Kumar, P.; Sharma, R.; Kumar, A. Regulatory interactions in phytohormone stress signaling implying plants resistance and resilience mechanisms. J. Plant Biochem. Biotechnol. 2021, 30, 813–828. [Google Scholar] [CrossRef]
- Lackman, P.; González-Guzmán, M.; Tilleman, S.; Carqueijeiro, I.; Pérez, A.C.; Moses, T.; Seo, M.; Kanno, Y.; Häkkinen, S.T.; Van Montagu, M.C. Jasmonate signaling involves the abscisic acid receptor PYL4 to regulate metabolic reprogramming in Arabidopsis and tobacco. Proc. Natl. Acad. Sci. USA 2011, 108, 5891–5896. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef]
- Guan, L.M.; Zhao, J.; Scandalios, J.G. Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 2000, 22, 87–95. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, J. Effect of Abscisic Acid on Active Oxygen Species, Antioxidative Defence System and Oxidative Damage in Leaves of Maize Seedlings. Plant Cell Physiol. 2001, 42, 1265–1273. [Google Scholar] [CrossRef]
- Cho, N.-K.; Lee, S.-E.; Choi, J.-S.; Hwang, K.-H.; Koo, S.-J.; Wang, H.-Y.; Kim, S.-M. Isolation of a New Herbicidal Compound Angelicin from Curly Dock (Rumex crispus L.). Korean J. Weed Sci. 2010, 30, 183–190. [Google Scholar] [CrossRef]
- Liu, H.-L.; Lee, Z.-X.; Chuang, T.-W.; Wu, H.-C. Effect of heat stress on oxidative damage and antioxidant defense system in white clover (Trifolium repens L.). Planta 2021, 254, 103. [Google Scholar] [CrossRef]
- Navari-Izzo, F.; Pinzino, C.; Quartacci, M.F.; Sgherri, C.L.M. Superoxide and Hydroxyl Radicai Generation, and Superoxide Dismutase in PII Membrane Fragments from Wheat. Free Radic. Res. 1999, 31, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A dye binding assay for protein. Anal. Biochem. 1976, 72, e54. [Google Scholar]
- Park, H.-S.; Kazerooni, E.A.; Kang, S.-M.; Al-Sadi, A.M.; Lee, I.-J. Melatonin enhances the tolerance and recovery mechanisms in Brassica juncea (L.) Czern. under saline conditions. Front. Plant Sci. 2021, 12, 593717. [Google Scholar] [CrossRef]
- Gam, H.-J.; Injamum-Ul-Hoque, M.; Kang, Y.; Ahsan, S.; Hasan, M.M.; Shaffique, S.; Kang, S.-M.; Lee, I.-J. Allelopathic effect of the methanol extract of the weed species-red sorrel (Rumex acetosella L.) on the growth, phytohormone content and antioxidant activity of the cover crop-white clover (Trifolium repens L.). BMC Plant Biol. 2024, 24, 523. [Google Scholar] [CrossRef]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.-M.; Yun, B.-W.; Lee, I.-J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef]
- Seskar, M.; Shulaev, V.; Raskin, I. Endogenous Methyl Salicylate in Pathogen-Inoculated Tobacco Plants1. Plant Physiol. 1998, 116, 387–392. [Google Scholar] [CrossRef]




| No. | Compound Name | Peak Area (%) |
|---|---|---|
| 1 | Methyl kolavenate | 35.68 |
| 2 | n-Hexadecanoic acid | 4.21 |
| 3 | Chondrillasterol | 3.49 |
| 4 | Carvyl angelate | 2.76 |
| 5 | Kolavenol | 2.30 |
| 6 | (E)-4,4-Dimethyl-2-pentene | 2.19 |
| 7 | 10(E),12(Z)-Conjugated linoleic acid | 1.57 |
| 8 | (E)-Longipinane | 1.44 |
| 9 | (Z,Z)-9,12-Octadecadienoic acid | 1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gam, H.-J.; Adhikari, A.; Kang, Y.; Injamum-Ul-Hoque, M.; Shaffique, S.; Woo, J.-I.; Jeon, J.R.; An, B.-K.; Back, M.Y.; Kim, K.-Y.; et al. Investigating the Allelopathic and Bioherbicidal Potential of Solidago altissima with a Focus on Chemical Signaling in Trifolium repens. Plants 2025, 14, 96. https://doi.org/10.3390/plants14010096
Gam H-J, Adhikari A, Kang Y, Injamum-Ul-Hoque M, Shaffique S, Woo J-I, Jeon JR, An B-K, Back MY, Kim K-Y, et al. Investigating the Allelopathic and Bioherbicidal Potential of Solidago altissima with a Focus on Chemical Signaling in Trifolium repens. Plants. 2025; 14(1):96. https://doi.org/10.3390/plants14010096
Chicago/Turabian StyleGam, Ho-Jun, Arjun Adhikari, Yosep Kang, Md. Injamum-Ul-Hoque, Shifa Shaffique, Ji-In Woo, Jin Ryeol Jeon, Byeong-Kwan An, Min Young Back, Ki-Yong Kim, and et al. 2025. "Investigating the Allelopathic and Bioherbicidal Potential of Solidago altissima with a Focus on Chemical Signaling in Trifolium repens" Plants 14, no. 1: 96. https://doi.org/10.3390/plants14010096
APA StyleGam, H.-J., Adhikari, A., Kang, Y., Injamum-Ul-Hoque, M., Shaffique, S., Woo, J.-I., Jeon, J. R., An, B.-K., Back, M. Y., Kim, K.-Y., Kang, S.-M., & Lee, I.-J. (2025). Investigating the Allelopathic and Bioherbicidal Potential of Solidago altissima with a Focus on Chemical Signaling in Trifolium repens. Plants, 14(1), 96. https://doi.org/10.3390/plants14010096







