The Physicochemical and Rheological Properties of Green Banana Flour–Wheat Flour Bread Substitutions
Abstract
:1. Introduction
2. Results and Discussion
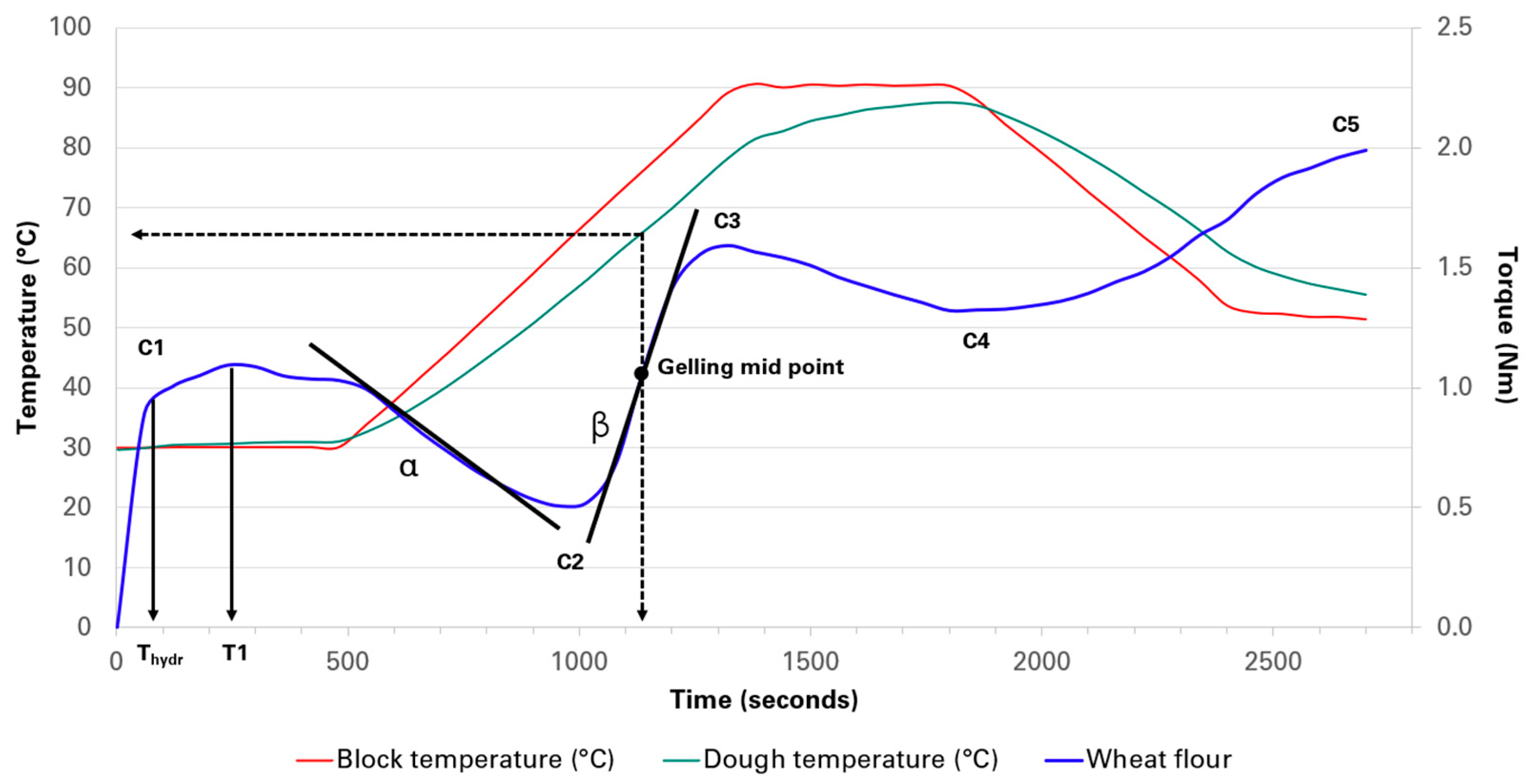
2.1. MixoLab Analysis
2.1.1. Flour Water Absorption Capacity and Stability
2.1.2. Gluten Weakening
| Samples | Substitution (%) | Thydr (min) | T1 (min) | C1 (Nm) | Water Absorption (%) | Amplitude (Nm) | Stability (min) | Slope-α (Nm/min) |
|---|---|---|---|---|---|---|---|---|
| Control bread | 0 | 3.63 ± 0.00 b | 7.08 ± 0.00 a | 1.10 ± 0.00 a | 62.8 ± 0.00 cd | 0.073 ± 0.00 a | 6.97 ± 0.00 cd | −0.095 ± 0.00 b |
| Cavendish | 5 | 4.85 ± 0.15 a | 4.19 ± 0.02 b | 1.10 ± 0.03 a | 61.7 ± 0.00 e | 0.092 ± 0.04 a | 6.87 ± 0.15 d | −0.100 ± 0.00 b |
| Ladyfinger | 1.13 ± 0.14 de | 5.91 ± 0.20 ab | 1.13 ± 0.03 a | 62.8 ± 0.00 cd | 0.101 ± 0.02 a | 7.04 ± 0.08 bd | −0.099 ± 0.00 b | |
| Ducasse | 1.11 ± 0.04 e | 5.28 ± 0.25 ab | 1.12 ± 0.03 a | 62.8 ± 0.52 d | 0.115 ± 0.03 a | 7.02 ± 0.04 cd | −0.100 ± 0.00 b | |
| Cavendish | 10 | 1.31 ± 0.04 ce | 5.66 ± 0.05 ab | 1.09 ± 0.01 a | 61.7 ± 0.00 e | 0.107 ± 0.01 a | 6.85 ± 0.02 d | −0.111 ± 0.01 b |
| Ladyfinger | 1.36 ± 0.06 ce | 5.91 ± 1.51 ab | 1.08 ± 0.01 a | 63.9 ± 0.00 b | 0.105 ± 0.01 a | 7.29 ± 0.09 a | −0.085 ± 0.00 b | |
| Ducasse | 1.57 ± 0.42 cd | 5.50 ± 1.16 ab | 1.10 ± 0.03 a | 62.8 ± 0.58 d | 0.114 ± 0.03 a | 7.16 ± 0.06 a-c | −0.102 ± 0.01 b | |
| Cavendish | 15 | 1.36 ± 0.03 ce | 6.54 ± 0.99 ab | 1.05 ± 0.02 a | 61.7 ± 0.00 e | 0.094 ± 0.03 a | 7.23 ± 0.08 ab | −0.105 ± 0.00 b |
| Ladyfinger | 1.70 ± 0.06 c | 7.68 ± 0.32 a | 0.94 ± 0.02 b | 67.0 ± 0.00 a | 0.109 ± 0.02 a | 7.31 ± 0.02 a | −0.055 ± 0.00 a | |
| Ducasse | 1.43 ± 0.05 ce | 6.14 ± 1.67 ab | 1.07 ± 0.08 a | 63.5 ± 0.00 bc | 0.110 ± 0.01 a | 7.26 ± 0.09 a | −0.090 ± 0.02 b | |
| Samples | Substitution (%) | C2 (Nm) | Slope-β (Nm/min) | C3 (Nm) | Time to C3 (min) | Gelling Mid-point (°C) | C4 (Nm) | C5 (Nm) |
| Control bread | 0 | 0.52 ± 0.00 a | 0.110 ± 0.00 d | 1.61 ± 0.00 c | 21.7 ± 0.00 c | 66.0 ± 0.00 d | 1.29 ± 0.00 cd | 1.89 ± 0.00 c |
| Cavendish | 5 | 0.49 ± 0.01 ac | 0.151 ± 0.02 cd | 1.71 ± 0.00 b | 22.5 ± 0.48 ab | 66.0 ± 0.01 d | 1.29 ± 0.00 cd | 1.95 ± 0.04 c |
| Ladyfinger | 0.51 ± 0.01 a | 0.155 ± 0.00 cd | 1.73 ± 0.00 b | 22.5 ± 0.04 ab | 68.0 ± 0.08 c | 1.37 ± 0.05 bc | 2.13 ± 0.09 b | |
| Ducasse | 0.50 ± 0.00 ab | 0.147 ± 0.03 cd | 1.74 ± 0.00 b | 22.3 ± 0.15 b | 66.0 ± 0.01 d | 1.26 ± 0.03 d | 1.86 ± 0.00 c | |
| Cavendish | 10 | 0.43 ± 0.01 d | 0.219 ± 0.00 b | 1.77 ± 0.01 ab | 22.9 ± 0.13 a | 69.2 ± 0.24 b | 1.38 ± 0.05 ab | 2.13 ± 0.08 b |
| Ladyfinger | 0.47 ± 0.00 bc | 0.174 ± 0.01 bc | 1.72 ± 0.00 b | 22.7 ± 0.18 ab | 68.1 ± 0.15 c | 1.41 ± 0.02 ab | 2.19 ± 0.04 ab | |
| Ducasse | 0.46 ± 0.00 c | 0.184 ± 0.03 bc | 1.75 ± 0.02 b | 22.6 ± 0.12 ab | 68.0 ± 0.01 c | 1.23 ± 0.07 d | 1.85 ± 0.09 c | |
| Cavendish | 15 | 0.43 ± 0.01 d | 0.325 ± 0.01 a | 1.84 ± 0.01 a | 22.8 ± 0.04 ab | 70.1 ± 0.09 a | 1.45 ± 0.01 a | 2.28 ± 0.05 a |
| Ladyfinger | 0.42 ± 0.02 d | 0.197 ± 0.00 bc | 1.70 ± 0.04 bc | 22.7 ± 0.05 ab | 68.1 ± 0.17 c | 1.45 ± 0.04 a | 2.22 ± 0.07 ab | |
| Ducasse | 0.42 ± 0.03 d | 0.229 ± 0.01 b | 1.74 ± 0.05 b | 22.6 ± 0.06 ab | 69.2 ± 0.23 b | 1.24 ± 0.06 d | 1.96 ± 0.02 c |
2.1.3. Starch Gelatinization and Gel Stability
2.1.4. Starch Retrogradation
2.2. Resistant and Non-Resistant Starch and Dietary Fiber Content in GBF-Enriched Bread
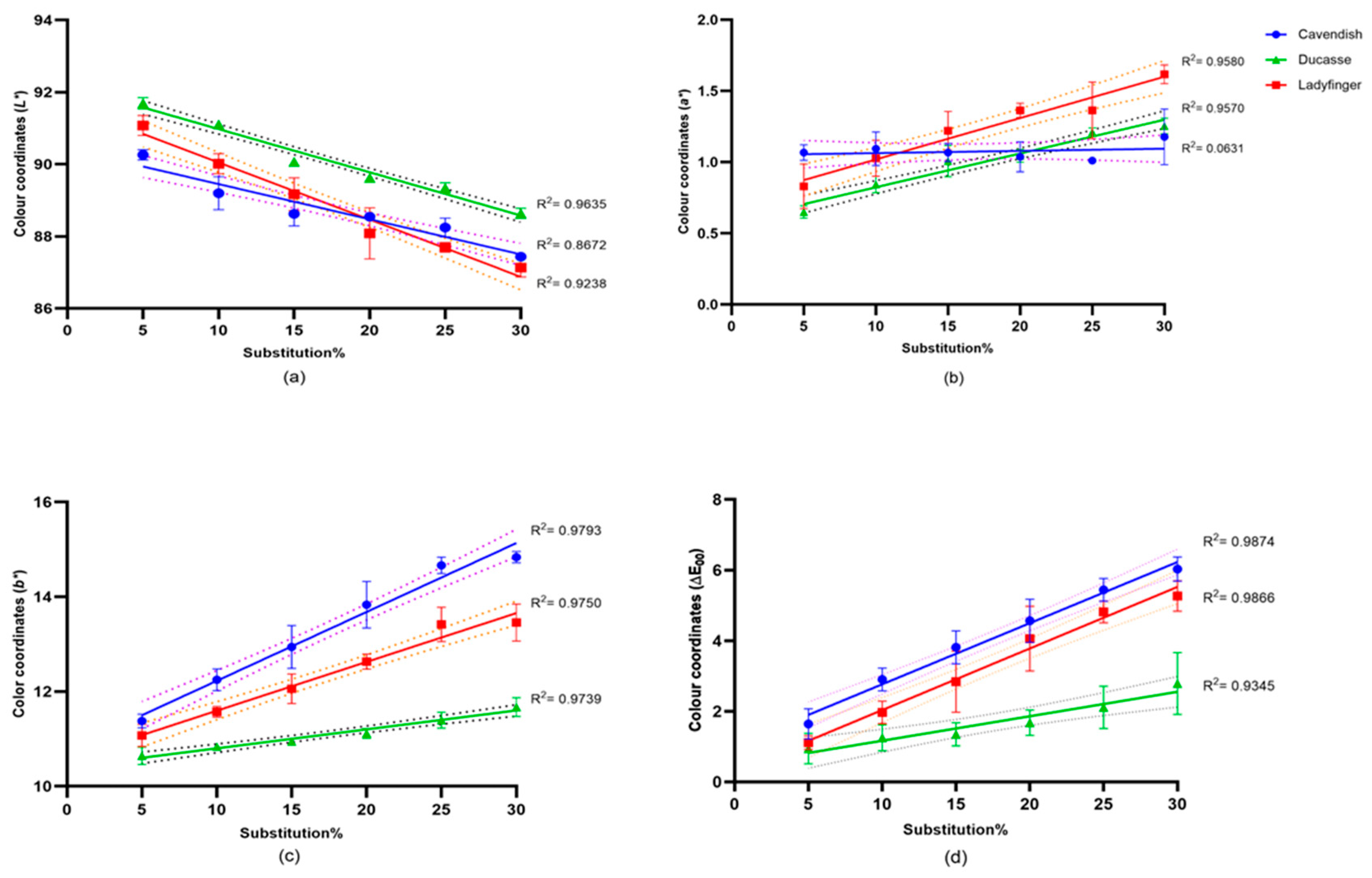
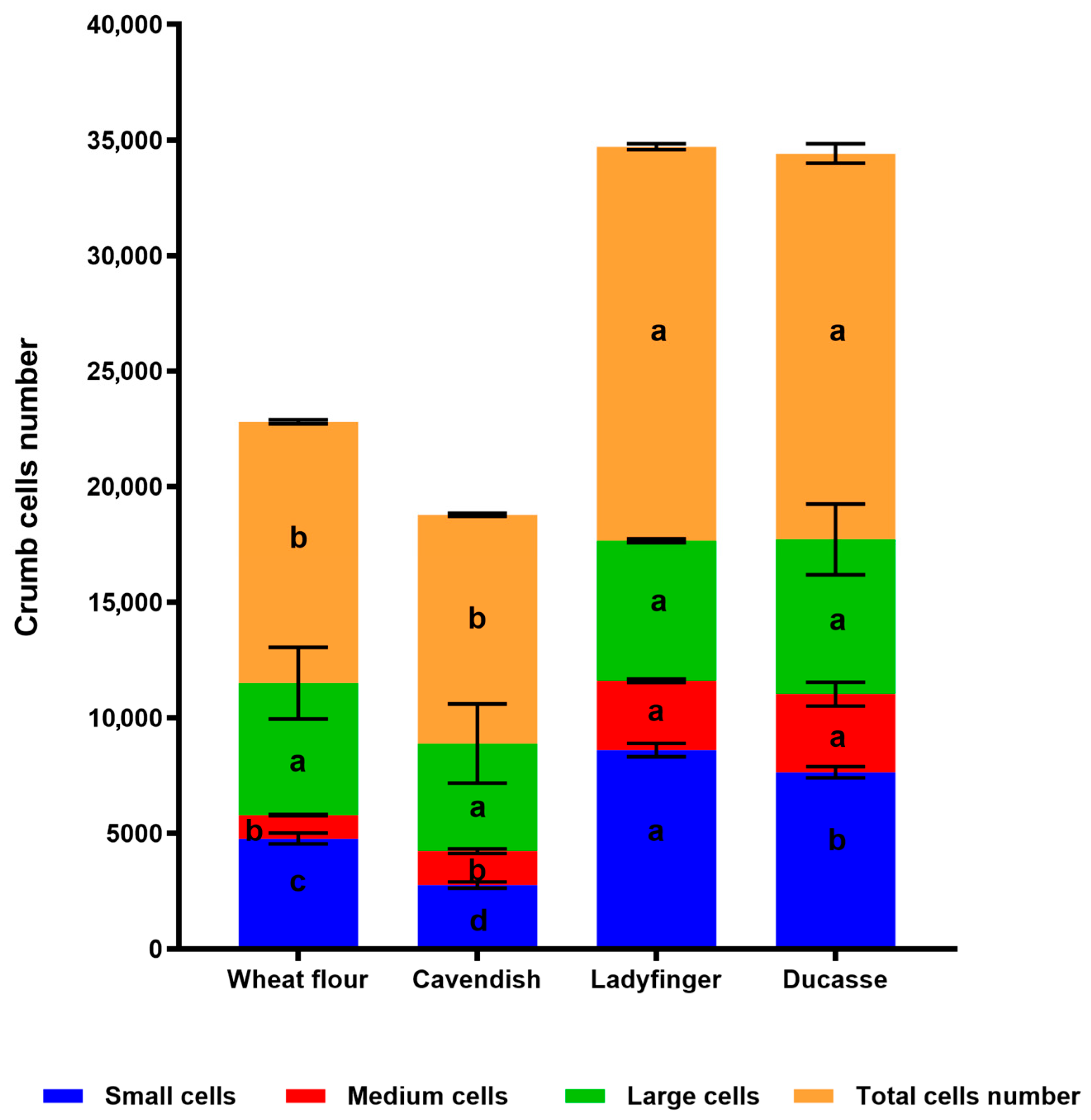
2.3. Flour and Bread Physical Properties
2.3.1. Flour and Bread Color
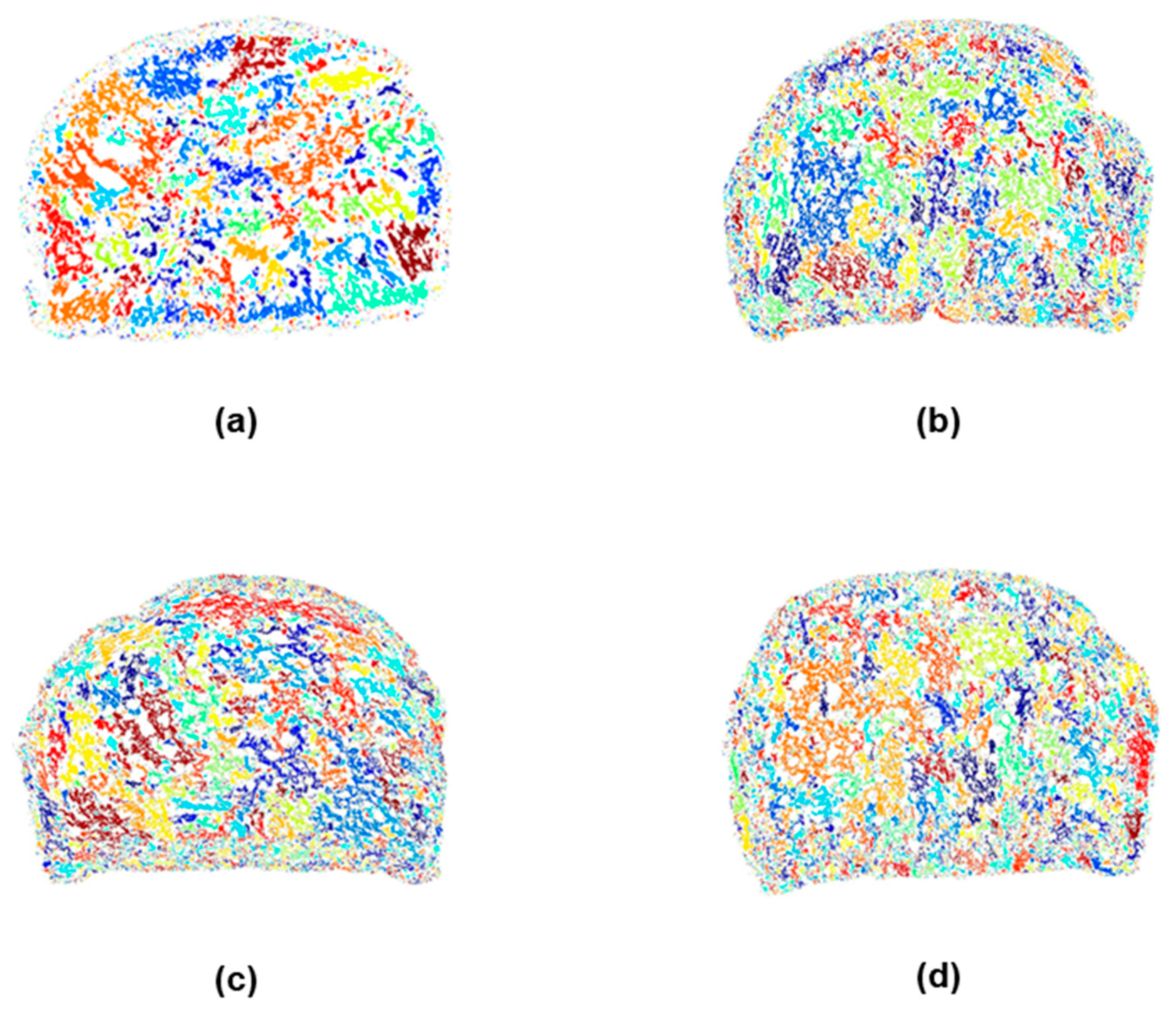
2.3.2. Crumb Cell Structure Analysis
3. Materials and Methods
3.1. Green Banana Flour Production
3.2. Banana Bread Preparation
3.3. Rheological Tests of Wheat Flour and GBF
3.4. RS and N-RS Analysis
3.5. Total Dietary Fiber Content (TDF)
3.6. Color Test
3.7. Digital Image Texture Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Slavin, J. Whole grains and human health. Nutr. Res. Rev. 2004, 17, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Aurore, G.; Parfait, B.; Fahrasmane, L. Bananas, raw materials for making processed food products. Trends Food Sci. Technol. 2009, 20, 78–91. [Google Scholar] [CrossRef]
- Bashmil, Y.M.; Ali, A.; Bk, A.; Dunshea, F.R.; Suleria, H.A.R. Screening and characterization of phenolic compounds from australian grown bananas and their antioxidant capacity. Antioxidants 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Vu, H.T.; Scarlett, C.J.; Vuong, Q.V. Phenolic compounds within banana peel and their potential uses: A review. J. Funct. Foods 2018, 40, 238–248. [Google Scholar] [CrossRef]
- Someya, S.; Yoshiki, Y.; Okubo, K. Antioxidant compounds from bananas (Musa cavendish). Food Chem. 2002, 79, 351–354. [Google Scholar] [CrossRef]
- Amini, A.; Khalili, L.; Keshtiban, A.K.; Homayouni, A. Resistant starch as a bioactive compound in colorectal cancer prevention. In Probiotics, Prebiotics, and Synbiotics: Bioactive Foods in Health Promotion; Ronald Ross Watson, V.R.P., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 773–780. [Google Scholar]
- Bashmil, Y.M.; Dunshea, F.R.; Appels, R.; Suleria, H.A.R. Bioaccessibility of Phenolic Compounds, Resistant Starch, and Dietary Fibers from Australian Green Banana during In Vitro Digestion and Colonic Fermentation. Molecules 2024, 29, 1535. [Google Scholar] [CrossRef]
- Akbar, D.; Babacan, H.; Marty, M.; Nguyen, T.; Rah-man, A.; Brown, P. The Banana Industry Sector Action Plan for Food Waste Reduction 2024 Technical Report; End Food Waste Australia: Urrbrae, Australia; CQU: Rockhampton, Australia; Australian Banana Growers’ Council: Rocklea, Australia, 2024. [Google Scholar]
- Agama-Acevedo, E.; Islas-Hernandez, J.J.; Osorio-Díaz, P.; Rendón-Villalobos, R.; Utrilla-Coello, R.G.; Angulo, O.; Bello-Pérez, L.A. Pasta with unripe banana flour: Physical, texture, and preference study. J. Food Sci. 2009, 74, S263–S267. [Google Scholar] [CrossRef]
- Amini Khoozani, A.; Kebede, B.; Birch, J.; Bekhit, A.E.-D.A. The effect of bread fortification with whole green banana flour on its physicochemical, nutritional and in vitro digestibility. Foods 2020, 9, 152. [Google Scholar] [CrossRef] [PubMed]
- de Souza, N.C.O.; de Oliveira, L.d.L.; de Alencar, E.R.; Moreira, G.P.; dos Santos Leandro, E.; Ginani, V.C.; Zandonadi, R.P. Textural, physical and sensory impacts of the use of green banana puree to replace fat in reduced sugar pound cakes. LWT 2018, 89, 617–623. [Google Scholar] [CrossRef]
- TÜrKer, B.; Savlak, N.; Kaşıkcı, M.B. Effect of green banana peel flour substitution on physical characteristics of gluten-free cakes. Curr. Res. Nutr. Food Sci. 2016, 4, 197–204. [Google Scholar] [CrossRef]
- Gomes, A.A.B.; Ferreira, M.E.; Pimentel, T.C. Bread with flour obtained from green banana with its peel as partial substitute for wheat flour: Physical, chemical and microbiological characteristics and acceptance. Int. Food Res. J. 2016, 23, 2214. [Google Scholar]
- Roman, L.; Gomez, M.; Hamaker, B.R.; Martinez, M.M. Banana starch and molecular shear fragmentation dramatically increase structurally driven slowly digestible starch in fully gelatinized bread crumb. Food Chem. 2019, 274, 664–671. [Google Scholar] [CrossRef]
- Sarawong, C.; Gutiérrez, Z.R.; Berghofer, E.; Schoenlechner, R. Effect of green plantain flour addition to gluten-free bread on functional bread properties and resistant starch content. Int. J. Food Sci. Technol. 2014, 49, 1825–1833. [Google Scholar] [CrossRef]
- Bchir, B.; Rabetafika, H.N.; Paquot, M.; Blecker, C. Effect of pear, apple and date fibres from cooked fruit by-products on dough performance and bread quality. Food Bioprocess Technol. 2014, 7, 1114–1127. [Google Scholar] [CrossRef]
- Bloksma, A.H.; Bushuk, W. Rheology and Chemistry of Doughs. In Wheat Chemistry and Technology, 3rd ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 1988; Volume II, pp. 131–217. [Google Scholar]
- Wang, X.; Appels, R.; Zhang, X.; Bekes, F.; Torok, K.; Tomoskozi, S.; Diepeveen, D.; Ma, W.; Islam, S. Protein-transitions in and out of the dough matrix in wheat flour mixing. Food Chem. 2017, 217, 542–551. [Google Scholar] [CrossRef]
- Mir, M.B.; Sablania, V.; Rajput, R.; Muzaffar, K. Understanding the Role of Additives in Gluten-Free Breads. In Gluten-Free Bread Technology; Mir, S.A., Shah, M.A., Hamdani, A.M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 121–154. [Google Scholar] [CrossRef]
- Li, P.-H.; Wang, C.-W.; Lu, W.-C.; Chan, Y.-J.; Wang, C.-C.R. Effect of resistant starch sources on the physical properties of dough and on the eating quality and glycemic index of salted noodles. Foods 2022, 11, 814. [Google Scholar] [CrossRef]
- Mohebbi, Z.; Homayouni, A.; Azizi, M.H.; Hosseini, S.J. Effects of beta-glucan and resistant starch on wheat dough and prebiotic bread properties. J. Food Sci. Technol. 2018, 55, 101–110. [Google Scholar] [CrossRef]
- Liu, N.; Ma, S.; Li, L.; Wang, X. Study on the effect of wheat bran dietary fiber on the rheological properties of dough. Grain Oil Sci. Technol. 2019, 2, 1–5. [Google Scholar] [CrossRef]
- Asif-Ul-Alam, S.M.; Islam, M.Z.; Hoque, M.M.; Monalisa, K. Effects of drying on the physicochemical and functional properties of green banana (Musa sapientum) flour and development of baked product. AJFST 2014, 2, 128–133. [Google Scholar] [CrossRef]
- Verbeke, C.; Debonne, E.; Versele, S.; Van Bockstaele, F.; Eeckhout, M. Technological Evaluation of Fiber Effects in Wheat-Based Dough and Bread. Foods 2024, 13, 2582. [Google Scholar] [CrossRef] [PubMed]
- Kundu, H.; Grewal, R.B.; Goyal, A.; Upadhyay, N.; Prakash, S. Effect of incorporation of pumpkin (Cucurbita moshchata) powder and guar gum on the rheological properties of wheat flour. J. Food Sci. Technol. 2014, 51, 2600–2607. [Google Scholar] [CrossRef]
- Mildner-Szkudlarz, S.; Bajerska, J.; Zawirska-Wojtasiak, R.; Górecka, D. White grape pomace as a source of dietary fibre and polyphenols and its effect on physical and nutraceutical characteristics of wheat biscuits. J. Sci. Food Agric. 2013, 93, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Xu, J.; Singh, M. Yeast leavened banana-bread: Formulation, processing, colour and texture analysis. Food Chem. 2010, 118, 620–626. [Google Scholar] [CrossRef]
- Ajila, C.M.; Leelavathi, K.; Rao, U.J.S.P. Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peel powder. J. Cereal Sci. 2008, 48, 319–326. [Google Scholar] [CrossRef]
- Chikpah, S.K.; Korese, J.K.; Hensel, O.; Sturm, B.; Pawelzik, E. Influence of blend proportion and baking conditions on the quality attributes of wheat, orange-fleshed sweet potato and pumpkin composite flour dough and bread: Optimization of processing factors. Discov. Food. 2023, 3, 2. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J.; Magala, M.; Kuchtová, V. Effect of apple pomace powder addition on farinographic properties of wheat dough and biscuits quality. Chem. Pap. 2014, 68, 1059–1065. [Google Scholar] [CrossRef]
- Miś, A.; Grundas, S.; Dziki, D.; Laskowski, J. Use of farinograph measurements for predicting extensograph traits of bread dough enriched with carob fibre and oat wholemeal. J. Food Eng. 2012, 108, 1–12. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, N.E.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Phenolic–protein interactions: Effects on food properties and health benefits. J. Med. Food. 2018, 21, 188–198. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Xu, H.; Zhang, T.; Lin, X.; Wu, X. Effect of covalent interaction with chlorogenic acid on the allergenic capacity of ovalbumin. J. Agric. Food Chem. 2018, 66, 9794–9800. [Google Scholar] [CrossRef]
- Girard, A.L.; Awika, J.M. Effects of edible plant polyphenols on gluten protein functionality and potential applications of polyphenol–gluten interactions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2164–2199. [Google Scholar] [CrossRef] [PubMed]
- Atwell, W.A. The terminology and methodology associated with basic starch phenomena. Cereal foods world 1988, 33, 306–311. [Google Scholar]
- Pereira, J.; Malairaj, S.; Brohi, S.A.; Boateng, E.F.; Zhang, W. Impact of unripe banana flour on water states, rheological behaviour and structural properties of myofibrillar protein composite gel. LWT 2020, 125, 109276. [Google Scholar] [CrossRef]
- Min, B.; Bae, I.Y.; Lee, H.G.; Yoo, S.-H.; Lee, S. Utilization of pectin-enriched materials from apple pomace as a fat replacer in a model food system. Bioresour. Technol. 2010, 101, 5414–5418. [Google Scholar] [CrossRef] [PubMed]
- Naivikul, O.; Arlai, A. The effect of drying methods on the characteristics and functional properties of unripe banana (Musa spp.) flour: Air drying, freeze drying and extrusion. Interdiscip. Environ. Rev. 2022, 17, 1–7. [Google Scholar]
- Yang, M.; Chang, L.; Jiang, F.; Zhao, N.; Zheng, P.; Simbo, J.; Yu, X.; Du, S.-k. Structural, physicochemical and rheological properties of starches isolated from banana varieties (Musa spp.). Food Chem. X 2022, 16, 100473. [Google Scholar] [CrossRef]
- Chávez-Salazar, A.; Bello-Pérez, L.A.; Agama-Acevedo, E.; Castellanos-Galeano, F.J.; Álvarez-Barreto, C.I.; Pacheco-Vargas, G. Isolation and partial characterization of starch from banana cultivars grown in Colombia. Int. J. Biol. Macromol. 2017, 98, 240–246. [Google Scholar] [CrossRef]
- Singh, J.; Dartois, A.; Kaur, L. Starch digestibility in food matrix: A review. Trends Food Sci. Technol. 2010, 21, 168–180. [Google Scholar] [CrossRef]
- Hoover, R. Composition, molecular structure, and physicochemical properties of tuber and root starches: A review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Grundy, M.M.L.; Edwards, C.H.; Mackie, A.R.; Gidley, M.J.; Butterworth, P.J.; Ellis, P.R. Re-evaluation of the mechanisms of dietary fibre and implications for macronutrient bioaccessibility, digestion and postprandial metabolism. Br. J. Nutr. 2016, 116, 816–833. [Google Scholar] [CrossRef] [PubMed]
- Gani, A.; Wani, S.M.; Masoodi, F.A.; Hameed, G. Whole-grain cereal bioactive compounds and their health benefits: A review. Int. J. Food Process. Technol. 2012, 3, 146–156. [Google Scholar] [CrossRef]
- Zobel, H.F. Molecules to granules: A comprehensive starch review. Starch-Stärke 1988, 40, 44–50. [Google Scholar] [CrossRef]
- Abd Karim, A.; Norziah, M.H.; Seow, C.C. Methods for the study of starch retrogradation. Food Chem. 2000, 71, 9–36. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N. Relationship of polymeric proteins and empirical dough rheology with dynamic rheology of dough and gluten from different wheat varieties. Food Hydrocoll. 2013, 33, 342–348. [Google Scholar] [CrossRef]
- Agama-Acevedo, E.; Pacheco-Vargas, G.; Gutierrez-Meraz, F.; Tovar, J.; Bello-Perez, L.A. Dietary fiber content, texture, and in vitro starch digestibility of different white bread crusts. J. Cereal Sci. 2019, 89, 102824. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Amaral, O.; Guerreiro, C.S.; Gomes, A.; Cravo, M. Resistant starch production in wheat bread: Effect of ingredients, baking conditions and storage. Eur. Food Res. Technol. 2016, 242, 1747–1753. [Google Scholar] [CrossRef]
- Juarez-Garcia, E.; Agama-Acevedo, E.; Sáyago-Ayerdi, S.G.; Rodriguez-Ambriz, S.L.; Bello-Perez, L.A. Composition, digestibility and application in breadmaking of banana flour. Plant Foods Hum. Nutr. 2006, 61, 131–137. [Google Scholar] [CrossRef]
- Eke-Ejiofor, J.; Kiin-Kabari, D.B. Effect of substitution on the functional properties of flour, proximate and sensory properties of wheat/plantain composite bread. Int. J. Agric. Sci. 2012, 2, 281–284. [Google Scholar]
- Sciarini, L.S.; Bustos, M.C.; Vignola, M.B.; Paesani, C.; Salinas, C.N.; Perez, G.T. A study on fibre addition to gluten free bread: Its effects on bread quality and in vitro digestibility. J. Food Sci. Technol. 2017, 54, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Roman, L.; Campanella, O.; Martinez, M.M. Shear-induced molecular fragmentation decreases the bioaccessibility of fully gelatinized starch and its gelling capacity. Carbohydr. Polym. 2019, 215, 198–206. [Google Scholar] [CrossRef]
- de Souza Viana, E.; dos Santos Souza, A.; Reis, R.C.; de Oliveira, V.J.d.S. Application of green banana flour for partial substitution of wheat flour in sliced bread. Semin. Cienc. Agrar. 2018, 39, 2399–2408. [Google Scholar]
- Agama-Acevedo, E.; Sañudo-Barajas, J.A.; Vélez De La Rocha, R.; González-Aguilar, G.A.; Bello-Perez, L.A. Potential of plantain peels flour (Musa paradisiaca L.) as a source of dietary fiber and antioxidant compound. CYTA—J. Food 2016, 14, 117–123. [Google Scholar] [CrossRef]
- Ezeogu, L.I.; Duodu, K.G.; Taylor, J.R.N. Effects of endosperm texture and cooking conditions on the in vitro starch digestibility of sorghum and maize flours. J. Cereal Sci. 2005, 42, 33–44. [Google Scholar] [CrossRef]
- Berti, C.; Riso, P.; Monti, L.D.; Porrini, M. In vitro starch digestibility and in vivo glucose response of gluten–free foods and their gluten counterparts. Eur. J. Nutr. 2004, 43, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castaño, M.; Lopera-Idarraga, J.; Pazmiño-Arteaga, J.; Gallardo-Cabrera, C. Evaluation of the behaviour of unripe banana flour with non-conventional flours in the production of gluten-free bread. Food Sci. Technol. Int. 2020, 26, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Royall, P.G.; Gaisford, S.; Williams, G.R.; Edwards, C.H.; Warren, F.J.; Flanagan, B.M.; Ellis, P.R.; Butterworth, P.J. Structural and enzyme kinetic studies of retrograded starch: Inhibition of α-amylase and consequences for intestinal digestion of starch. Carbohydr. Polym. 2017, 164, 154–161. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J.H. Digestion of the polysaccharides of some cereal foods in the human small intestine. Am. J. Clin. Nutr. 1985, 42, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Hasjim, J.; Ai, Y.; Jane, J.l. Novel applications of amylose-lipid complex as resistant starch type 5. In Resistant Starch: Sources, Applications and Health Benefits; Wiley Online Library: Hoboken, NJ, USA, 2013; pp. 79–94. [Google Scholar]
- Kawai, K.; Takato, S.; Sasaki, T.; Kajiwara, K. Complex formation, thermal properties, and in-vitro digestibility of gelatinized potato starch–fatty acid mixtures. Food Hydrocoll. 2012, 27, 228–234. [Google Scholar] [CrossRef]
- Segundo, C.; Román, L.; Gómez, M.; Martínez, M.M. Mechanically fractionated flour isolated from green bananas (M. cavendishii var. nanica) as a tool to increase the dietary fiber and phytochemical bioactivity of layer and sponge cakes. Food Chem. 2017, 219, 240–248. [Google Scholar] [PubMed]
- Mao, W.W.; Kinsella, J.E. Amylase activity in banana fruit: Properties and changes in activity with ripening. J. Food Sci. 1981, 46, 1400–1403. [Google Scholar] [CrossRef]
- Pico, J.; Xu, K.; Guo, M.; Mohamedshah, Z.; Ferruzzi, M.G.; Martinez, M.M. Manufacturing the ultimate green banana flour: Impact of drying and extrusion on phenolic profile and starch bioaccessibility. Food Chem. 2019, 297, 124990. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, W.R.; Hughes, J.G.; Cockman, R.W.; Small, D.M. The effects of temperature on the crystalline properties and resistant starch during storage of white bread. Food Chem. 2017, 228, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.R.; Cui, G.; Rigg, B. The development of the CIE 2000 colour-difference formula: CIEDE2000. Color Res. Appl. 2001, 26, 340–350. [Google Scholar] [CrossRef]
- Sharma, G.; Wu, W.; Dalal, E.N. The CIEDE2000 color-difference formula: Implementation notes, supplementary test data, and mathematical observations. Color Res. Appl. 2005, 30, 21–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Ruan, C.; Cheng, Z.; Zhou, Y.; Liang, J. Mixolab behavior, quality attributes and antioxidant capacity of breads incorporated with Agaricus bisporus. J. Food Sci. Technol. 2019, 56, 3921–3929. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Wang, M.; Zheng, J.; Ou, S. Positive and negative effects of polyphenol incorporation in baked foods. Food Chem. 2019, 284, 90–99. [Google Scholar] [CrossRef]
- Çelik, E.E.; Gökmen, V. Formation of Maillard reaction products in bread crust-like model system made of different whole cereal flours. Eur. Food Res. Technol. 2020, 246, 1207–1218. [Google Scholar] [CrossRef]
- Ruderman, M.; Appels, R.; Howell, K. Application of digital image analysis to assess the texture of bread products. In Proceedings of the Australasian Grain Science Association Annual Meeting, Melbourne, Australia, 14 August 2024. [Google Scholar]
- Umbaugh, S.E. Computer Vision and Image Analysis: Digital Image Processing and Analysis; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Arp, C.G.; Correa, M.J.; Ferrero, C. High-amylose resistant starch as a functional ingredient in breads: A technological and microstructural approach. Food Bioprocess Technol. 2018, 11, 2182–2193. [Google Scholar] [CrossRef]
- Van Hung, P.; Yamamori, M.; Morita, N. Formation of enzyme-resistant starch in bread as affected by high-amylose wheat flour substitutions. Cereal Chem. 2005, 82, 690–694. [Google Scholar] [CrossRef]
- Alcântara, R.G.d.; Fukumasu, H.; Raspantini, P.C.F.; Raspantini, L.E.R.; Steel, C.J.; Oliveira, L.d.C.; Carvalho, R.A.d.; Vanin, F.M. Baking effect on resistant starch digestion from composite bread produced with partial wheat flour substitution. J. Food Qual. 2020, 2020, 9245035. [Google Scholar] [CrossRef]
- de Alcântara, R.G.; de Carvalho, R.A.; Vanin, F.M. Evaluation of wheat flour substitution type (corn, green banana and rice flour) and concentration on local dough properties during bread baking. Food Chem. 2020, 326, 126972. [Google Scholar] [CrossRef] [PubMed]
- Tapre, A.R.; Jain, R.K. Study of advanced maturity stages of banana. IJAERS 2012, 1, 272–274. [Google Scholar]
- AACC. Approved Methods of the American Association of Cereal Chemists; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- McCleary, B.V.; McNally, M.; Rossiter, P. Measurement of resistant starch by enzymatic digestion in starch and selected plant materials: Collaborative study. J. AOAC Int. 2002, 85, 1103–1111. [Google Scholar] [CrossRef]
- McCleary, B.V.; Sloane, N.; Draga, A.; Lazewska, I. Measurement of total dietary fiber using AOAC Method 2009.01 (AACC International Approved Method 32-45.01): Evaluation and updates. Cereal Chem. 2013, 90, 396–414. [Google Scholar] [CrossRef]
- Mokrzycki, W.S.; Tatol, M. Colour difference ΔEA survey. Mach. Graph. Vis 2011, 20, 383–411. [Google Scholar]


| Samples | Substitution (%) | N-RS (g/100 g) | RS (g/100 g) | TS (g/100 g) | TDF (g/100 g) |
|---|---|---|---|---|---|
| Control bread | 0 | 64.0 ± 0.14 f | 1.32 ± 0.01 f | 65.3 ± 0.14 g | 3.8 ± 0.39 g |
| Cavendish | 5 | 62.9 ± 0.06 gh | 1.59 ± 0.01 e | 64.5 ± 0.07 h | 5.9 ± 0.24 f |
| Ladyfinger | 5 | 62.6 ± 0.07 h | 1.76 ± 0.01 d | 64.4 ± 0.08 h | 3.8 ± 0.38 g |
| Ducasse | 5 | 63.1 ± 0.07 g | 1.90 ± 0.01 c | 65.0 ± 0.07 g | 6.4 ± 0.08 d |
| Cavendish | 10 | 72.0 ± 0.18 b | 1.89 ± 0.01 c | 73.9 ± 0.18 b | 5.9 ± 0.18 e |
| Ladyfinger | 10 | 70.8 ± 0.24 c | 1.90 ± 0.01 c | 72.7 ± 0.24 c | 7.1 ± 0.11 c |
| Ducasse | 10 | 68.9 ± 0.07 d | 1.89 ± 0.01 c | 70.8 ± 0.07 e | 5.9 ± 0.28 ef |
| Cavendish | 15 | 69.1 ± 0.13 d | 2.26 ± 0.01 b | 71.4 ± 0.14 d | 8.0 ± 0.39 b |
| Ladyfinger | 15 | 81.7 ± 0.13 a | 2.56 ± 0.02 a | 84.3 ± 0.15 a | 9.1 ± 0.45 a |
| Ducasse | 15 | 65.1 ± 0.24 e | 2.58 ± 0.01 a | 67.7 ± 0.25 f | 7.1 ± 0.42 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashmil, Y.M.; Bekes, F.; Ruderman, M.; Suleria, H.A.R.; Appels, R.; Dunshea, F.R. The Physicochemical and Rheological Properties of Green Banana Flour–Wheat Flour Bread Substitutions. Plants 2025, 14, 207. https://doi.org/10.3390/plants14020207
Bashmil YM, Bekes F, Ruderman M, Suleria HAR, Appels R, Dunshea FR. The Physicochemical and Rheological Properties of Green Banana Flour–Wheat Flour Bread Substitutions. Plants. 2025; 14(2):207. https://doi.org/10.3390/plants14020207
Chicago/Turabian StyleBashmil, Yasmeen M., Frank Bekes, Michael Ruderman, Hafiz A. R. Suleria, Rudi Appels, and Frank R. Dunshea. 2025. "The Physicochemical and Rheological Properties of Green Banana Flour–Wheat Flour Bread Substitutions" Plants 14, no. 2: 207. https://doi.org/10.3390/plants14020207
APA StyleBashmil, Y. M., Bekes, F., Ruderman, M., Suleria, H. A. R., Appels, R., & Dunshea, F. R. (2025). The Physicochemical and Rheological Properties of Green Banana Flour–Wheat Flour Bread Substitutions. Plants, 14(2), 207. https://doi.org/10.3390/plants14020207










