Distribution of Vegetation and Soil Seed Banks Across Habitat Types in Paddy Fields Under Different Farming Practices
Abstract
1. Introduction
2. Results
2.1. Distribution of Vegetation by Habitat Type
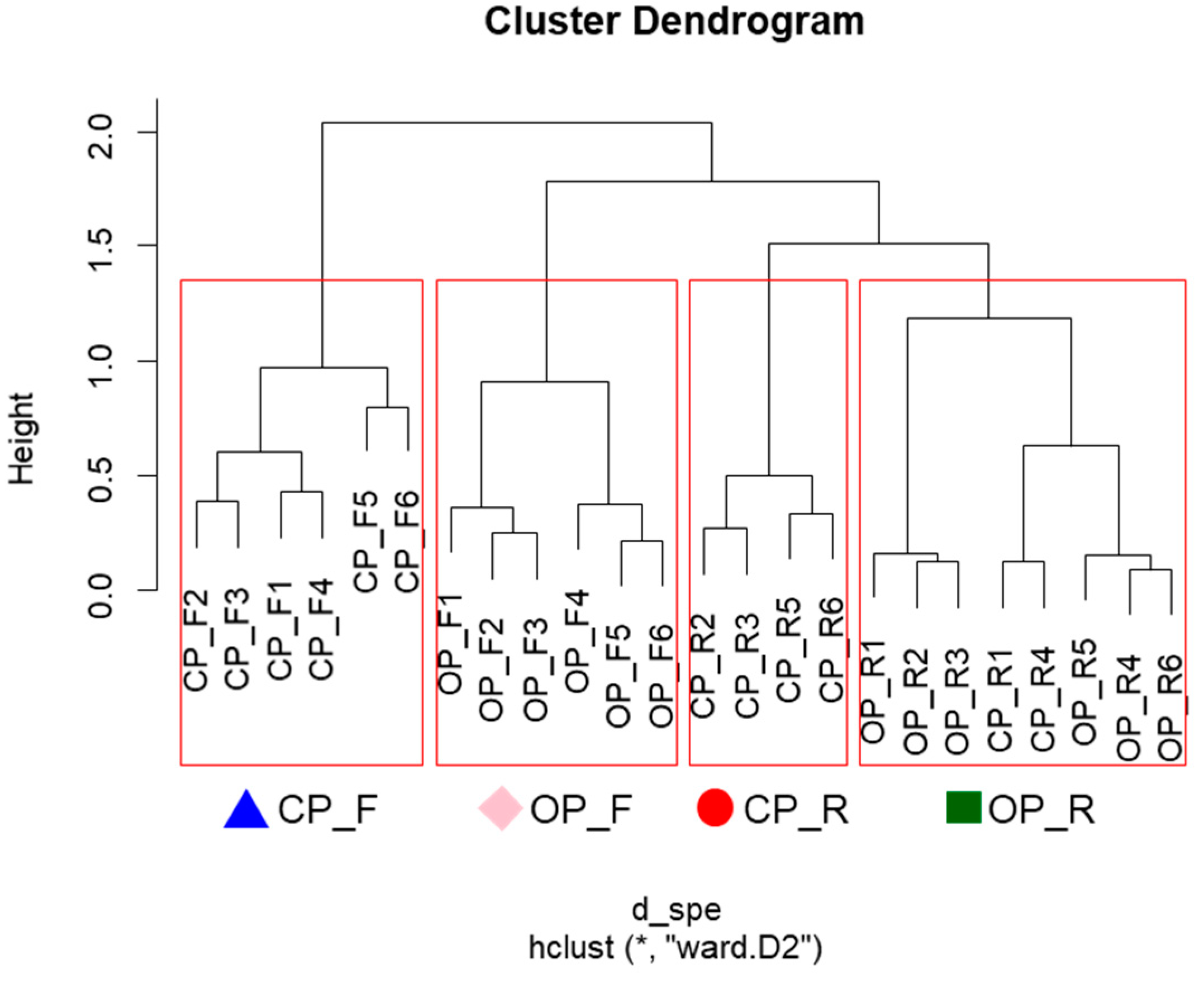
2.2. Distribution of Soil Seed Banks by Habitat Type
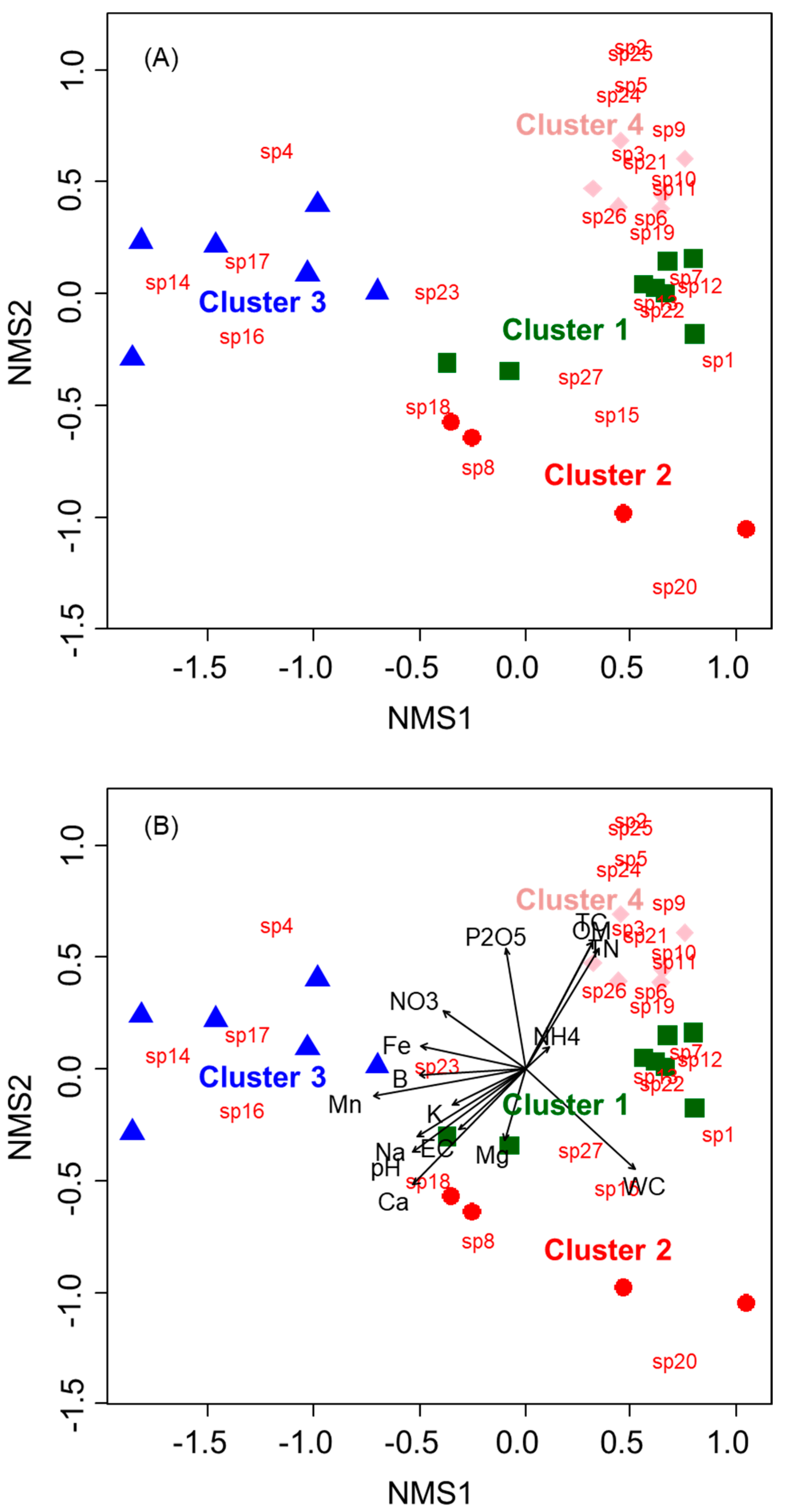
2.3. Relationship Between the Distribution of Soil Seed Banks and Soil Environmental Factors
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Field Survey
4.3. Soil Sampling for Soil Seed Bank and Soil Environmental Factor Analyses
4.4. Evaluation of Soil Seed Bank Characteristics
4.5. Soil Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elouafi, I. Why Biodiversity Matters in Agriculture and Food Systems. Science 2024, 386, eads8197. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Adair, E.C.; Cardinale, B.J.; Byrnes, J.E.K.; Hungate, B.A.; Matulich, K.L.; Matulich, K.L.; Gonzalez, A.; Duffy, J.E.; Gamfeldt, L.; et al. A Global Synthesis Reveals Biodiversity Loss as a Major Driver of Ecosystem Change. Nature 2012, 486, 105–108. [Google Scholar] [CrossRef]
- Klein, A.M.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of Pollinators in Changing Landscapes for World Crops. Proc. R. Soc. B Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Mori, A.S.; Dee, L.E.; Gonzalez, A.; Ohashi, H.; Cowles, J.; Wright, A.J.; Loreau, M.; Hautier, Y.; Newbold, T.; Reich, P.B.; et al. Biodiversity–Productivity Relationships Are Key to Nature-Based Climate Solutions. Nat. Clim. Change 2021, 11, 543–550. [Google Scholar] [CrossRef]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Whyte, K.P. Climate Change Effects on Biodiversity, Ecosystems, Ecosystem Services, and Natural Resource Management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef]
- Wittwer, R.A.; Bender, S.F.; Hartman, K.; Hydbom, S.; Lima, R.A.; Loaiza, V.; Van Der Heijden, M.G. Organic and Conservation Agriculture Promote Ecosystem Multifunctionality. Sci. Adv. 2021, 7, eabg6995. [Google Scholar] [CrossRef]
- Tscharntke, T.; Clough, Y.; Wanger, T.C.; Jackson, L.; Motzke, I.; Perfecto, I.; Whitbread, A. Global Food Security, Biodiversity Conservation, and the Future of Agricultural Intensification. Biol. Conserv. 2012, 151, 53–59. [Google Scholar] [CrossRef]
- Mäder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil Fertility and Biodiversity in Organic Farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; De Ruiter, P.C.; Van Der Putten, W.H.; Birkhofer, K.; Hedlund, K. Intensive Agriculture Reduces Soil Biodiversity across Europe. Glob. Change Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological Intensification: Harnessing Ecosystem Services for Food Security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Michael, D.R.; Wood, J.T.; O’Loughlin, T.; Lindenmayer, D.B. Influence of Land Sharing and Land Sparing Strategies on Patterns of Vegetation and Terrestrial Vertebrate Richness and Occurrence in Australian Endangered Eucalypt Woodlands. Agric. Ecosyst. Environ. 2016, 227, 24–32. [Google Scholar] [CrossRef]
- HLPE. Other Innovative Approaches for Sustainable Agriculture and Food Systems that Enhance Food Security and Nutrition; Report by the High-Level Panel of Experts on Food Security and Nutrition of the Committee on World Food Security; UN Food and Agriculture Organization: Rome, Italy, 2019. [Google Scholar]
- Bautze, D.; Karanja, E.; Musyoka, M.; Rüegg, J.; Goldmann, E.; Kiboi, M.; Adamtey, N. Closing the Crop Yield Gap between Organic and Conventional Farming Systems in Kenya: Long-Term Trial Research Indicates Agronomic Viability. J. Agric. Food Res. 2024, 18, 101499. [Google Scholar] [CrossRef]
- Royo, A.A.; Ristau, T.E. Stochastic and Deterministic Processes Regulate Spatio-Temporal Variation in Seed Bank Diversity. J. Veg. Sci. 2013, 24, 724–734. [Google Scholar] [CrossRef]
- Choi, Y.S.; Kim, J.G. Seed Bank Has Potential for the Restoration of Insectivorous Plants in Janggun Montane Wetland. Ecol. Eng. 2022, 182, 106728. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, G.; Zhao, M.; Wang, M.; Jiang, M. Direct and Indirect Effects of Soil Salinization on Soil Seed Banks in Salinizing Wetlands in the Songnen Plain, China. Sci. Total Environ. 2022, 819, 152035. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Baskin, C.C.; Baskin, J.M.; Pakeman, R.J.; Huang, Z.; Gao, R.; Cornelissen, J.H. Global Patterns of Potential Future Plant Diversity Hidden in Soil Seed Banks. Nat. Commun. 2021, 12, 7023. [Google Scholar] [CrossRef]
- Ooi, M.K. Seed Bank Persistence and Climate Change. Seed Sci. Res. 2012, 22, S53–S60. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin III, F.S. The Mineral Nutrition of Wild Plants Revisited: A Re-Evaluation of Processes and Patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar]
- Furey, G.N.; Tilman, D. Plant Biodiversity and the Regeneration of Soil Fertility. Proc. Natl. Acad. Sci. USA 2021, 118, e2111321118. [Google Scholar] [CrossRef]
- Tilman, D.; Wedin, D.; Knops, J. Productivity and Sustainability Influenced by Biodiversity in Grassland Ecosystems. Nature 1996, 379, 718–720. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant Diversity Increases Soil Microbial Activity and Soil Carbon Storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil Carbon Sequestration Accelerated by Restoration of Grassland Biodiversity. Nat. Commun. 2019, 10, 718. [Google Scholar] [CrossRef]
- Lawler, S.P. Rice Fields as Temporary Wetlands: A Review. Isr. J. Zool. 2001, 47, 513–528. [Google Scholar] [CrossRef]
- Choi, S.H.; Choi, G.; Nam, H.K. Impact of Rice Paddy Agriculture on Habitat Usage of Migratory Shorebirds at the Rice Paddy Scale in Korea. Sci. Rep. 2022, 12, 5762. [Google Scholar] [CrossRef]
- Bang, J.H.; Bae, M.J.; Lee, E.J. Plant Distribution along an Elevational Gradient in a Macrotidal Salt Marsh on the West Coast of Korea. Aquat. Bot. 2018, 147, 52–60. [Google Scholar] [CrossRef]
- Wu, C.F.; Wang, H.H.; Chen, S.H.; Trac, L.V.T. Assessing the Efficiency of Bird Habitat Conservation Strategies in Farmland Ecosystems. Ecol. Model. 2024, 492, 110732. [Google Scholar] [CrossRef]
- Agidie, A.; Wondie, A.; Beneberu, G. Wetland Disturbance Level and Macrophyte Composition in Natural Wetlands and Paddy Fields in the Eastern Part of Lake Tana, Ethiopia. Heliyon 2024, 10, e40701. [Google Scholar] [CrossRef] [PubMed]
- Dalzochio, M.S.; Baldin, R.; Stenert, C.; Maltchik, L. Can Organic and Conventional Agricultural Systems Affect Wetland Macroinvertebrate Taxa in Rice Fields? Basic Appl. Ecol. 2016, 17, 220–229. [Google Scholar] [CrossRef]
- Luo, Y.; Fu, H.; Traore, S. Biodiversity Conservation in Rice Paddies in China: Toward Ecological Sustainability. Sustainability 2014, 6, 6107–6124. [Google Scholar] [CrossRef]
- Jin, Y.N.; Yu, K.S.; Ri, U.J.; Song, C.N. Methods and Effectiveness of Using Apple Snails (Pomacea sp.) in Organic Rice Farming. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2022, 92, 495–504. [Google Scholar] [CrossRef]
- Qi, Y.; Li, J.; Yan, B.; Deng, Z.; Fu, G. Impact of Herbicides on Wild Plant Diversity in Agro-Ecosystems: A Review. Biodivers. Sci. 2016, 24, 228–236. [Google Scholar] [CrossRef]
- Bang, J.H.; Park, J.H.; Lee, Y.M.; Chang, C.L.; Hong, S.J. Comparison of Soil Seed Bank and Soil Characteristics in Conventional Paddy Field and Organic Paddy Field. J. Wet. Res. 2023, 25, 237–247. [Google Scholar]
- Middleton, B.A. Soil Seed Banks and the Potential Restoration of Forested Wetlands after Farming. J. Appl. Ecol. 2003, 40, 1025–1034. [Google Scholar] [CrossRef]
- Wang, G.D.; Wang, M.; Lu, X.G.; Jiang, M. Effects of Farming on the Soil Seed Banks and Wetland Restoration Potential in Sanjiang Plain, Northeastern China. Ecol. Eng. 2015, 77, 265–274. [Google Scholar] [CrossRef]
- Gomes, M.P.; Richardi, V.S.; Bicalho, E.M.; da Rocha, D.C.; Navarro-Silva, M.A.; Soffiatti, P.; Garcia, Q.S.; Sant’Anna-Santos, B.F. Effects of Ciprofloxacin and Roundup on Seed Germination and Root Development of Maize. Sci. Total Environ. 2019, 651, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.P.; da Silva Cruz, F.V.; Bicalho, E.M.; Borges, F.V.; Fonseca, M.B.; Juneau, P.; Garcia, Q.S. Effects of Glyphosate Acid and the Glyphosate-Commercial Formulation (Roundup) on Dimorphandra wilsonii Seed Germination: Interference of Seed Respiratory Metabolism. Environ. Pollut. 2017, 220, 452–459. [Google Scholar] [CrossRef]
- Ruthrof, K.X.; Hopkins, A.J.; Danks, M.; O’Hara, G.; Bell, R.; Henry, D.; Standish, R.; Tibbett, M.; Howieson, J.; Burgess, T.; et al. Rethinking Soil Water Repellency and Its Management. Plant Ecol. 2019, 220, 977–984. [Google Scholar] [CrossRef]
- Van Der Weiden, M.J.J.; van Haperen, A.M.; Kanters, T.J.; Wassen, M.J. Ecohydrogeochemistry of the Slikken van Flakkee, a Former Tidal Wetland in the Netherlands. Plant Ecol. 2023, 224, 417–434. [Google Scholar] [CrossRef]
- Eskelinen, A.; Jessen, M.T.; Bahamonde, H.A.; Bakker, J.D.; Borer, E.T.; Caldeira, M.C.; Harpole, W.S.; Jia, M.; Lannes, L.S.; Nogueira, C.; et al. Herbivory and Nutrients Shape Grassland Soil Seed Banks. Nat. Commun. 2023, 14, 3949. [Google Scholar] [CrossRef]
- Amiaud, B.; Touzard, B. The Relationships between Soil Seed Bank, Aboveground Vegetation, and Disturbances in Old Embanked Marshlands of Western France. Flora 2004, 199, 25–35. [Google Scholar] [CrossRef]
- Kiss, R.; Deák, B.; Török, P.; Tóthmérész, B.; Valkó, O. Grassland Seed Bank and Community Resilience in a Changing Climate. Restor. Ecol. 2018, 26, S141–S150. [Google Scholar] [CrossRef]
- Tóth, Á.; Deák, B.; Kelemen, A.; Kiss, R.; Lukács, K.; Bátori, Z.; Valkó, O. Vertical Stratification of the Soil Seed Bank in Wet Grasslands and Its Implications for Restoration. Community Ecol. 2024. [Google Scholar] [CrossRef]
- Liuqing, Y.; Yongliang, L.; Mingyao, W.; Xiufu, Z.; Shiwen, H.; Qing, X. Plant Diversity Research in Rice Field Habitat. Chin. J. Rice Sci. 1998, 12, 149. [Google Scholar]
- Han, X.; Hu, C.; Chen, Y.; Qiao, Y.; Liu, D.; Fan, J.; Li, S.; Zhang, Z. Soil Nitrogen Sequestration in a Long-Term Fertilizer Experiment in Central China. Span. J. Agric. Res. 2020, 18, e1102. [Google Scholar] [CrossRef]
- Liang, B.C.; MacKenzie, A.F.; Schnitzer, M.; Monreal, C.M.; Voroney, P.R.; Beyaert, R.P. Management-Induced Change in Labile Soil Organic Matter under Continuous Corn in Eastern Canadian Soils. Biol. Fertil. Soils 1997, 26, 88–94. [Google Scholar] [CrossRef]
- Bang, J.H.; Lee, E.J. Differences in Crab Burrowing and Halophyte Growth by Habitat Types in a Korean Salt Marsh. Ecol. Indic. 2019, 98, 599–607. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Shao, Y.; Wang, J.; Chen, Z.; Roß-Nickoll, M.; Schäffer, A. Conversion of Rice Field Ecosystems from Conventional to Ecological Farming: Effects on Pesticide Fate, Ecotoxicity and Soil Properties. Environ. Manag. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bless, A.; Davila, F.; Plant, R. A Genealogy of Sustainable Agriculture Narratives: Implications for the Transformative Potential of Regenerative Agriculture. Agric. Hum. Values 2023, 40, 1379–1397. [Google Scholar] [CrossRef]
- Duque, T.S.; Pinheiro, R.A.; Souza, I.M.; Silva, G.G.; Soares, M.A.; Dos Santos, J.B. Herbicides and Bio-Inputs: Compatibility and Challenges for Sustainable Agriculture. Chemosphere 2024, 369, 143878. [Google Scholar] [CrossRef]
- Tang, F.H.; Lenzen, M.; McBratney, A.; Maggi, F. Risk of Pesticide Pollution at the Global Scale. Nat. Geosci. 2021, 14, 206–210. [Google Scholar] [CrossRef]
- Barroso, G.M.; Dos Santos, E.A.; Pires, F.R.; Galon, L.; Cabral, C.M.; Dos Santos, J.B. Phytoremediation: A Green and Low-Cost Technology to Remediate Herbicides in the Environment. Chemosphere 2023, 334, 138943. [Google Scholar] [CrossRef]
- Lorente, C.; Causape, J.; Glud, R.N.; Hancke, K.; Merchan, D.; Muniz, S.; Val, J.; Navarro, E. Impacts of Agricultural Irrigation on Nearby Freshwater Ecosystems: The Seasonal Influence of Triazine Herbicides in Benthic Algal Communities. Sci. Total Environ. 2015, 503, 151–158. [Google Scholar] [CrossRef]
- Osland, M.J.; Stevens, P.W.; Lamont, M.M.; Brusca, R.C.; Hart, K.M.; Waddle, J.H.; Seminoff, J.A. Tropicalization of Temperate Ecosystems in North America: The Northward Range Expansion of Tropical Organisms in Response to Warming Winter Temperatures. Glob. Change Biol. 2021, 27, 3009–3034. [Google Scholar] [CrossRef] [PubMed]
- Delcour, I.; Spanoghe, P.; Uyttendaele, M. Literature Review: Impact of Climate Change on Pesticide Use. Food Res. Int. 2015, 68, 7–15. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Jin, Z.; Smith, P.; Barrett, C.B.; Zhu, Y.G.; Zhuang, M. Climate Change Exacerbates the Environmental Impacts of Agriculture. Science 2024, 385, eadn3747. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Nam, H.K.; Yoo, J.C. Characteristics of Population Dynamics and Habitat Use of Shorebirds in Rice Fields during Spring Migration. Korean J. Environ. Agric. 2014, 33, 334–343. [Google Scholar]
- Lee, H.; Alday, J.G.; Cho, K.H.; Lee, E.J.; Marrs, R.H. Effects of Flooding on the Seed Bank and Soil Properties in a Conservation Area on the Han River, South Korea. Ecol. Eng. 2014, 70, 102–113. [Google Scholar] [CrossRef]
- Korea National Arboretum. Checklist of Vascular Plants in Korea (Native Plants); Korea National Arboretum: Pocheon, Republic of Korea, 2020; p. 1006. [Google Scholar]
- NIAS. Methods of Analysis of Soil and Plant; National Institute of Agricultural Science, RDA: Suwon, Republic of Korea, 2000. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, P.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]

| CP_F (%) | CP_R (%) | OP_F (%) | OP_R (%) | |
|---|---|---|---|---|
| Bare ground | 97.2 (0.9) | 11.7 (1.7) | 3.8 (1.2) | 19.2 (2.0) |
| Oryza sativa L. | - | 88.3 (1.7) | - | 80.8 (2.0) |
| Digitaria ciliaris (Retz.) Koeler | 0.5 (0.2) | - | 72.5 (7.5) | - |
| Persicaria lapathifolia (L.) Delarbre | - | - | 9.3 (5.5) | - |
| Commelina communis L. | - | - | 6.3 (5.7) | - |
| Setaria pumila (Poir.) Roem. & Schult. | - | - | 4.7 (2.2) | - |
| Portulaca oleracea L. | 1.7 (0.7) | - | - | - |
| Cyperus iria L. | - | - | 1.2 (0.8) | - |
| Eclipta thermalis Bunge | - | - | 0.8 (0.2) | - |
| Lactuca indica L. | - | - | 0.8 (0.8) | - |
| Cardamine flexuosa With. | 0.5 (0.2) | - | - | - |
| Echinochloa crus-galli (L.) P.Beauv. var. echinatum (Willd.) Honda | - | - | 0.5 (0.2) | - |
| Rorippa palustris (L.) Besser | 0.2 (0.2) | - | - | - |
| No. | Scientific Name | CP_F | CP_R | OP_F | OP_R |
|---|---|---|---|---|---|
| sp1 | Bidens tripartita L. | 0 (0) | 0 (0) | 0 (0) | 28 (28) |
| sp2 | Galium spurium L. var. echinospermum (Wallr.) Desp. | 0 (0) | 0 (0) | 85 (85) | 0 (0) |
| sp3 | Ranunculus sceleratus L. | 0 (0) | 0 (0) | 28 (28) | 0 (0) |
| sp4 | Senecio vulgaris L. | 57 (57) | 0 (0) | 0 (0) | 0 (0) |
| sp5 | Oxalis corniculata L. | 0 (0) | 0 (0) | 170 (139) | 0 (0) |
| sp6 | Acalypha australis L. | 0 (0) | 0 (0) | 113 (72) | 85 (85) |
| sp7 | Alopecurus aequalis Sobol. | 0 (0) | 0 (0) | 0 (0) | 11,928 (3050) |
| sp8 | Erigeron canadensis L. | 0 (0) | 113 (57) | 0 (0) | 57 (57) |
| sp9 | Fimbristylis littoralis Gaudich. | 0 (0) | 0 (0) | 1587 (684) | 57 (36) |
| sp10 | Digitaria ciliaris (Retz.) Koeler | 0 (0) | 0 (0) | 2040 (337) | 680 (124) |
| sp11 | Lindernia procumbens (Krock.) Philcox | 0 (0) | 0 (0) | 708 (217) | 368 (52) |
| sp12 | Vicia villosa Roth | 0 (0) | 0 (0) | 0 (0) | 142 (52) |
| sp13 | Stellaria alsine Grimm | 0 (0) | 170 (62) | 1558 (772) | 8188 (3829) |
| sp14 | Eragrostis multicaulis Steud. | 1700 (670) | 198 (142) | 57 (57) | 0 (0) |
| sp15 | Poa annua L. | 113 (84) | 5582 (1858) | 907 (327) | 18,105 (4465) |
| sp16 | Rorippa palustris (L.) Besser | 1502 (742) | 453 (195) | 0 (0) | 0 (0) |
| sp17 | Portulaca oleracea L. | 2408 (762) | 227 (84) | 85 (38) | 0 (0) |
| sp18 | Cyperus difformis L. | 0 (0) | 28 (28) | 0 (0) | 0 (0) |
| sp19 | Ludwigia epilobioides Maxim. | 0 (0) | 0 (0) | 113 (84) | 85 (38) |
| sp20 | Eleusine indica (L.) Gaertn. | 0 (0) | 85 (58) | 28 (28) | 0 (0) |
| sp21 | Cerastium glomeratum Thuill. | 0 (0) | 0 (0) | 992 (403) | 113 (72) |
| sp22 | Mazus pumilus (Burm.f.) Steenis | 0 (0) | 113 (36) | 3938 (1165) | 1388 (360) |
| sp23 | Centipeda minima (L.) A. Braun & Asch. | 1020 (600) | 255 (137) | 397 (218) | 170 (88) |
| sp24 | Cyperus iria L. | 0 (0) | 0 (0) | 397 (251) | 0 (0) |
| sp25 | Euchiton japonicus (Thunb.) Holub | 0 (0) | 0 (0) | 57 (57) | 0 (0) |
| sp26 | Eclipta thermalis Bunge | 113 (57) | 85 (58) | 1190 (116) | 652 (135) |
| sp27 | Cardamine flexuosa With. | 85 (85) | 340 (116) | 538 (167) | 510 (139) |
| Total seed density (seeds m−2) | 6998 | 7649 | 14,988 | 42,556 |
| Soil Factors | NMS1 | NMS2 | r2 | p |
|---|---|---|---|---|
| Water Content (%) | 0.75720 | −0.65319 | 0.6129 | 0.001 |
| Organic Matter (g kg−1) | 0.48723 | 0.87327 | 0.5493 | 0.001 |
| pH | −0.81769 | −0.57566 | 0.5498 | 0.001 |
| EC (dS m−1) | −0.74982 | −0.66164 | 0.2234 | 0.073 |
| TN (%) | 0.54882 | 0.83594 | 0.5341 | 0.001 |
| TC (%) | 0.48691 | 0.87345 | 0.5502 | 0.001 |
| NH4+ (mg kg−1) | 0.75857 | 0.65159 | 0.0282 | 0.736 |
| NO3− (mg kg−1) | −0.83592 | 0.54884 | 0.2828 | 0.024 |
| P2O5 (mg kg−1) | −0.16212 | 0.98677 | 0.3870 | 0.006 |
| K+ (cmolc kg−1) | −0.90600 | −0.42329 | 0.1860 | 0.124 |
| Ca2+ (cmolc kg−1) | −0.71413 | −0.70001 | 0.7103 | 0.001 |
| Mg2+ (cmolc kg−1) | −0.29794 | −0.95459 | 0.1438 | 0.210 |
| Na+ (cmolc kg−1) | −0.86108 | −0.50847 | 0.4564 | 0.005 |
| Fe (%) | −0.97887 | 0.20450 | 0.3277 | 0.016 |
| Mn (mg kg−1) | −0.98540 | −0.17023 | 0.6909 | 0.001 |
| B (mg kg−1) | −0.99840 | −0.05663 | 0.3243 | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, J.H.; An, N.-H.; Lee, Y.-M.; Park, J.-H.; Kong, M.-J.; Hong, S.-J. Distribution of Vegetation and Soil Seed Banks Across Habitat Types in Paddy Fields Under Different Farming Practices. Plants 2025, 14, 177. https://doi.org/10.3390/plants14020177
Bang JH, An N-H, Lee Y-M, Park J-H, Kong M-J, Hong S-J. Distribution of Vegetation and Soil Seed Banks Across Habitat Types in Paddy Fields Under Different Farming Practices. Plants. 2025; 14(2):177. https://doi.org/10.3390/plants14020177
Chicago/Turabian StyleBang, Jeong Hwan, Nan-Hee An, Young-Mi Lee, Jong-Ho Park, Min-Jae Kong, and Sung-Jun Hong. 2025. "Distribution of Vegetation and Soil Seed Banks Across Habitat Types in Paddy Fields Under Different Farming Practices" Plants 14, no. 2: 177. https://doi.org/10.3390/plants14020177
APA StyleBang, J. H., An, N.-H., Lee, Y.-M., Park, J.-H., Kong, M.-J., & Hong, S.-J. (2025). Distribution of Vegetation and Soil Seed Banks Across Habitat Types in Paddy Fields Under Different Farming Practices. Plants, 14(2), 177. https://doi.org/10.3390/plants14020177






