Citrus Yellow Vein Clearing Virus Infection in Lemon Influences Host Preference of the Citrus Whitefly by Affecting the Host Metabolite Composition
Abstract
:1. Introduction
2. Results
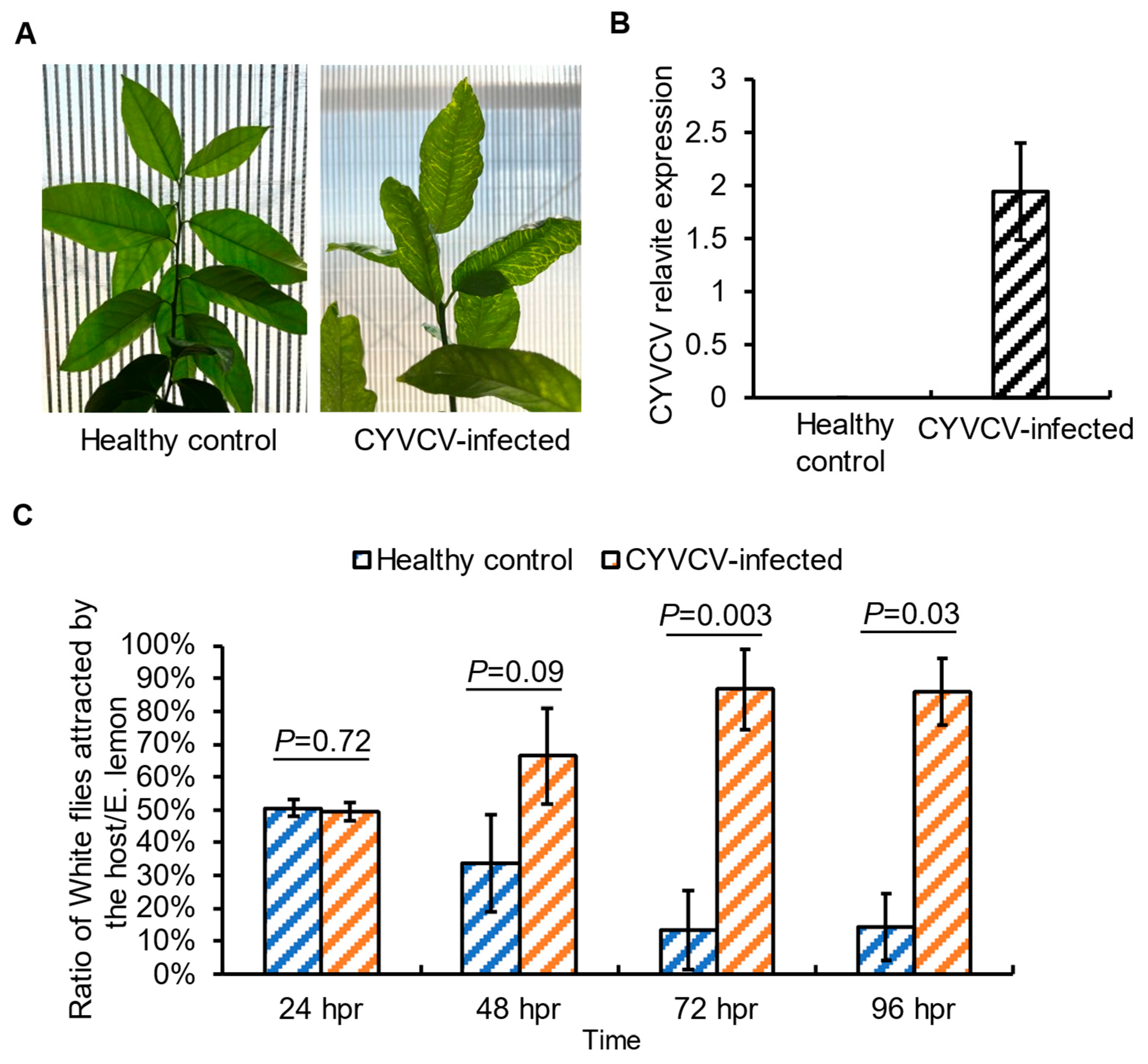
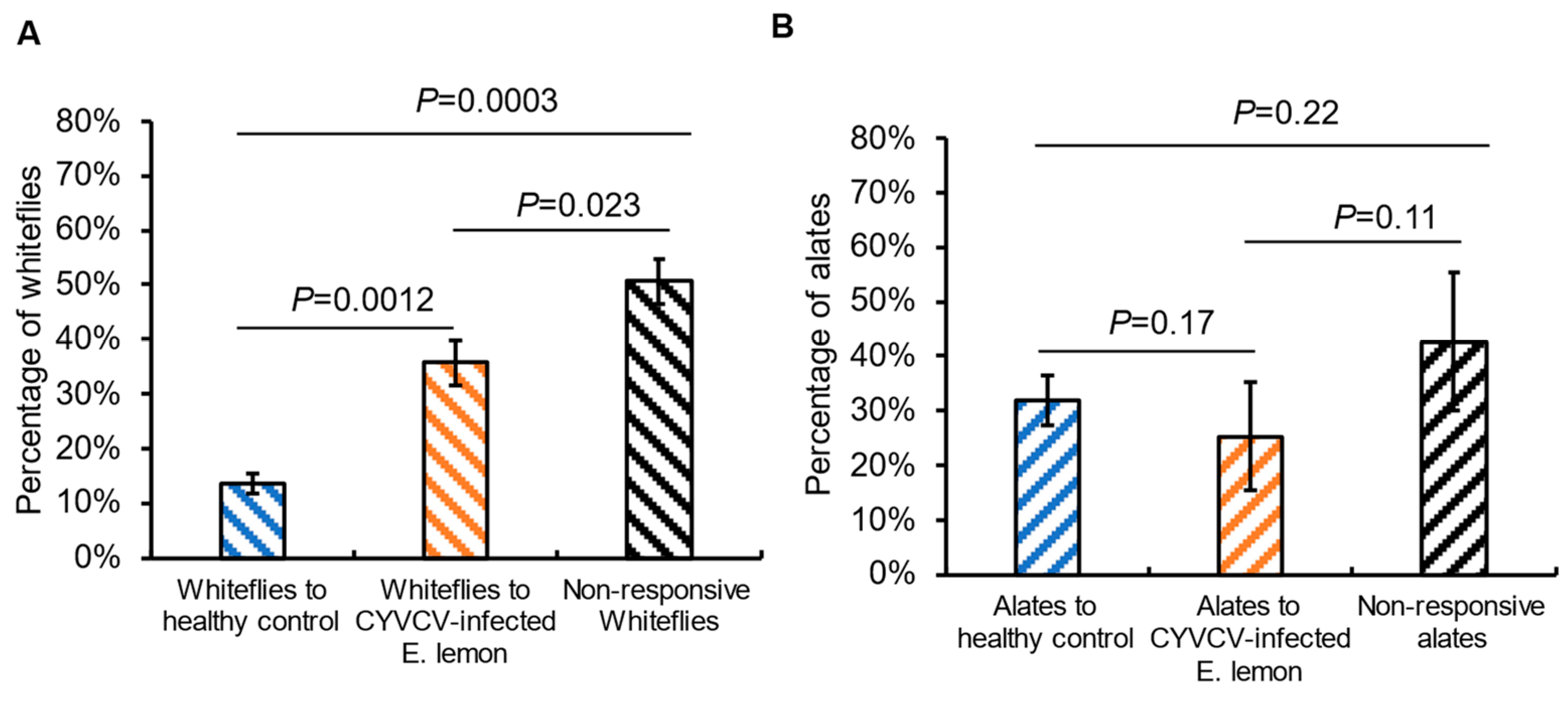
2.1. Plant Infection by CYVCV Alters the Settling Behavior of Non-Viruliferous Citrus Whiteflies
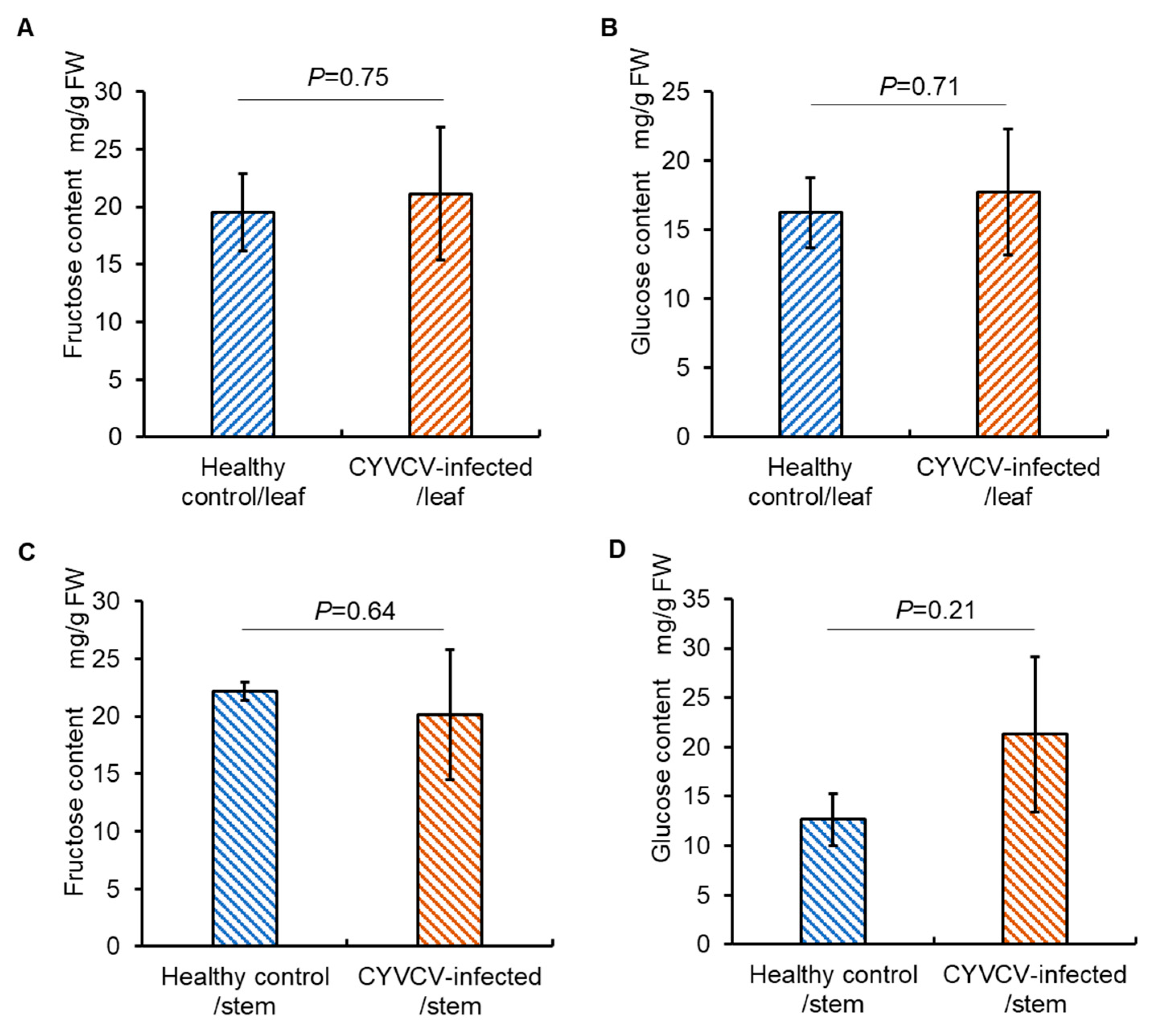
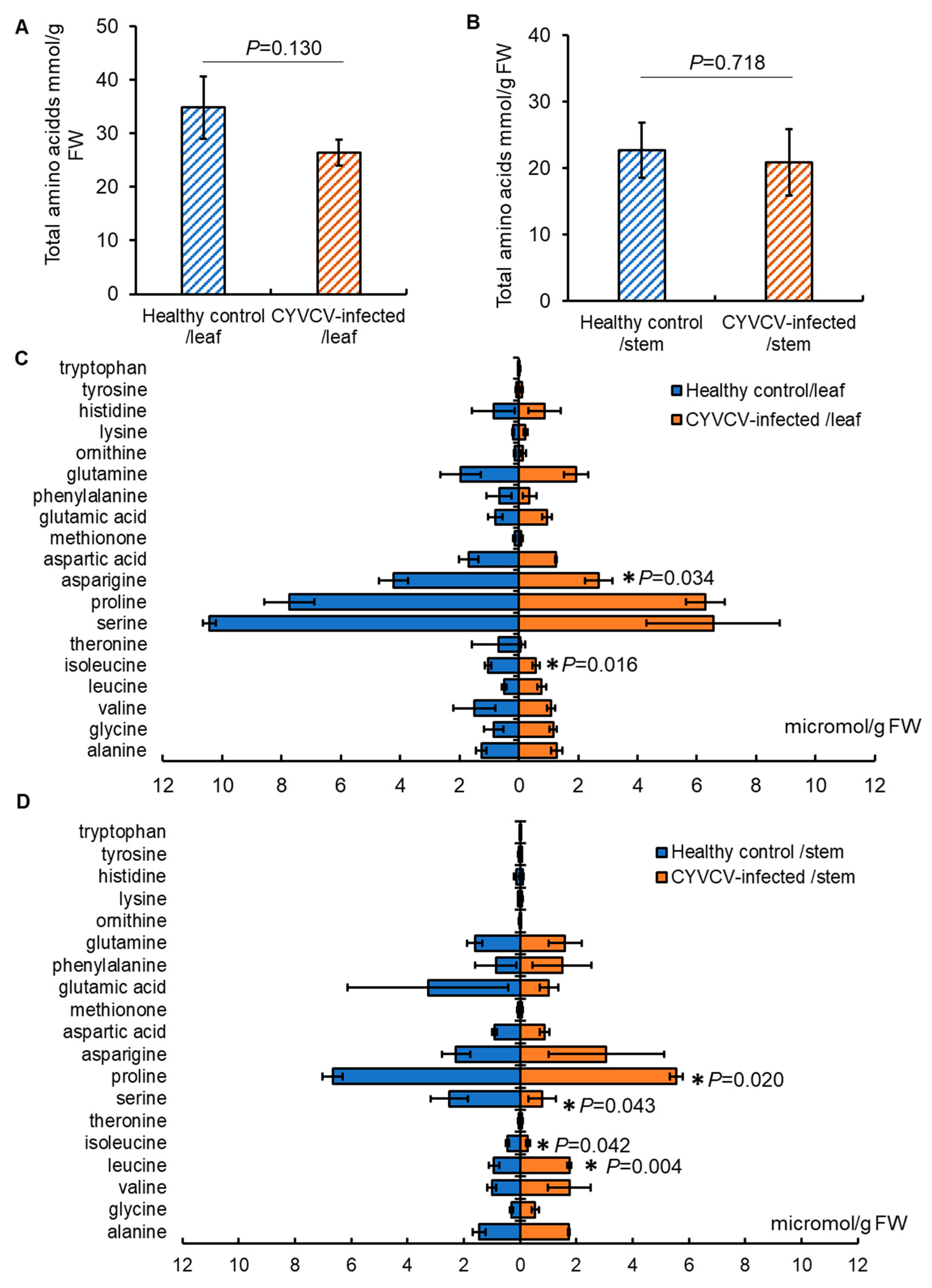
2.2. CYVCV Infection Modulates the Profiles of Amino Acids, but Not Sugars in Eureka Lemon
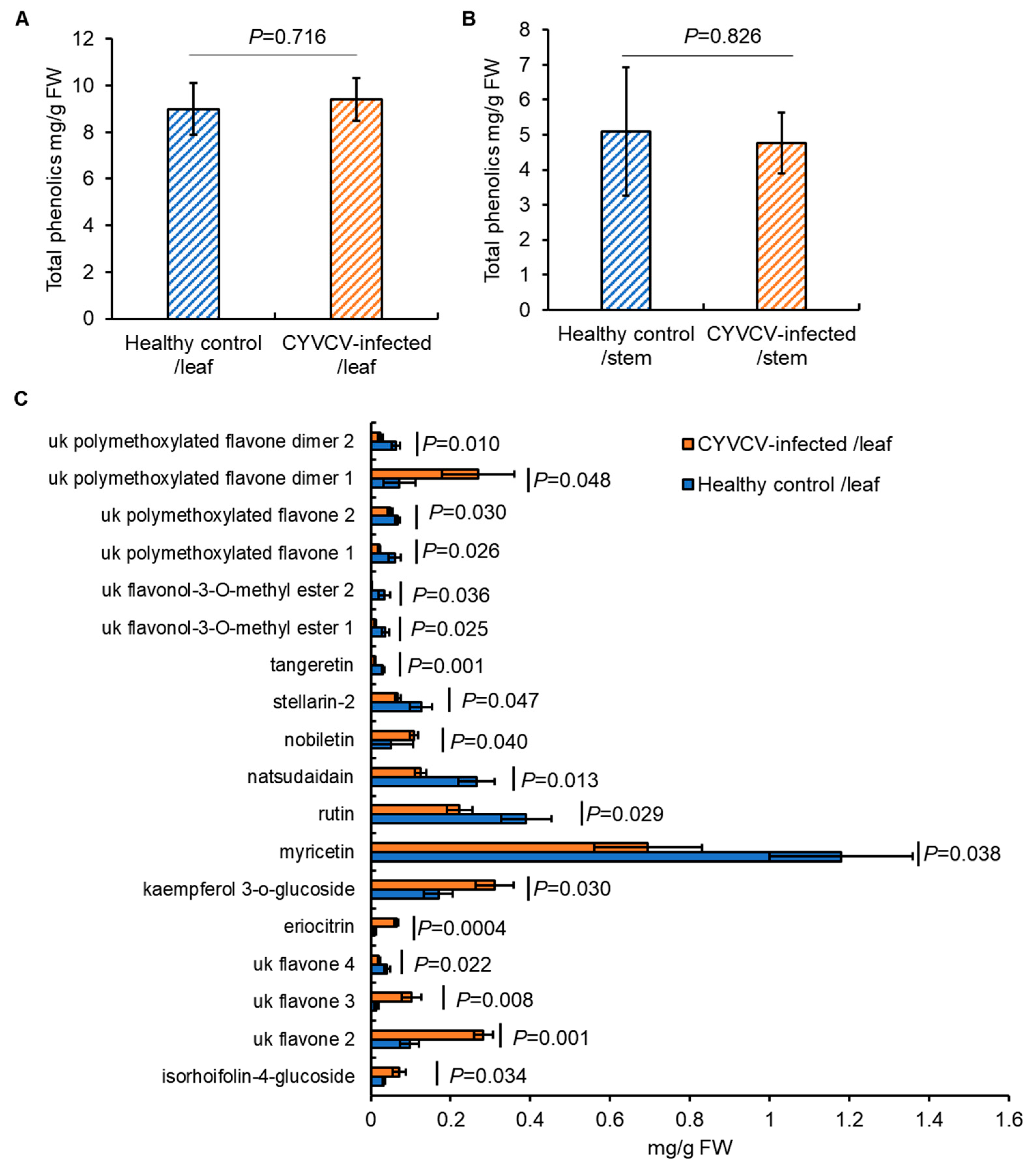
2.3. CYVCV Infection Alters the Content of Specific Soluble Phenolics in Eureka Lemon
2.4. CYVCV Infection Influences the Metabolite Profiles of Volatile Terpenoid
2.5. Citrus Whiteflies Exhibit a Preference to the Air Current Carring Volatile Compounds Released from CYVCV-Infected Plants Other than the Healthy Control in Y-Tube Olfactometer Bioassays
2.6. CYVCV-Induced VOCs Profile Changes in Lemon Tree Have Limited Effect on Olfactory Response of Spirea Aphids
3. Discussion
3.1. CYVCV Infection in Citrus Plant May Alter the Orientation Behavior of Citrus Whitefly
3.2. The Alternation of Citrus Non-Volatile Metabolites Profile Induced by CYVCV Infection Plant May Affect the Orientation Behavior of Citrus Whitefly
3.3. VOCs Released from CYVCV-Infected Plants May Play a Role in Attracting Citrus Whiteflies
4. Materials and Methods
4.1. Plants, Virus, and Insects
4.2. CYVCV Detection by RT-qPCR
4.3. Free-Choice Bioassays
4.4. Y-Tube Olfactometer Bioassay
4.5. Chemical Analyses
4.6. Statistic Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietzgen, R.G.; Mann, K.S.; Johnson, K.N. Plant Virus-Insect Vector Interactions: Current and Potential Future Research Directions. Viruses 2016, 8, 303. [Google Scholar] [CrossRef]
- Tatineni, S.; Hein, G.L. Plant Viruses of Agricultural Importance: Current and Future Perspectives of Virus Disease Management Strategies. Phytopathology 2023, 113, 117–141. [Google Scholar] [CrossRef]
- Roossinck, M.J. A new look at plant viruses and their potential beneficial roles in crops. Mol. Plant Pathol. 2015, 16, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Bosque-Pérez, N.A.; Davis, T.S. Insect-Borne Plant Pathogens and Their Vectors: Ecology, Evolution, and Complex Interactions. Ann. Rev. Entomol. 2018, 63, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Ferrari, M.J.; Roossinck, M.J. Manipulation of aphid behavior by a persistent plant virus. J. Virol. 2019, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhang, Z.; Zhang, C.; Zhou, X.; Zhang, D.; Liu, Y. The molecular mechanism of efficient transmission of plant viruses in variable virus–vector–plant interactions. Horti. Plant J. 2021, 7, 501–508. [Google Scholar] [CrossRef]
- Fang, Y.; Jiao, X.; Xie, W.; Wang, S.; Wu, Q.; Shi, X.; Chen, G.; Su, Q.; Yang, X.; Pan, H.; et al. Tomato yellow leaf curl virus alters the host preferences of its vector Bemisia tabaci. Sci. Rep. 2013, 3, 2876. [Google Scholar] [CrossRef] [PubMed]
- Rajabaskar, D.; Bosque-Pérez, N.A.; Eigenbrode, S.D. Preference by a virus vector for infected plants is reversed after virus acquisition. Virus Res. 2014, 186, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Biochemical and physiological mechanisms underlying effects of Cucumber mosaic virus on host-plant traits that mediate transmission by aphid vectors. Plant Cell Environ. 2014, 37, 1427–1439. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Deceptive chemical signals induced by a plant virus attract insect vectors to inferior hosts. Proc. Natl. Acad. Sci. USA 2010, 107, 3600–3605. [Google Scholar] [CrossRef] [PubMed]
- Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M.; Hardie, J. The Chemical Ecology of Aphids. Ann. Rev. Entomol. 1992, 37, 67–90. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Coutinho, T.A.; Gershenzon, J. Roles of plant volatiles in defense against microbial pathogens and microbial exploitation of volatiles. Plant Cell Environ. 2019, 42, 2827–2843. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, E.S.; Bosque-Pérez, N.A.; Berger, P.H.; Zemetra, R.S.; Ding, H.; Eigenbrode, S.D. Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to Barley yellow dwarf virus–infected transgenic and untransformed wheat. Environ. Entomol. 2004, 33, 1207–1216. [Google Scholar] [CrossRef]
- Chang, X.; Guo, Y.; Ren, Y.; Li, Y.; Wang, F.; Ye, G. Virus-induced plant volatiles promote virus acquisition and transmission by insect vectors. Int. J. Mol. Sci. 2023, 24, 1777. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Weldegergis, B.T.; Li, J.; Jung, C.; Qu, J.; Sun, Y.; Qian, H.; Tee, C.; van Loon, J.J.A.; Dicke, M.; et al. Virulence factors of Geminivirus interact with MYC2 to subvert plant resistance and promote vector performance. Plant Cell 2014, 26, 4991–5008. [Google Scholar] [CrossRef]
- Luan, J.-B.; Yao, D.-M.; Zhang, T.; Walling, L.L.; Yang, M.; Wang, Y.-J.; Liu, S.-S. Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 2013, 16, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Qi, T.; Li, W.-X.; Tian, H.; Gao, H.; Wang, J.; Ge, J.; Yao, R.; Ren, C.; Wang, X.-B.; et al. Viral effector protein manipulates host hormone signaling to attract insect vectors. Cell Res. 2017, 27, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Prager, S.M.; Wallis, C.; Trumble, J.T. Indirect effects of one plant pathogen on the transmission of a second pathogen and the behavior of its potato psyllid vector. Environ. Entomol. 2015, 44, 1065–1075. [Google Scholar] [CrossRef]
- Prager, S.; Wallis, C.M.; Jones, M.; Novy, R.; Trumble, J.T. Examining the potential role of foliar chemistry in imparting potato germplasm tolerance to potato psyllid, green peach aphid, and zebra chip disease. J. Econ. Entomol. 2018, 111, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.M.; Galarneau, E.R.A. Phenolic compound induction in plant-microbe and plant-insect interactions: A meta-analysis. Front. Plant Sci. 2020, 11, 580753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Y.; Qian, X.; Zhang, X.; Li, X.; Sun, X. Recent advances in the specialized metabolites mediating resistance to insect pests and pathogens in tea plants (Camellia sinensis). Plants 2024, 13, 323. [Google Scholar] [CrossRef] [PubMed]
- Jin, T.; Kim, J.K.; Byun, H.S.; Choi, H.S.; Cha, B.; Kwak, H.R.; Kim, M. Occurrence and multiplex PCR detection of Citrus yellow vein clearing virus in Korea. Plant Path. J. 2024, 40, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, H.; Hurst, J.; Timko, M.P.; Zhou, C. Recent advances on Citrus yellow vein clearing virus in citrus. Hort. Plant J. 2020, 6, 216–222. [Google Scholar] [CrossRef]
- Sun, Y.; Yokomi, R. Genotype sequencing and phylogenetic analysis revealed the origins of Citrus yellow vein clearing virus California isolates. Viruses 2024, 16, 188. [Google Scholar] [CrossRef]
- Sun, Y.; Yokomi, R. The Discovery of a Citrus yellow vein clearing virus Hacienda Heights isolate diversifies the geological origins of the virus in California, United States. Viruses 2024, 16, 1479. [Google Scholar] [CrossRef]
- Bin, Y.; Xu, J.; Duan, Y.; Ma, Z.; Zhang, Q.; Wang, C.; Su, Y.; Jiang, Q.; Song, Z.; Zhou, C. The titer of Citrus yellow vein clearing virus is positively associated with the severity of symptoms in infected citrus seedlings. Plant Dis. 2022, 106, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yokomi, R. Whole genome sequence of Citrus yellow vein clearing virus CA1 isolate. BMC Res. Notes 2023, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, H.M.; Cao, M.J.; Wang, X.F.; Jin, X.; Liu, K.H.; Zhou, C.Y. Occurrence, distribution, and molecular characterization of Citrus yellow vein clearing virus in China. Plant Dis. 2017, 101, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Onelge, N.; Satar, S.; Elibuyuk, O.; Bozan, O.; Kamberoolu, M. Transmission studies on Citrus yellow vein clearing virus. In Proceedings of the 18th Conference, IOCV 2011 International Organization of Citrus Virologists, Riverside, CA, USA, 8 November 2010; Available online: https://iocv.ucr.edu/sites/default/files/2020-06/Onelge_et_al.pdf (accessed on 16 January 2025).
- Zhang, Y.; Wang, Y.; Wang, Q.; Cao, M.; Zhou, C.; Zhou, Y. Identification of Aphis spiraecola as a vector of Citrus yellow vein clearing virus. Eur. J. Plant Pathol. 2018, 152, 841–844. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Liu, C.H.; Wang, Q.; Wang, Y.L.; Zhou, C.Y.; Zhou, Y. Identification of Dialeurodes citri as a vector of Citrus yellow vein clearing virus in China. Plant Dis. 2019, 103, 65–68. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, M.C.; Rambla, J.L.; Alamar, M.C.; Gutiérrez, A.; Granell, A. Comparative analysis of the volatile fraction of fruit juice from different citrus species. PLoS ONE 2011, 6, e22016. [Google Scholar] [CrossRef] [PubMed]
- Simons, T.; McNeil, C.; Pham, V.D.; Wang, S.; Wang, Y.; Slupsky, C.; Guinard, J.-X. Chemical and sensory analysis of commercial Navel oranges in California. NPJ Sci. Food 2019, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schutz, S.; de Both, M.T.J.; Haring, M.A.; Schuurink, R.C. The Role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Chen, G.; Mescher, M.C.; Peng, Z.; Xie, W.; Wang, S.; Wu, Q.; Liu, J.; Li, C.; Wang, W.; et al. Whitefly aggregation on tomato is mediated by feeding-induced changes in plant metabolites that influence the behaviour and performance of conspecifics. Funct. Ecol. 2018, 32, 1180–1193. [Google Scholar] [CrossRef]
- Saad, K.A.; Mohamad Roff, M.N.; Hallett, R.H.; Idris, A.B. Aphid-induced defences in chilli affect preferences of the whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Sci. Rep. 2015, 5, 13697. [Google Scholar] [CrossRef] [PubMed]
- Wagan, T.A.; Cai, W.; Hua, H. Repellency, toxicity, and anti-oviposition of essential oil of Gardenia jasminoides and its four major chemical components against whiteflies and mites. Sci. Rep. 2018, 8, 9375. [Google Scholar] [CrossRef] [PubMed]
- Darshanee, H.L.C.; Ren, H.; Ahmed, N.; Zhang, Z.F.; Liu, Y.H.; Liu, T.X. Volatile-mediated attraction of greenhouse whitefly Trialeurodes vaporariorum to tomato and e32ggplant. Front. Plant Sci. 2017, 8, 1285. [Google Scholar] [CrossRef] [PubMed]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2012, 2, 578. [Google Scholar] [CrossRef] [PubMed]
- Warnasooriya, P.G.A.S.; Balagalla, D.N.; Jayasinghe, W.H. Viruliferous and non-viruliferous aphids behave differently to enhance the spread of the virus. Entomol. Exp. Appl. 2024, 172, 42–49. [Google Scholar] [CrossRef]
- Gautam, S.; Gadhave, K.R.; Buck, J.W.; Dutta, B.; Coolong, T.; Adkins, S.; Srinivasan, R. Virus-virus interactions in a plant host and in a hemipteran vector: Implications for vector fitness and virus epidemics. Virus Res. 2020, 286, 198069. [Google Scholar] [CrossRef] [PubMed]
- Mauck, K.; Bosque-Pérez, N.A.; Eigenbrode, S.D.; De Moraes, C.M.; Mescher, M.C. Transmission mechanisms shape pathogen effects on host–vector interactions: Evidence from plant viruses. Funct. Ecol. 2012, 26, 1162–1175. [Google Scholar] [CrossRef]
- Vosman, B.; van’t Westende, W.P.C.; Henken, B.; van Eekelen, H.D.L.M.; deVos, R.C.H.; Voorrips, R.E. Broad spectrum insect resistance and metabolites in close relatives of the cultivated tomato. Euphytica 2018, 214, 46. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Ding, H.; Shiel, P.; Berger, P.H. Volatiles from potato plants infected with potato leafroll virus attract and arrest the virus vector, Myzus persicae (Homoptera: Aphididae). Proc. R. Soc. Lond. B 2002, 269, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Alvarez, J.M.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Influence of hairy nightshade Solanum sarrachoides (Sendtner) and Potato leafroll virus (Luteoviridae: Polerovirus) on the host preference of Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ. Entomol. 2006, 35, 546–553. [Google Scholar] [CrossRef]
- Abrahamian, P.; Tian, T.; Posis, K.; Guo, Y.Y.; Yu, D.; Blomquist, C.L.; Wei, G.; Adducci, B.A.; Vidalakis, G.; Bodaghi, S.; et al. Genetic analysis of the emerging citrus yellow vein clearing virus reveals a divergent virus population in American isolates. Plant Dis. 2023, 108, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Krugner, R.; Johnson, M.W.; Daane, K.M.; Morse, J.G. Olfactory responses of the egg parasitoid, Gonatocerus ashmeadi Girault (Hymenoptera: Mymaridae), to host plants infested by Homalodisca vitripennis (Germar) (Hemiptera: Cicadellidae). Biol. Control 2008, 47, 8–15. [Google Scholar] [CrossRef]
- Wallis, C.M.; Gorman, Z.; Rattner, R.; Hajeri, S.; Yokomi, R. Amino acid, sugar, phenolic, and terpenoid profiles are capable of distinguishing Citrus tristeza virus infection status in citrus cultivars: Grapefruit, lemon, mandarin, and sweet orange. PLoS ONE 2022, 17, e0268255. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-D.; Wallis, C.M.; Krugner, R.; Yokomi, R. Citrus Yellow Vein Clearing Virus Infection in Lemon Influences Host Preference of the Citrus Whitefly by Affecting the Host Metabolite Composition. Plants 2025, 14, 288. https://doi.org/10.3390/plants14020288
Sun Y-D, Wallis CM, Krugner R, Yokomi R. Citrus Yellow Vein Clearing Virus Infection in Lemon Influences Host Preference of the Citrus Whitefly by Affecting the Host Metabolite Composition. Plants. 2025; 14(2):288. https://doi.org/10.3390/plants14020288
Chicago/Turabian StyleSun, Yong-Duo, Christopher M. Wallis, Rodrigo Krugner, and Raymond Yokomi. 2025. "Citrus Yellow Vein Clearing Virus Infection in Lemon Influences Host Preference of the Citrus Whitefly by Affecting the Host Metabolite Composition" Plants 14, no. 2: 288. https://doi.org/10.3390/plants14020288
APA StyleSun, Y.-D., Wallis, C. M., Krugner, R., & Yokomi, R. (2025). Citrus Yellow Vein Clearing Virus Infection in Lemon Influences Host Preference of the Citrus Whitefly by Affecting the Host Metabolite Composition. Plants, 14(2), 288. https://doi.org/10.3390/plants14020288





