Plant Adaptation and Soil Shear Strength: Unraveling the Drought Legacy in Amorpha fruticosa
Abstract
1. Introduction
2. Materials and Methods
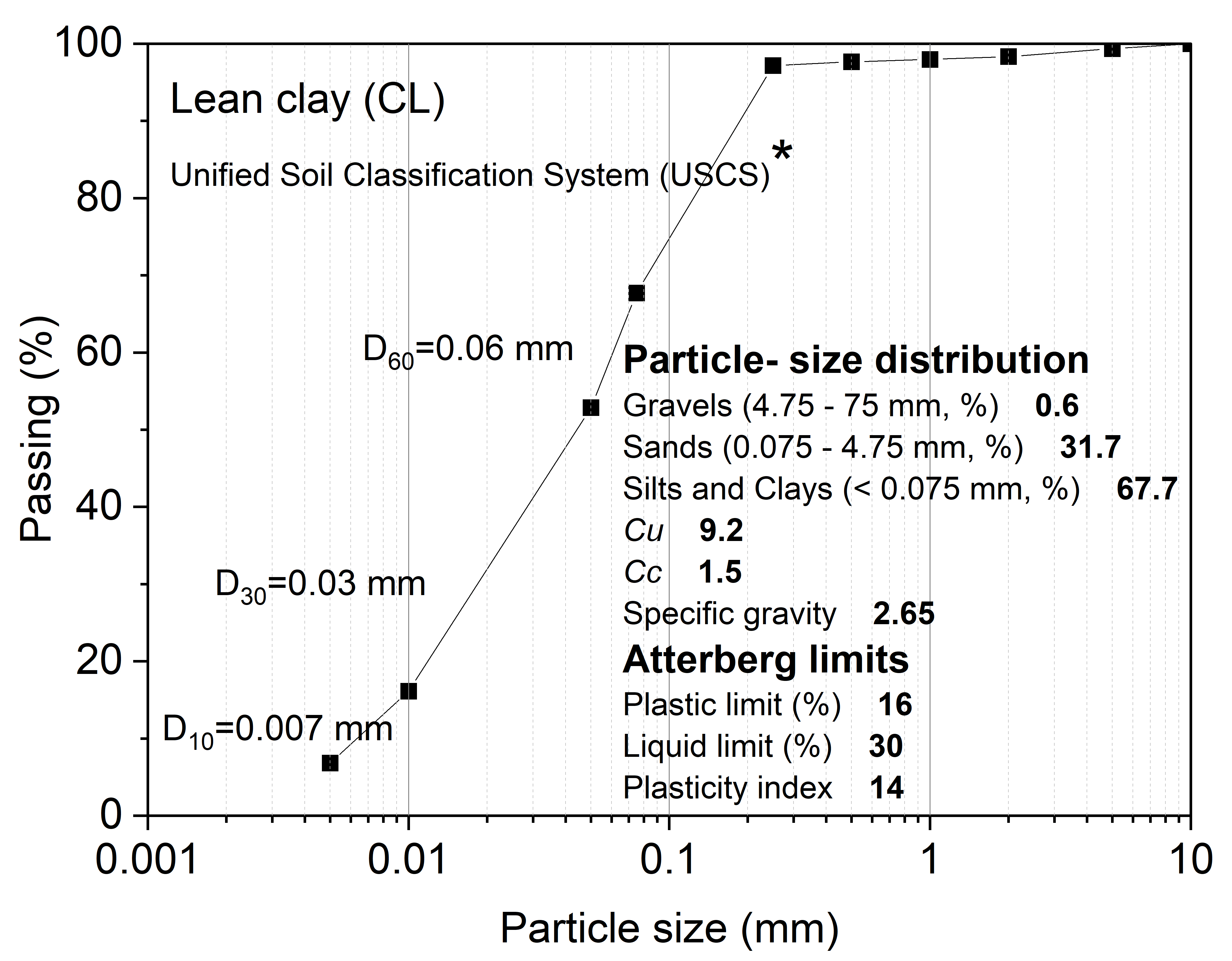
2.1. Experimental Materials and Designs
2.2. Direct Shear Tests
2.3. Prediction of Soil Shear Strength by the Wu and Waldron Model (WWM)
2.4. Plant Morphology
2.5. Gas Exchange
2.6. Root Chemistry
2.7. Statistical Analyses
3. Results
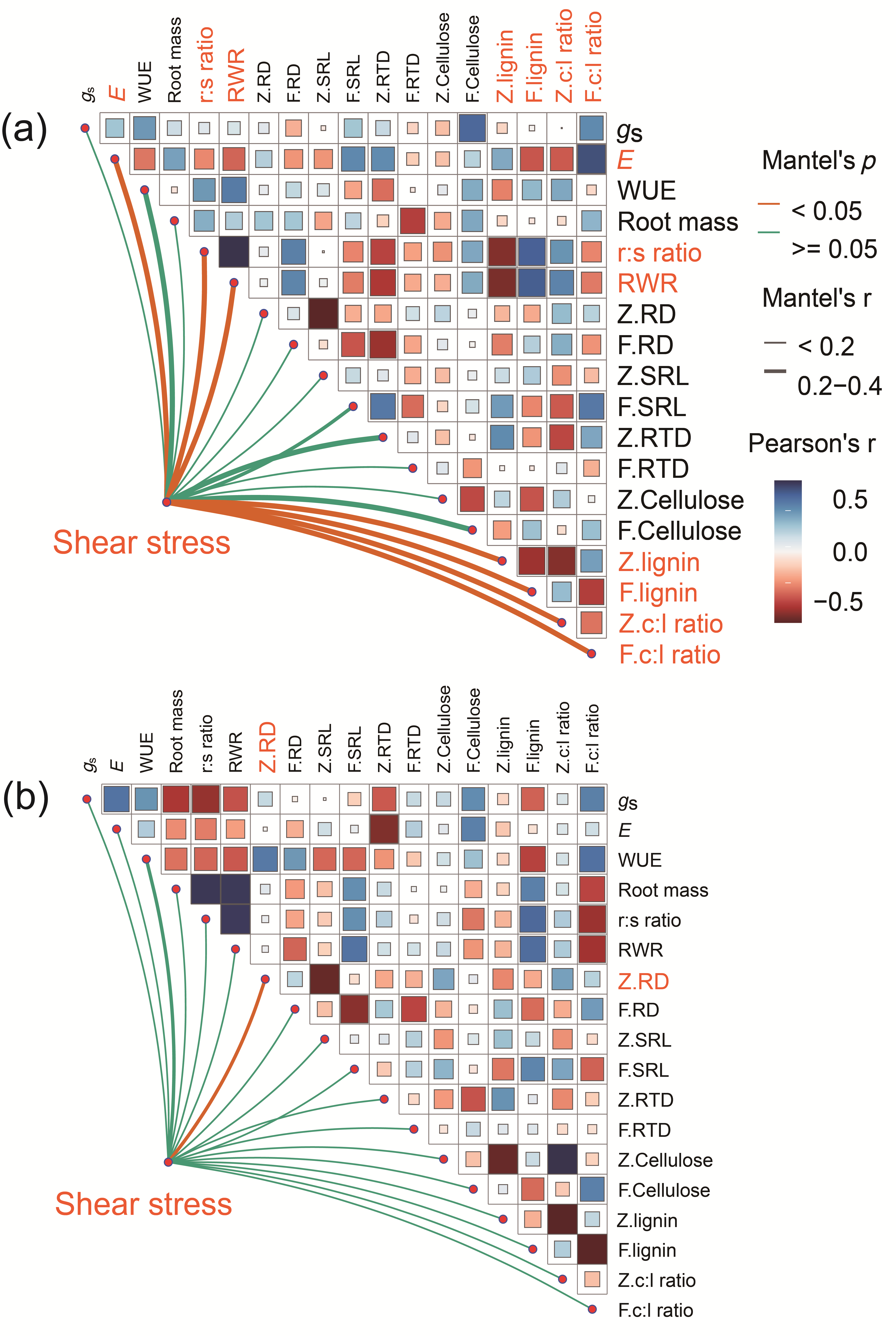
3.1. Legacy Effects of Drought on Root Reinforcement
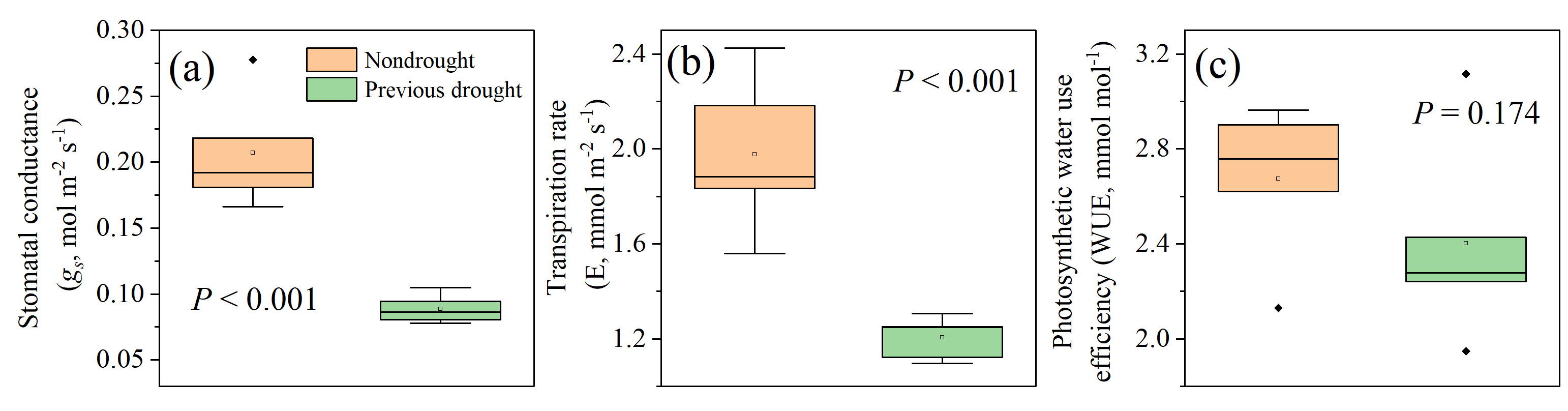
3.2. Legacy Effects of Drought on Plant Morphological Traits and Gas Exchange
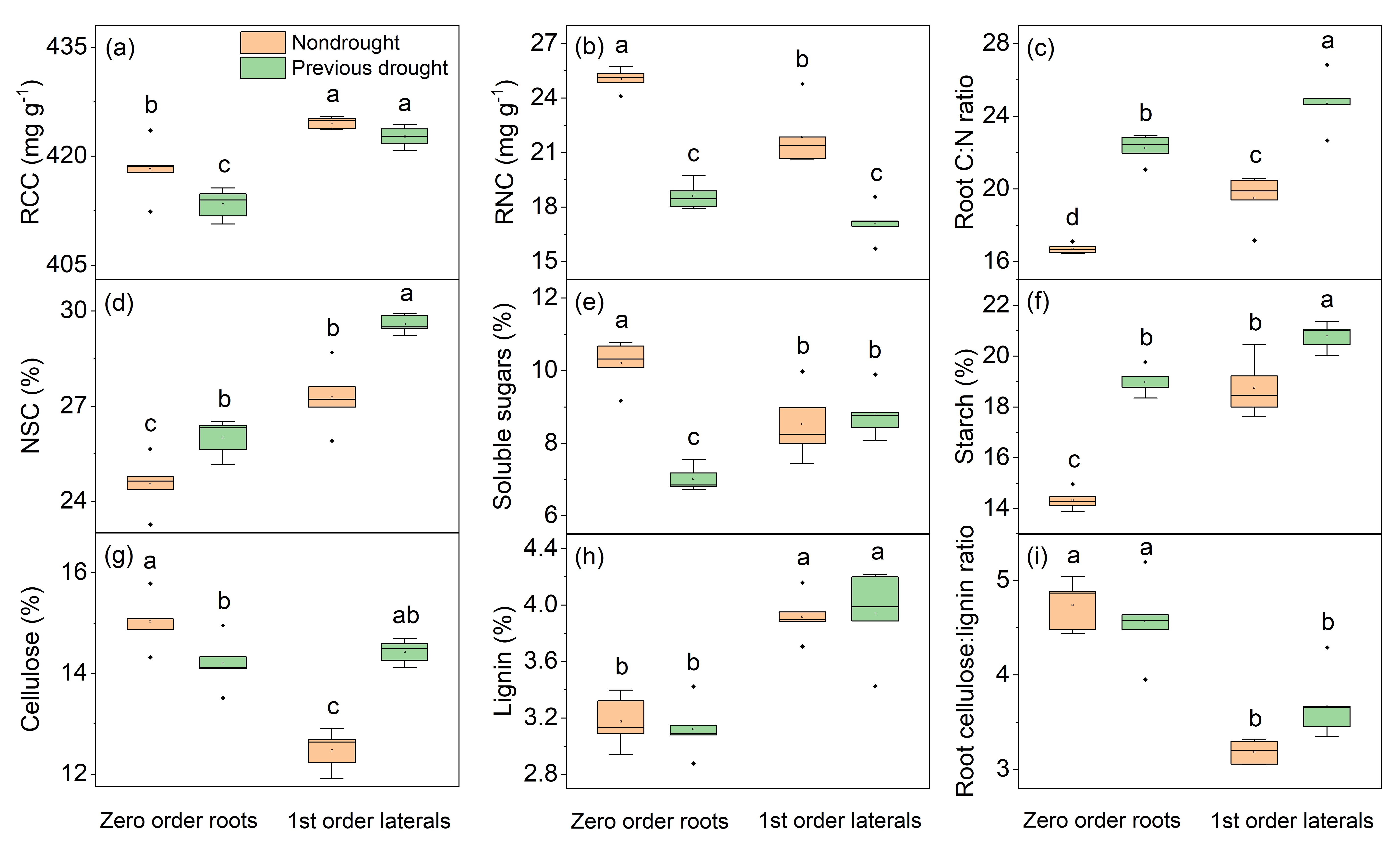
3.3. Legacy Effects of Drought on Root Chemical Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, N.; Lu, Y.; Zhou, H.; Deng, M.; Han, D. Combined impacts of antecedent earthquakes and droughts on disastrous debris flows. J. Mt. Sci. 2014, 11, 1507–1520. [Google Scholar] [CrossRef]
- Gariano, S.L.; Guzzetti, F. Landslides in a changing climate. Earth-Sci. Rev. 2016, 162, 227–252. [Google Scholar] [CrossRef]
- McGuire, L.A.; Rengers, F.K.; Kean, J.W.; Coe, J.A.; Mirus, B.B.; Baum, R.L.; Godt, J.W. Elucidating the role of vegetation in the initiation of rainfall-induced shallow landslides: Insights from an extreme rainfall event in the Colorado Front Range. Geophys. Res. Lett. 2016, 43, 9084–9092. [Google Scholar] [CrossRef]
- Ghestem, M.; Veylon, G.; Bernard, A.; Vanel, Q.; Stokes, A. Influence of plant root system morphology and architectural traits on soil shear resistance. Plant Soil 2014, 377, 43–61. [Google Scholar] [CrossRef]
- Li, B.V.; Jenkins, C.N.; Xu, W. Strategic protection of landslide vulnerable mountains for biodiversity conservation under land-cover and climate change impacts. Proc. Natl. Acad. Sci. USA 2022, 119, e2113416118. [Google Scholar] [CrossRef]
- Masi, E.B.; Segoni, S.; Tofani, V. Root reinforcement in slope stability models: A review. Geosciences 2021, 11, 212. [Google Scholar] [CrossRef]
- Vergani, C.; Giadrossich, F.; Buckley, P.; Conedera, M.; Pividori, M.; Salbitano, F.; Rauch, H.S.; Lovreglio, R.; Schwarz, M. Root reinforcement dynamics of European coppice woodlands and their effect on shallow landslides: A review. Earth-Sci. Rev. 2017, 167, 88–102. [Google Scholar] [CrossRef]
- Fan, Y.; Miguez-Macho, G.; Jobbágy, E.G.; Jackson, R.B.; Otero-Casal, C. Hydrologic regulation of plant rooting depth. Proc. Natl. Acad. Sci. USA 2017, 114, 10572–10577. [Google Scholar] [CrossRef] [PubMed]
- Khalilnejad, A.; Ali, F.H.; Osman, N. Contribution of the root to slope stability. Geotech. Geol. Eng. 2012, 30, 277–288. [Google Scholar] [CrossRef]
- Veylon, G.; Ghestem, M.; Stokes, A.; Bernard, A. Quantification of mechanical and hydric components of soil reinforcement by plant roots. Can. Geotech. J. 2015, 52, 1839–1849. [Google Scholar] [CrossRef]
- Kim, J.H.; Fourcaud, T.; Jourdan, C.; Maeght, J.; Mao, Z.; Metayer, J.; Meylan, L.; Pierret, A.; Rapidel, B.; Roupsard, O.; et al. Vegetation as a driver of temporal variations in slope stability: The impact of hydrological processes. Geophys. Res. Lett. 2017, 44, 4897–4907. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Song, B.; Xue, P.; Zhang, W.; Peth, S.; Hill, R.L.; Horn, R. Characterizing uncertainty in process-based hydraulic modeling, exemplified in a semiarid Inner Mongolia steppe. Geoderma 2023, 440, 116713. [Google Scholar] [CrossRef]
- Zi, J.; Liu, T.; Zhang, W.; Pan, X.; Ji, H.; Zhu, H. Quantitatively characterizing sandy soil structure altered by MICP using multi-level thresholding segmentation algorithm. J. Rock Mech. Geotech. Eng. 2024, 16, 4285–4299. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, L.; Jiang, J. Why fine tree roots are stronger than thicker roots: The role of cellulose and lignin in relation to slope stability. Geomorphology 2014, 206, 196–202. [Google Scholar] [CrossRef]
- Giadrossich, F.; Cohen, D.; Schwarz, M.; Ganga, A.; Marrosu, R.; Pirastru, M.; Capra, G.F. Large roots dominate the contribution of trees to slope stability. Earth Surf. Process. Landf. 2019, 44, 1602–1609. [Google Scholar] [CrossRef]
- Fry, E.L.; Evans, A.L.; Sturrock, C.J.; Bullock, J.M.; Bardgett, R.D. Root architecture governs plasticity in response to drought. Plant Soil 2018, 433, 189–200. [Google Scholar] [CrossRef]
- Fromm, H. Root plasticity in the pursuit of water. Plants 2019, 8, 236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L. Root-soil-water hydrological interaction and its impact on slope stability. Georisk Assess. Manag. Risk Eng. Syst. Geohazards 2019, 13, 349–359. [Google Scholar] [CrossRef]
- Kamchoom, V.; Boldrin, D.; Leung, A.K.; Sookkrajang, C.; Likitlersuang, S. Biomechanical properties of the growing and decaying roots of Cynodon dactylon. Plant Soil 2022, 471, 193–210. [Google Scholar] [CrossRef]
- Kamchoom, V.; Leung, A.K.; Boldrin, D.; Sakolpanya, T.; Wu, Z.; Likitlersuang, S. Shearing behaviour of vegetated soils with growing and decaying roots. Can. Geotech. J. 2022, 59, 2067–2084. [Google Scholar] [CrossRef]
- Ray, R.L.; Jacobs, J.M.; Ballestero, T.P. Regional landslide susceptibility: Spatiotemporal variations under dynamic soil moisture conditions. Nat. Hazards 2011, 59, 1317–1337. [Google Scholar] [CrossRef]
- Uhlemann, S.; Chambers, J.; Wilkinson, P.; Maurer, H.; Merritt, A.; Meldrum, P.; Kuras, O.; Gunn, D.; Smith, A.; Dijkstra, T. Four-dimensional imaging of moisture dynamics during landslide reactivation. J. Geophys. Res. Earth Surf. 2017, 122, 398–418. [Google Scholar] [CrossRef]
- Handwerger, A.L.; Huang, M.; Fielding, E.J.; Booth, A.M.; Bürgmann, R. A shift from drought to extreme rainfall drives a stable landslide to catastrophic failure. Sci. Rep. 2019, 9, 1569. [Google Scholar] [CrossRef]
- Kayadelen, C.; Tekinsoy, M.A.; Taşkıran, T. Influence of matric suction on shear strength behavior of a residual clayey soil. Environ. Geol. 2007, 53, 891–901. [Google Scholar] [CrossRef]
- Çokça, E.; Tilgen, H.P. Shear strength-suction relationship of compacted Ankara clay. Appl. Clay Sci. 2010, 49, 400–404. [Google Scholar] [CrossRef]
- Filho, O.A.; Fernandes, M.A. Landslide analysis of unsaturated soil slopes based on rainfall and matric suction data. Bull. Eng. Geol. Environ. 2018, 78, 4167–4185. [Google Scholar] [CrossRef]
- Yang, Z.; Cai, H.; Shao, W.; Huang, D.; Uchimura, T.; Lei, X.; Tian, H.; Qiao, J. Clarifying the hydrological mechanisms and thresholds for rainfall-induced landslide: In situ monitoring of big data to unsaturated slope stability analysis. Bull. Eng. Geol. Environ. 2018, 78, 2139–2150. [Google Scholar] [CrossRef]
- Ni, J.J.; Leung, A.K.; Ng, C.W.W.; Shao, W. Modelling hydro-mechanical reinforcements of plants to slope stability. Comput. Geotech. 2018, 95, 99–109. [Google Scholar] [CrossRef]
- Wang, C.; Fu, B.; Zhang, L.; Xu, Z. Soil moisture–plant interactions: An ecohydrological review. J. Soils Sediments 2018, 19, 1–9. [Google Scholar] [CrossRef]
- ASTM D2487-17; Standard Practice for Classification of Soils for Engineering Purposes. ASTM International: West Conshohocken, PA, USA, 2020.
- Guo, H.; Chen, X.; Song, D.; Mu, Q.; Sadeghi, H.; Jiang, H.; Lv, M. Effects of solar radiation and fine roots on suction of Amorpha fruticosa vegetated soil. J. Mt. Sci. 2023, 20, 1790–1804. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Song, D.; Lv, M.; Guo, H.; Sadeghi, H. Characteristics of root-permeated soil under simple-shear and direct-shear conditions. J. Mt. Sci. 2023, 20, 2422–2435. [Google Scholar] [CrossRef]
- Wu, T.H.; McKinnell, W.P.; Swanston, D.N. Strength of the tree roots and landslides on Prince of Wales Island, Alaska. Can. Geotech. J. 1979, 16, 19–33. [Google Scholar] [CrossRef]
- Mao, Z. Root reinforcement models: Classification, criticism and perspectives. Plant Soil 2022, 472, 17–28. [Google Scholar] [CrossRef]
- Bai, L.; Liu, J.; Hu, J.; Zhang, X.; Li, S. Deformation characteristics of the straight roots of Amorpha fruticosa. Arid Zone Res. 2021, 38, 1111–1119. [Google Scholar]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A starting guide to root ecology: Strengthening ecological concepts and standardising root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.A.; Boerner, R. Foliar nutrient dynamics and nutrient use efficiency in Cornus florida. Oecologia 1985, 66, 602–606. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Change 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Marquis, R.J.; Newell, E.; Villegas, A. Non-structural carbohydrate accumulation and use in an understorey rain-forest shrub and relevance for the impact of leaf herbivory. Funct. Ecol. 1997, 11, 636–643. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate storage enhances seedling shade and stress tolerance in a neotropical forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Leavitt, S.W.; Danzer, S.R. Method for batch processing small wood samples to holocellulose for stable-carbon isotope analysis. Anal. Chem. 1993, 65, 87–89. [Google Scholar] [CrossRef]
- Chaves, A.V.; Waghorn, G.C.; Tavendale, M.H. A simplified method for lignin measurement in a range of forage species. J. New Zealand Grassl. 2002, 64, 129–133. [Google Scholar] [CrossRef]
- Van Soest, P.J. Development of a comprehensive system of feed analyses and its application to forages. J. Anim. Sci. 1967, 26, 119–128. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, X.; Nie, Y.; Bai, S.H.; Zhou, L.; Shao, J.; Cheng, W.; Wang, J.; Hu, F.; Fu, Y. Drought-induced changes in root biomass largely result from altered root morphological traits: Evidence from a synthesis of global field trials. Plant Cell Environ. 2018, 41, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Khuder, H.; Stokes, A.; Danjon, F.; Gouskou, K.; Lagane, F. Is it possible to manipulate root anchorage in young trees? Plant Soil 2007, 294, 87–102. [Google Scholar] [CrossRef]
- Stokes, A.; Lucas, A.; Jouneau, L. Plant biomechanical strategies in response to frequent disturbance: Uprooting of Phyllostachys nidularia (Poaceae) growing on landslide-prone slopes in Sichuan, China. Am. J. Bot. 2007, 94, 1129–1136. [Google Scholar] [CrossRef]
- Wu, T.H. Root reinforcement of soil: Review of analytical models, test results, and applications to design. Can. Geotech. J. 2013, 50, 259–274. [Google Scholar] [CrossRef]
- Liu, X.; Lan, H.; Li, L.; Cui, P. An ecological indicator system for shallow landslide analysis. Catena 2022, 214, 106211. [Google Scholar] [CrossRef]
- Qin, M.; Cui, P.; Jiang, Y.; Guo, J.; Zhang, G.; Ramzan, M. Occurrence of shallow landslides triggered by increased hydraulic conductivity due to tree roots. Landslides 2022, 19, 2593–2604. [Google Scholar] [CrossRef]
- Danjon, F.; Barker, D.H.; Drexhage, M.; Stokes, A. Using three-dimensional plant root architecture in models of shallow-slope stability. Ann. Bot. 2008, 101, 1281–1293. [Google Scholar] [CrossRef] [PubMed]
- Pollen, N.; Simon, A.; Collison, A. Advances in assessing the mechanical and hydrologic effects of riparian vegetation on streambank stability. In Riparian Vegetation and Fluvial Geomorphology; Bennett, S.J., Simon, A., Eds.; American Geophysical Union: Washington, DC, USA, 2004; Volume 8, pp. 125–139. [Google Scholar]
- Hales, T.C.; Miniat, C.F. Soil moisture causes dynamic adjustments to root reinforcement that reduce slope stability. Earth Surf. Process. Landf. 2016, 42, 803–813. [Google Scholar] [CrossRef]
- Cislaghi, A.; Alterio, E.; Fogliata, P.; Rizzi, A.; Lingua, E.; Vacchiano, G.; Bischetti, G.B.; Sitzia, T. Effects of tree spacing and thinning on root reinforcement in mountain forests of the European Southern Alps. For. Ecol. Manag. 2021, 482, 118873. [Google Scholar] [CrossRef]
- Deljouei, A.; Cislaghi, A.; Abdi, E.; Borz, S.A.; Majnounian, B.; Hales, T.C. Implications of hornbeam and beech root systems on slope stability: From field and laboratory measurements to modelling methods. Plant Soil 2022, 483, 547–572. [Google Scholar] [CrossRef]
- Flepp, G.; Robyr, R.; Scotti, R.; Giadrossich, F.; Conedera, M.; Vacchiano, G.; Fischer, C.; Ammann, P.; May, D.; Schwarz, M. Temporal dynamics of root reinforcement in European Spruce forests. Forests 2021, 12, 815. [Google Scholar] [CrossRef]
- Coombes, A.; Martin, J.; Slater, D. Defining the allometry of stem and crown diameter of urban trees. Urban For. Urban Gree. 2019, 44, 126421. [Google Scholar] [CrossRef]
- Guasconi, D.; Manzoni, S.; Hugelius, G. Climate-dependent responses of root and shoot biomass to drought duration and intensity in grasslands–a meta-analysis. Sci. Total Environ. 2023, 903, 166209. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Rehschuh, R.; Cecilia, A.; Zuber, M.; Faragó, T.; Baumbach, T.; Hartmann, H.; Jansen, S.; Mayr, S.; Ruehr, N. Drought-induced xylem embolism limits the recovery of leaf gas exchange in Scots pine. Plant Physiol. 2020, 184, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhong, Y.; Shangguan, Z. A meta-analysis of leaf gas exchange and water status responses to drought. Sci. Rep. 2016, 6, 20917. [Google Scholar] [CrossRef]
- Yu, L.; Huang, Z.; Tang, S.; Korpelainen, H.; Li, C. Populus euphratica males exhibit stronger drought and salt stress resistance than females. Environ. Exp. Bot. 2023, 205, 105114. [Google Scholar] [CrossRef]
- Skelton, R.P.; Brodribb, T.J.; McAdam, S.A.M.; Mitchell, P.J. Gas exchange recovery following natural drought is rapid unless limited by loss of leaf hydraulic conductance: Evidence from an evergreen woodland. New Phytol. 2017, 215, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.W.W.; Ni, J.J.; Leung, A.K.; Wang, Z.J. A new and simple water retention model for root-permeated soils. Géotech. Lett. 2016, 6, 106–111. [Google Scholar] [CrossRef]
- Pallewattha, M.; Indraratna, B.; Heitor, A.; Rujikiatkamjorn, C. Shear strength of a vegetated soil incorporating both root reinforcement and suction. Transp. Geotech. 2019, 18, 72–82. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural carbon in woody plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]


| Height (m) | DBH (mm) | Plant Mass (g) | Root Mass (g) | Stem Mass (g) | Leaf Mass (g) | Shoot Mass (g) | Root/Shoot Ratio | |
|---|---|---|---|---|---|---|---|---|
| Non-drought | 1.99 ± 0.09 | 10.7 ± 0.93 | 343 ± 14.2 | 179 ± 6.18 | 133 ± 11.3 | 31.2 ± 3.92 | 164 ± 13.7 | 1.10 ± 0.10 |
| Previous drought | 1.69 ± 0.12 | 7.00 ± 0.81 | 227 ± 8.96 | 107 ± 6.84 | 95.4 ± 1.51 | 24.1 ± 1.80 | 119 ± 2.45 | 0.90 ± 0.04 |
| F-value | 0.491 | 0.146 | 1.15 | 0.017 | 4.52 | 1.46 | 3.97 | 3.96 |
| p-value | ns | * | ** | ** | * | ns | * | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Chen, X.; Xu, G.; Chen, J.; Song, D.; Lv, M.; Guo, H.; Chen, J. Plant Adaptation and Soil Shear Strength: Unraveling the Drought Legacy in Amorpha fruticosa. Plants 2025, 14, 179. https://doi.org/10.3390/plants14020179
Jiang H, Chen X, Xu G, Chen J, Song D, Lv M, Guo H, Chen J. Plant Adaptation and Soil Shear Strength: Unraveling the Drought Legacy in Amorpha fruticosa. Plants. 2025; 14(2):179. https://doi.org/10.3390/plants14020179
Chicago/Turabian StyleJiang, Hao, Xiaoqing Chen, Gang Xu, Jiangang Chen, Dongri Song, Ming Lv, Hanqing Guo, and Jingyi Chen. 2025. "Plant Adaptation and Soil Shear Strength: Unraveling the Drought Legacy in Amorpha fruticosa" Plants 14, no. 2: 179. https://doi.org/10.3390/plants14020179
APA StyleJiang, H., Chen, X., Xu, G., Chen, J., Song, D., Lv, M., Guo, H., & Chen, J. (2025). Plant Adaptation and Soil Shear Strength: Unraveling the Drought Legacy in Amorpha fruticosa. Plants, 14(2), 179. https://doi.org/10.3390/plants14020179






