Seasonal Pattern of Endo-β-Mannanase Activity During Germination of Jeffersonia dubia, Exhibiting Morphophysiological Dormancy
Abstract
1. Introduction
2. Results
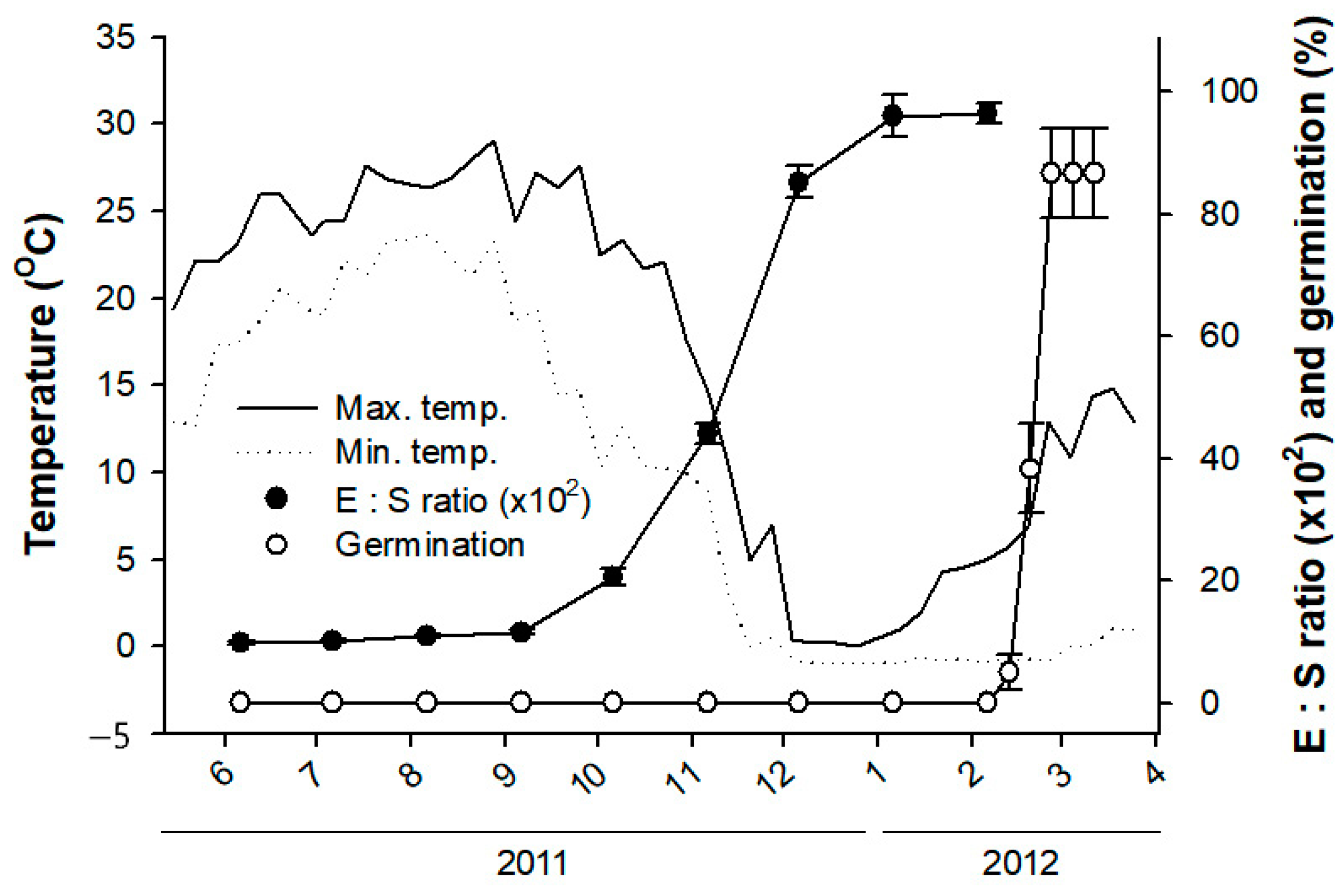
2.1. Embryo Elongation and Germination in a Field Condition
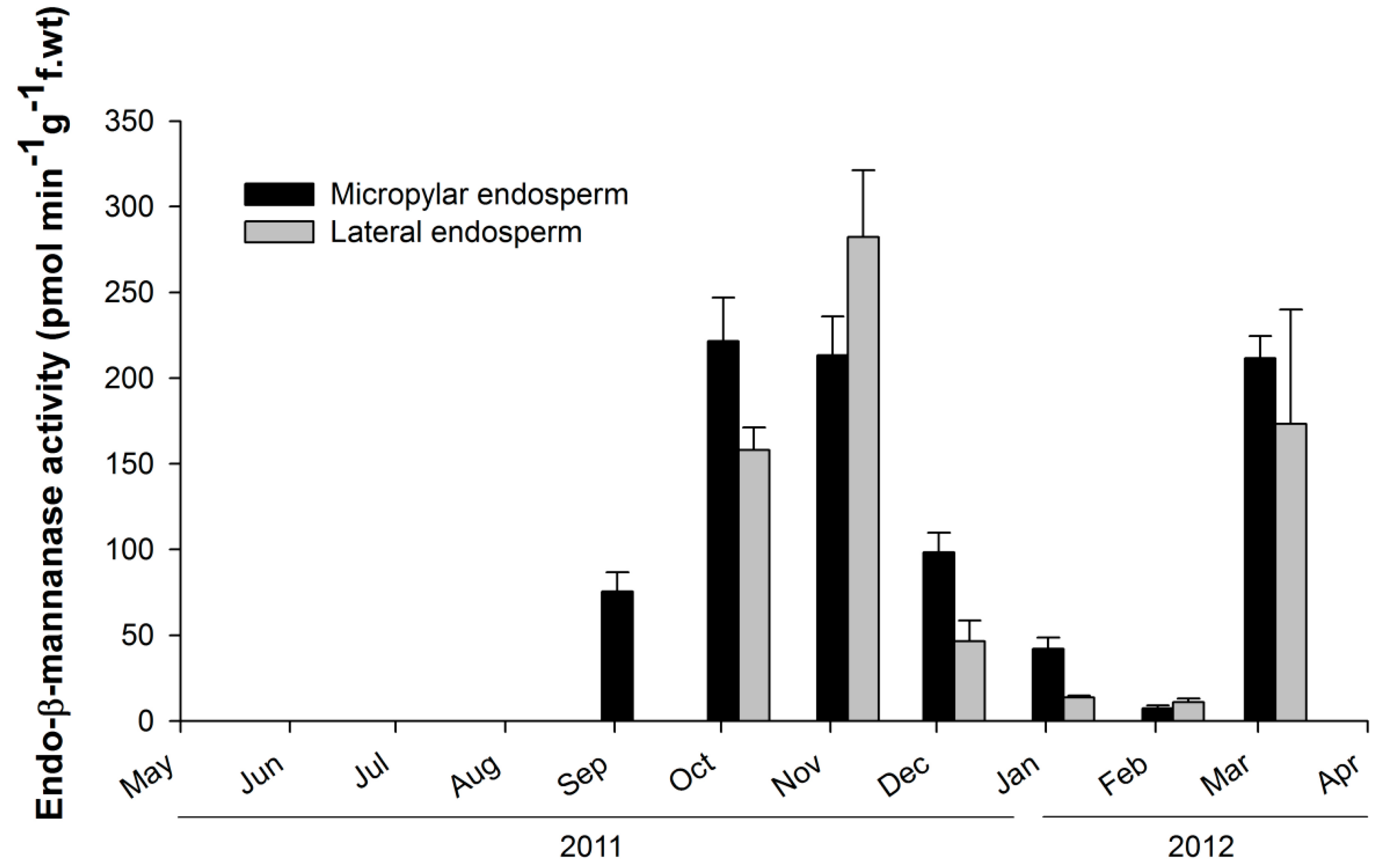
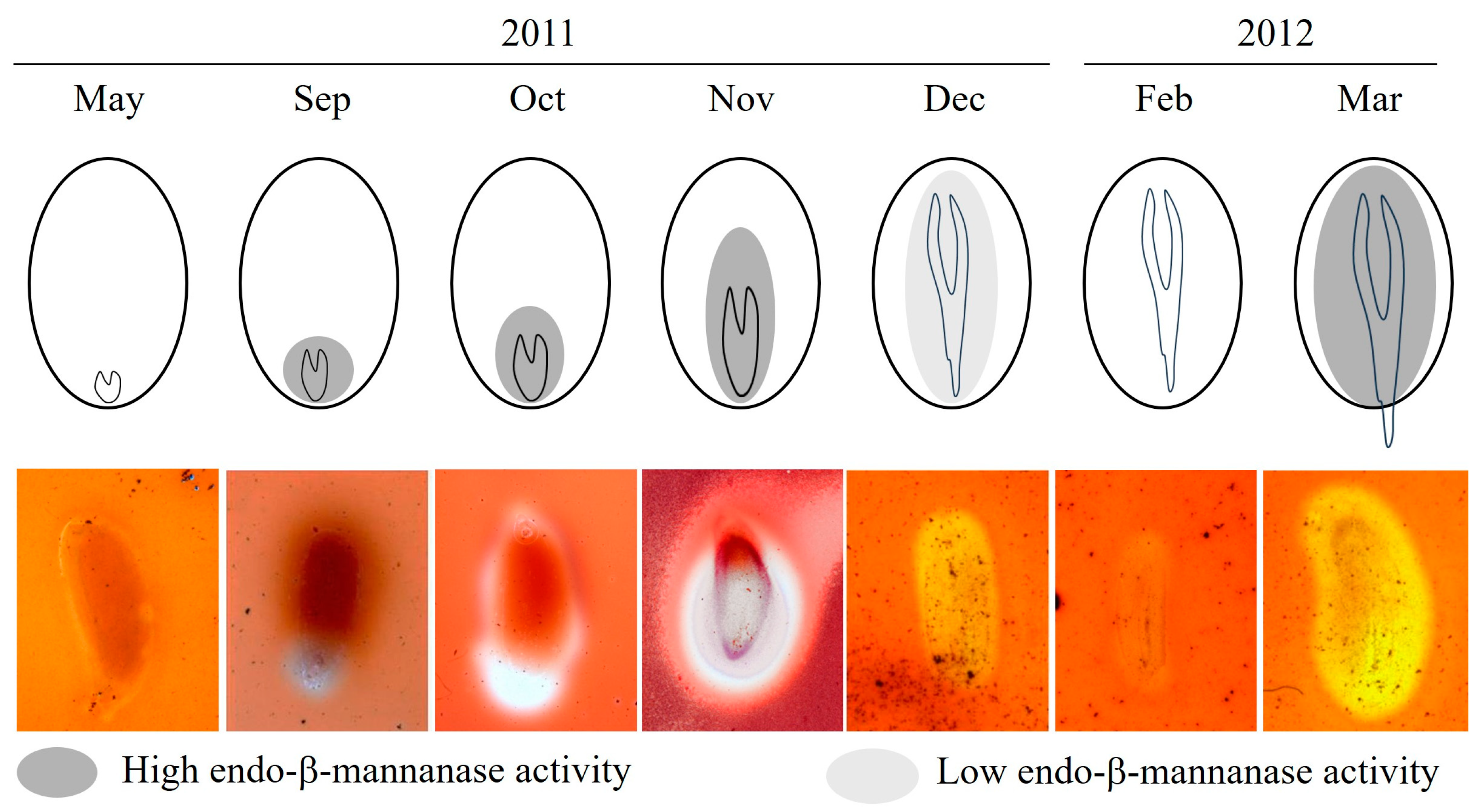
2.2. Observation of Endo-β-Mannanase Activity
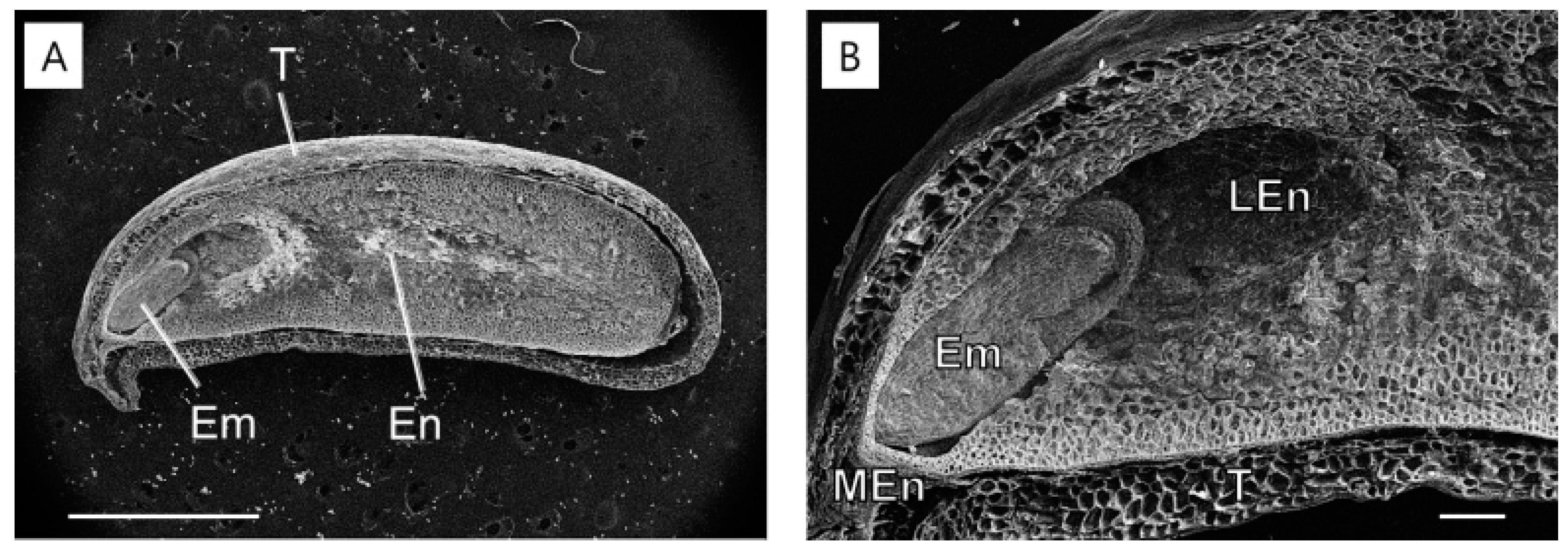
2.3. Structural Changes in the Endosperm
3. Discussion
4. Materials and Methods
4.1. Seeds
4.2. Embryo Elongation and Germination in a Field Condition
4.3. Endo-β-Mannanase Extraction and Assay
4.4. Structural Changes in the Endosperm
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Groot, S.P.; Kieliszewska-Rokicka, B.; Vermeer, E.; Karssen, C.M. Gibberellin-induced hydrolysis of endosperm cell walls in gibberellin-deficient tomato seeds prior to radicle protrusion. Planta 1988, 174, 500–504. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055. [Google Scholar] [CrossRef] [PubMed]
- Groot, S.; Karssen, C. Gibberellins regulate seed germination in tomato by endosperm weakening: A study with gibberellin-deficient mutants. Planta 1987, 171, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.; Cantliffe, D.; Huber, D.; Nell, T. Gibberellic acid stimulated degradation of endosperm in pepper. J. Am. Soc. Hortic. Sci. 1985, 110, 61–65. [Google Scholar] [CrossRef]
- Nonogaki, H.; Morohashi, Y. An endo-β-mannanase develops exclusively in the micropylar endosperm of tomato seeds prior to radicle emergence. Plant Physiol. 1996, 110, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Still, D.W.; Bradford, K.J. Endo-β-mannanase activity from individual tomato endosperm caps and radicle tips in relation to germination rates. Plant Physiol. 1997, 113, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Toorop, P.E.; Bewley, J.D.; Hilhorst, H.W. Endo-β-mannanase isoforms are present in the endosperm and embryo of tomato seeds, but are not essentially linked to the completion of germination. Planta 1996, 200, 153–158. [Google Scholar] [CrossRef]
- Cavasin, P.Y.; dos Santos, H.O.; Oliveira, T.F.; Pereira, J.A.; da Gama, A.B.N.; Pereira, W.V.S. Endo-β-mannanase and superoxide dismutase as enzymatic markers for lettuce seeds thermotolerance. J. Seed Sci. 2023, 45, e202345008. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Z.; Yang, X.; He, Y.; Wang, X. Production of endo-β-mannanase, superoxide radicals, hydrogen peroxide, and peroxidase in the micropylar endosperm of pepper seed during germination is determined by the endosperm itself. Sci. Hortic. 2022, 294, 110757. [Google Scholar] [CrossRef]
- Watkins, J.T.; Cantliffe, D.J. Mechanical resistance of the seed coat and endosperm during germination of Capsicum annuum at low temperature. Plant Physiol. 1983, 72, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Leubner-Metzger, G.; Frundt, C.; Vogeli-Lange, R.; Meins, F., Jr. Class I β-1, 3-glucanases in the endosperm of tobacco during germination. Plant Physiol. 1995, 109, 751–759. [Google Scholar] [CrossRef]
- Welbaum, G.E.; Muthui, W.J.; Wilson, J.H.; Grayson, R.L.; Fell, R.D. Weakening of muskmelon perisperm envelope tissue 4. J. Exp. Bot. 1995, 46, 391–400. [Google Scholar] [CrossRef]
- Rodolfo, S.A.; de Miguel, L.; Mercuri, O. Phytochrome control of cellulase activity in Datura ferox L. seeds and its relationship with germination. J. Exp. Bot. 1986, 37, 1574–1580. [Google Scholar] [CrossRef]
- Orłowska, A.; Kępczyński, J. KAR1-dormancy release in Avena fatua caryopses includes increased AfMAN gene expression and ENDO-β-MANNANASE activity in the coleorhiza and radicle. J. Plant Physiol. 2024, 303, 154363. [Google Scholar] [CrossRef]
- Müller, K.; Tintelnot, S.; Leubner-Metzger, G. Endosperm-limited Brassicaceae seed germination: Abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol. 2006, 47, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, S.E.E.; da Silva, E.A.A.; Davide, A.C.; José, A.C.; Silva, A.T.; Fraiz, A.C.R.; Faria, J.M.R.; Hilhorst, H.W. Mechanism and control of Genipa americana seed germination. Physiol. Plant. 2012, 144, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Nikolaeva, M.G. Physiology of Deep Dormancy in Seeds; Israel Program for Scientific Translations Jerusalem: Springfield, VA, USA, 1969. [Google Scholar]
- Choi, K.G.; Takahashi, N. Studies of seed germination in Panax ginseng C.A. Meyer (1) The effect of germination inhibitors in fruits on dormancy breaking. Bull. Inst. Agric. Res. Tohoku Univ. 1977, 28, 145–157. [Google Scholar]
- Hidayati, S.N.; Baskin, J.M.; Baskin, C.C. Morphophysiological dormancy in seeds of two North American and one Eurasian species of Sambucus (Caprifoliaceae) with underdeveloped spatulate embryos. Am. J. Bot. 2000, 87, 1669–1678. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Seed germination ecophysiology of Jeffersonia diphylla, a perennial herb of mesic deciduous forests. Am. J. Bot. 1989, 76, 1073–1080. [Google Scholar] [CrossRef]
- Rhie, Y.H.; Lee, S.Y.; Kim, K.S. Seed dormancy and germination in Jeffersonia dubia (Berberidaceae) as affected by temperature and gibberellic acid. Plant Biol. 2015, 17, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Rhie, Y.H.; Lee, S.Y. Seed dormancy and germination of Epimedium koreanum Nakai. Sci. Hortic. 2020, 272, 109600. [Google Scholar] [CrossRef]
- Liu, C.-P.; Chen, S.-Y.; Baskin, C.C.; Chien, C.-T. Non-deep simple and deep simple morphophysiological dormancy in seeds of three species of Ilex from subtropical and tropical regions of Taiwan. Seed Sci. Res. 2023, 33, 50–57. [Google Scholar] [CrossRef]
- Arens, H.; Fischer, H.; Leyck, S.; Römer, A.; Ulbrich, B. Antiinflammatory compounds from Plagiorhegma dubium cell culture. Planta Medica 1985, 51, 52–56. [Google Scholar] [CrossRef]
- Jeong, B.R.; Sivanesan, I. Micropropagation, berberine content and antitumor activity of Jeffersonia dubia (Maxim.) Benth et Hook. Plant Cell Tissue Organ Cult. 2016, 124, 453–458. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Chakraborty, S.; Singh, S. Berberine: A potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs 2010, 19, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.C. The comparative internal morphology of seeds. Am. Midl. Nat. 1946, 36, 513–660. [Google Scholar] [CrossRef]
- Nascimento, W.M.; Cantliffe, D.J.; Huber, D.J. Endo-β-mannanase activity and seed germination of thermosensitive and thermotolerant lettuce genotypes in response to seed priming. Seed Sci. Res. 2001, 11, 255–264. [Google Scholar]
- Homrichhausen, T.M.; Hewitt, J.R.; Nonogaki, H. Endo-β-mannanase activity is associated with the completion of embryogenesis in imbibed carrot (Daucus carota L.) seeds. Seed Sci. Res. 2003, 13, 219–227. [Google Scholar] [CrossRef]
- Da Silva, E.A.; De Melo, D.L.; Davide, A.C.; De Bode, N.; Abreu, G.B.; Faria, J.M.; Hilhorst, H.W. Germination ecophysiology of Annona crassiflora seeds. Ann. Bot. 2007, 99, 823–830. [Google Scholar] [CrossRef]
- Kondo, T.; Sato, C.; Baskin, J.M.; Baskin, C.C. Post-dispersal embryo development, germination phenology, and seed dormancy in Cardiocrinum cordatum var. glehnii (Liliaceae s. str.), a perennial herb of the broadleaved deciduous forest in Japan. Am. J. Bot. 2006, 93, 849–859. [Google Scholar] [CrossRef]
- Gong, X.; Bassel, G.W.; Wang, A.; Greenwood, J.S.; Bewley, J.D. The emergence of embryos from hard seeds is related to the structure of the cell walls of the micropylar endosperm, and not to endo-β-mannanase activity. Ann. Bot. 2005, 96, 1165–1173. [Google Scholar] [CrossRef]
- Ren, Y.; Bewley, J.D.; Wang, X. Protein and gene expression patterns of endo-β-mannanase following germination of rice. Seed Sci. Res. 2008, 18, 139–149. [Google Scholar] [CrossRef]
- Nonogaki, H.; Nomaguchi, M.; Morohashi, Y.; Matsushima, H. Development and localization of endo-β-mannanase in the embryo of germinating and germinated tomato seeds. J. Exp. Bot. 1998, 49, 1501–1507. [Google Scholar]
- Gong, X.; Bewley, J.D. Sorting out the LeMANs: Endo-β-mannanase genes and their encoded proteins in tomato. Seed Sci. Res. 2007, 17, 143–154. [Google Scholar] [CrossRef]
- Nonogaki, H.; Nomaguchi, M.; Okumoto, N.; Kaneko, Y.; Matsushima, H.; Morohashi, Y. Temporal and spatial pattern of the biochemical activation of the endosperm during and following imbibition of tomato seeds. Physiol. Plant. 1998, 102, 236–242. [Google Scholar] [CrossRef]
- Toorop, P.E.; van Aelst, A.C.; Hilhorst, H.W. The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germination is under control of ABA. J. Exp. Bot. 2000, 51, 1371–1379. [Google Scholar] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Downie, B.; Hilhorst, H.W.; Bewley, J.D. A new assay for quantifying endo-β-D-mannanase activity using Congo Red dye. Phytochemistry 1994, 36, 829–835. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.H.; Lee, S.Y.; Rhie, Y.H. Seasonal Pattern of Endo-β-Mannanase Activity During Germination of Jeffersonia dubia, Exhibiting Morphophysiological Dormancy. Plants 2025, 14, 251. https://doi.org/10.3390/plants14020251
Kwon YH, Lee SY, Rhie YH. Seasonal Pattern of Endo-β-Mannanase Activity During Germination of Jeffersonia dubia, Exhibiting Morphophysiological Dormancy. Plants. 2025; 14(2):251. https://doi.org/10.3390/plants14020251
Chicago/Turabian StyleKwon, Young Hyun, Seung Youn Lee, and Yong Ha Rhie. 2025. "Seasonal Pattern of Endo-β-Mannanase Activity During Germination of Jeffersonia dubia, Exhibiting Morphophysiological Dormancy" Plants 14, no. 2: 251. https://doi.org/10.3390/plants14020251
APA StyleKwon, Y. H., Lee, S. Y., & Rhie, Y. H. (2025). Seasonal Pattern of Endo-β-Mannanase Activity During Germination of Jeffersonia dubia, Exhibiting Morphophysiological Dormancy. Plants, 14(2), 251. https://doi.org/10.3390/plants14020251







