ABA and Pre-Harvest Sprouting Differences in Knockout Lines of OsPHS3 Encoding Carotenoid Isomerase via CRISPR/Cas9 in Rice
Abstract
1. Introduction
2. Results
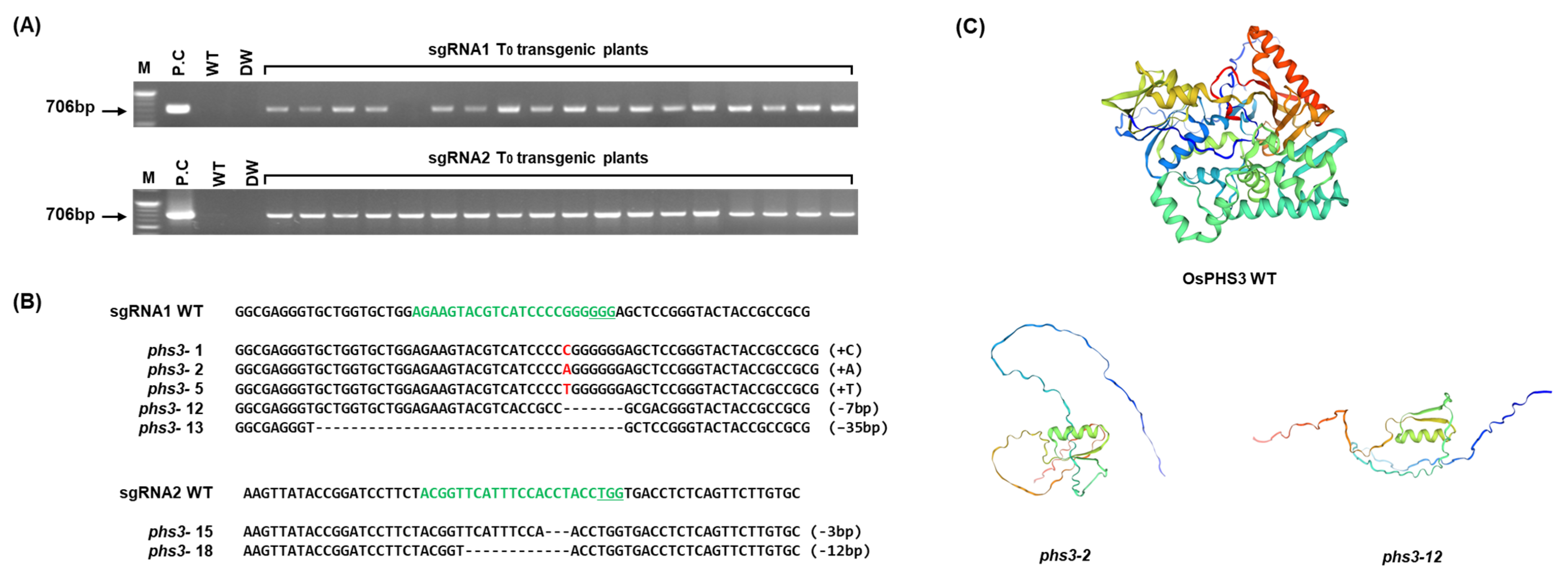
2.1. Confirmation and Expression of PHS3 Gene Encoding Carotenoid Isomerase in Rice
2.2. Generation of OsPHS3 Knockout Homozygous Mutants
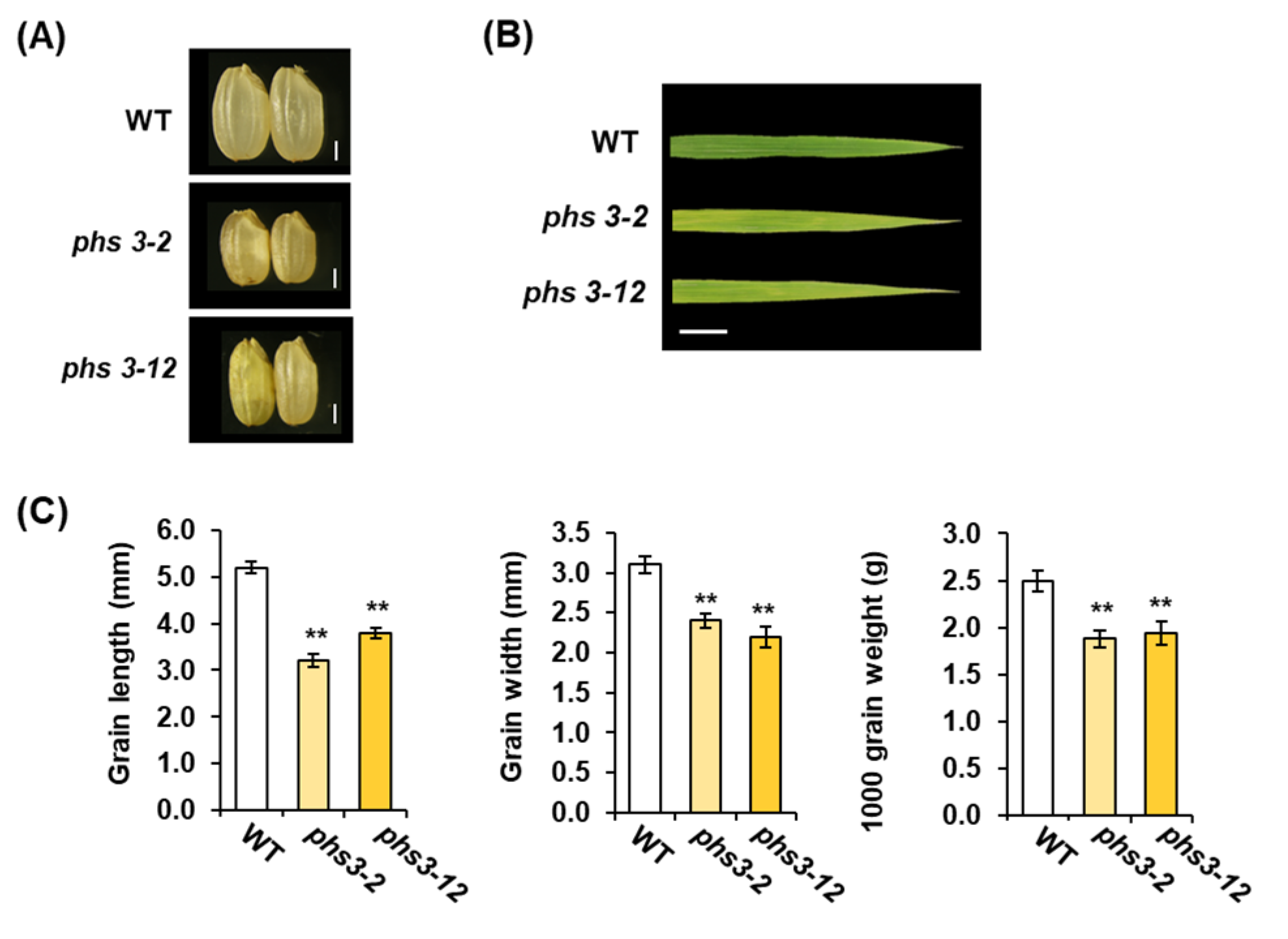
2.3. Agronomic Traits of phs3-2 and phs3-12 Null Lines
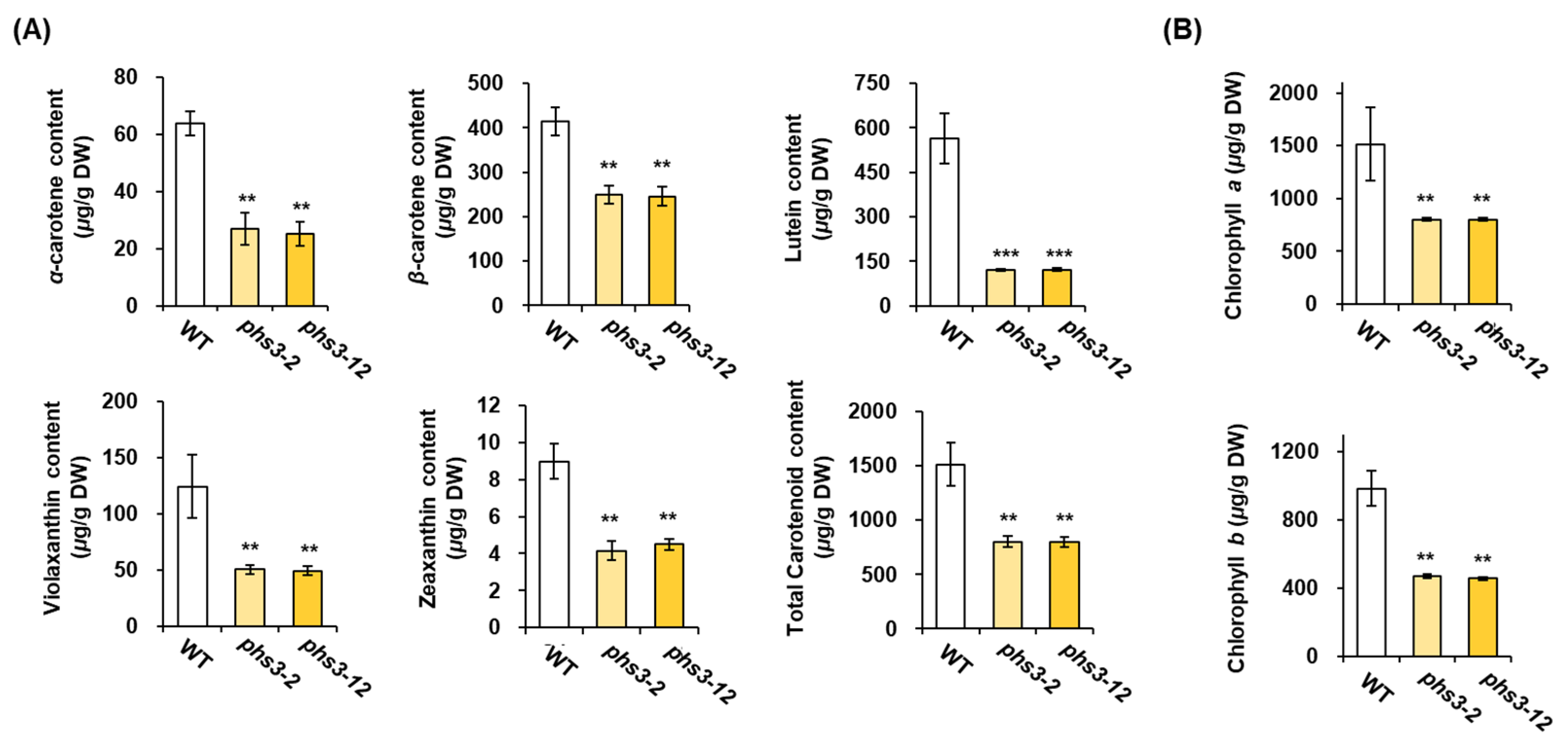
2.4. Carotenoid Profiles of phs3-2 and phs3-12 Null Lines
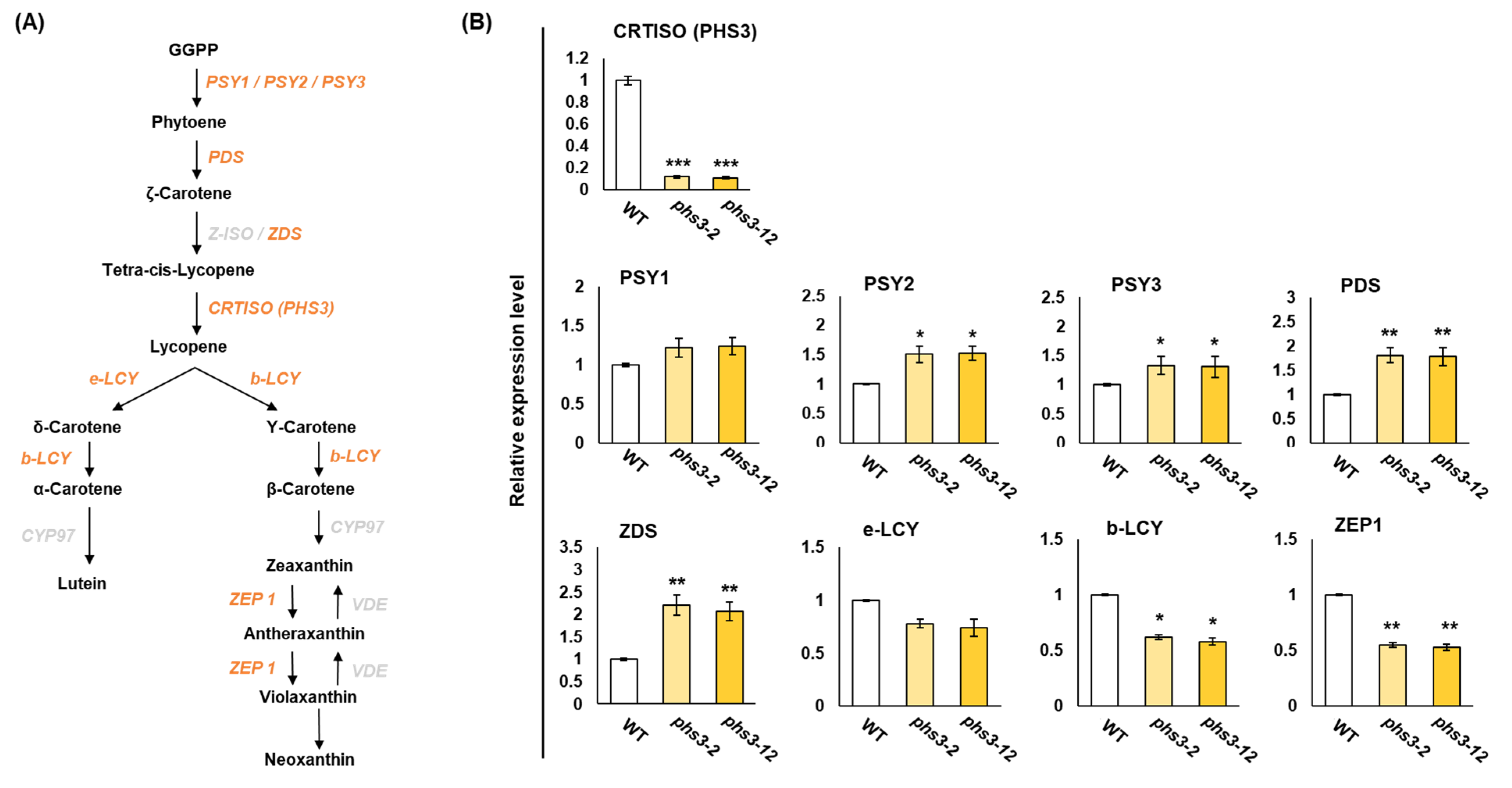
2.5. Expression Profiling of Carotenoid Biosynthesis Genes
2.6. ABA and Pre-Harvest Sprouting in phs3-2 and phs3-12 Null Lines
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growing Conditions
4.2. Selection of Gene Target Site and CRISPR/Cas9 Vector Construction
4.3. Generation and Selection of Transgenic Rice Plants
4.4. Deep Sequencing Analysis of Target Genes
4.5. Carotenoid Extraction and HPLC Analysis
4.6. Gene Expression Profiling by qRT-PCR Analysis
4.7. Test of Pre-Harvest Sprouting
4.8. Determination of ABA
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siebenmorgen, T.J.; Grigg, B.C.; Lanning, S.B. Impacts of preharvest factors during kernel development on rice quality and functionality. Annu. Rev. Food Sci. Technol. 2013, 4, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Zhang, H.; Lu, J.; Si, H.; Ma, C. Genetic improvement of wheat with pre-harvest sprouting resistance in China. Genes 2023, 14, 837. [Google Scholar] [CrossRef] [PubMed]
- Gubler, F.; Millar, A.A.; Jacobsen, J.V. Dormancy release, ABA and pre-harvest sprouting. Curr. Opin. Plant Biol. 2005, 8, 183–187. [Google Scholar] [CrossRef]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef]
- McCarty, D.R. Genetic control and integration of maturation and germination pathways in seed development. Annu. Rev. Plant Biol. 1995, 46, 71–93. [Google Scholar] [CrossRef]
- Assmann, S.M.; Snyder, J.A.; Lee, Y.R.J. ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant Cell Environ. 2000, 23, 387–395. [Google Scholar] [CrossRef]
- Paek, N.C.; Lee, B.M.; Bai, D.G.; Smith, J.D. Inhibition of germination gene expression by Viviparous-1 and ABA during maize kernel development. Mol. Cells 1998, 8, 336–342. [Google Scholar] [CrossRef]
- Cunningham, F.X., Jr.; Gantt, E. Genes and enzymes of carotenoid biosynthesis in plants. Annu. Rev. Plant Biol. 1998, 49, 557–583. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A. Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol. 2003, 131, 1591–1601. [Google Scholar] [CrossRef]
- Xiong, L.; Zhu, J.K. Regulation of abscisic acid biosynthesis. Plant Physiol. 2003, 133, 29–36. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid Metabolism in Plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Murillo, C.; Wurtzel, E.T. Maize Y9 encodes a product essential for 15-cis-zeta-carotene isomerization. Plant Physiol. 2007, 144, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, F.; Wurtzel, E.T. Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol. 2010, 153, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kreunen, S.S.; Cuttriss, A.J.; DellaPenna, D.; Pogson, B.J. Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 2002, 14, 321–332. [Google Scholar] [CrossRef]
- Isaacson, T.; Ohad, I.; Beyer, P.; Hirschberg, J. Analysis in vitro of the enzyme CRTISO establishes a poly-cis-carotenoid biosynthesis pathway in plants. Plant Physiol. 2004, 136, 4246–4255. [Google Scholar] [CrossRef]
- Auldridge, M.E.; McCarty, D.R.; Klee, H.J. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Curr. Opin. Plant Biol. 2006, 9, 315–321. [Google Scholar] [CrossRef]
- Isaacson, T.; Ronen, G.; Zamir, D.; Hirschberg, J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-Carotene and xanthophylls in plants. Plant Cell 2002, 14, 333–342. [Google Scholar] [CrossRef]
- Chai, C.; Fang, J.; Liu, Y.; Tong, H.; Gong, Y.; Wang, Y.; Liu, M.; Wang, Q.; Qian, Z.; Cheng, C.; et al. ZEBRA2, encoding a carotenoid isomerase, is involved in photoprotection in rice. Plant Mol. Biol. 2011, 75, 211–221. [Google Scholar] [CrossRef]
- Han, S.H.; Sakuraba, Y.; Koh, H.J.; Paek, N.C. Leaf variegation in the rice zebra2 mutant is caused by photoperiodic accumulation of tetra-Cis-Lycopene and singlet oxygen. Mol. Cells 2012, 33, 87–97. [Google Scholar] [CrossRef]
- Liu, L.; Xie, T.; Peng, P.; Qiu, H.; Zhao, J.; Fang, J.; Patil, S.B.; Wang, Y.; Fang, S.; Chu, j.; et al. Mutations in the MIT3 gene encoding a caroteniod isomerase lead to increased tiller number in rice. Plant Sci. 2018, 267, 1–10. [Google Scholar] [CrossRef]
- Zhang, F.; Wen, Y.; Guo, X. CRISPR/Cas9 for genome editing: Progress, implications and challenges. Hum. Mol. Genet. 2014, 23, R40–R46. [Google Scholar] [CrossRef]
- Longxing, T.; Xi, W.; Huijuan, T.; Haisheng, C.; Changdeng, Y.; Jieyun, Z.; Kangle, Z. A physiological study on pre-harvest sprouting in rice. Zuo Wu Xue Bao 2006, 32, 728–733. [Google Scholar]
- Biddulph, T.B.; Plummer, J.A.; Setter, T.L.; Mares, D.J. Seasonal conditions influence dormancy and preharvest sprouting tolerance of wheat (Triticum aestivum L.) in the field. Field Crops Res. 2008, 107, 116–128. [Google Scholar] [CrossRef]
- Chen, C.X.; Cai, S.B.; Bai, G.H. A major QTL controlling seed dormancy and pre-harvest sprouting resistance on chromosome 4A in a Chinese wheat landrace. Mol. Breed. 2008, 21, 351–358. [Google Scholar] [CrossRef]
- Gao, X.; Hu, C.H.; Li, H.Z.; Yao, Y.J.; Meng, M.; Dong, J.; Zhao, W.C.; Chen, Q.J.; Li, X.Y. Factors affecting pre-harvest sprouting resistance in wheat (Triticum aestivum L.): A review. J. Anim. Plant Sci. 2013, 23, 556–565. [Google Scholar]
- Rasul, G.; Humphreys, G.D.; Wu, J.; Brûlé-Babel, A.; Fofana, B.; Glover, K.D. Evaluation of preharvest sprouting traits in a collection of spring wheat germplasm using genotype and genotype× environment interaction model. Plant Breed. 2012, 131, 244–251. [Google Scholar] [CrossRef]
- Efremov, G.I.; Shchennikova, A.V.; Kochieva, E.Z. Functional Diversification of the Carotenoid-cis-trans-Isomerases CrtISO, CrtISO-L1, and CrtISO-L2 in Tomato Species (Solanum, Section Lycopersicon). Dokl. Biochem. Biophys. 2022, 507, 340–344. [Google Scholar] [CrossRef]
- Eun, C.H.; Kim, S.U.; Kim, I.J. Regulatory cis-elements on citrus peel-specific expressed gene, CuCRTISO-like, promoter respond to hormones and abiotic stresses in transgenic Arabidopsis. Plant Biotechnol. Rep. 2017, 11, 63–69. [Google Scholar] [CrossRef]
- Welsch, R.; Beyer, P.; Hugueney, P.; Kleinig, H.; von Lintig, J. Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 2000, 211, 846–854. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Tan, B.C.; Gage, D.A.; Zeevaart, J.A.; McCarty, D.R. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 1997, 276, 1872–1874. [Google Scholar] [CrossRef]
- Jung, Y.J.; Lee, H.J.; Kim, J.H.; Kim, D.H.; Kim, H.K.; Cho, Y.G.; Kang, K.K. CRISPR/Cas9-targeted mutagenesis of F3′ H, DFR and LDOX, genes related to anthocyanin biosynthesis in black rice (Oryza sativa L.). Plant Biotechnol. Rep. 2019, 13, 521–531. [Google Scholar] [CrossRef]
- Park, J.; Bae, S.; Kim, J.S. Cas-Designer: A web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.T.; Ryu, J.; Choi, M.K.; Kweon, J.; Kang, B.C.; Kim, S.G. A simple, flexible and high-throughput cloning system for plant genome editing via CRISPR-Cas system. J. Integr. Plant Biol. 2016, 58, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.J.; Bae, S.; Lee, G.J.; Seo, P.J.; Cho, Y.G.; Kang, K.K. A novel method for high-frequency genome editing in rice, using the CRISPR/Cas9 system. J. Plant Biotechnol. 2017, 44, 89–96. [Google Scholar] [CrossRef]
- Nishimura, A.; Aichi, I.; Matsuoka, M. A protocol for Agrobacterium-mediated transformation in rice. Nat. Protoc. 2006, 1, 2796–2802. [Google Scholar] [CrossRef]
- Park, J.; Lim, K.; Kim, J.S.; Bae, S. Cas-analyzer: An online tool for assessing genome editing results using NGS data. Bioinformatics 2017, 33, 286–288. [Google Scholar] [CrossRef]
- Kim, H.J.; Koo, K.A.; Park, W.S.; Kang, D.M.; Kim, H.S.; Lee, B.Y.; Goo, Y.M.; Kim, J.H.; Lee, M.K.; Woo, D.K.; et al. Anti-obesity activity of anthocyanin and carotenoid extracts from color-fleshed sweet potatoes. J. Food Biochem. 2020, 44, 11. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Luo, J.; Shen, G.; Yan, J.; He, C.; Zhang, H. AtCHIP functions as an E3 ubiquitin ligase of protein phosphatase 2A subunits and alters plant response to abscisic acid treatment. Plant J. 2006, 46, 649–657. [Google Scholar] [CrossRef]
- Duncan, D.B. Multiple range and multiple F tests. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]



| Line | Plant Height (cm) | Culm Diameter (mm) | Panicle Length (cm) | Number of Tillers |
|---|---|---|---|---|
| WT | 119.8 ± 10.4 | 6.1 ± 0.13 | 22.58 ± 0.86 | 13.8 ± 0.08 |
| phs3-2 | 82.3 ± 6.7 | 4.7 ± 0.12 | 20.41 ± 0.47 | 16.2 ± 0.04 |
| phs3-12 | 84.1 ± 3.4 | 4.6 ± 0.20 | 21.09 ± 0.55 | 16.3 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.-J.; Go, J.; Kim, J.-Y.; Lee, H.-J.; Kim, J.-H.; Lee, H.-M.; Cho, Y.-G.; Kang, K.-K. ABA and Pre-Harvest Sprouting Differences in Knockout Lines of OsPHS3 Encoding Carotenoid Isomerase via CRISPR/Cas9 in Rice. Plants 2025, 14, 345. https://doi.org/10.3390/plants14030345
Jung Y-J, Go J, Kim J-Y, Lee H-J, Kim J-H, Lee H-M, Cho Y-G, Kang K-K. ABA and Pre-Harvest Sprouting Differences in Knockout Lines of OsPHS3 Encoding Carotenoid Isomerase via CRISPR/Cas9 in Rice. Plants. 2025; 14(3):345. https://doi.org/10.3390/plants14030345
Chicago/Turabian StyleJung, Yu-Jin, Jiyun Go, Jin-Young Kim, Hyo-Ju Lee, Jong-Hee Kim, Hye-Mi Lee, Yong-Gu Cho, and Kwon-Kyoo Kang. 2025. "ABA and Pre-Harvest Sprouting Differences in Knockout Lines of OsPHS3 Encoding Carotenoid Isomerase via CRISPR/Cas9 in Rice" Plants 14, no. 3: 345. https://doi.org/10.3390/plants14030345
APA StyleJung, Y.-J., Go, J., Kim, J.-Y., Lee, H.-J., Kim, J.-H., Lee, H.-M., Cho, Y.-G., & Kang, K.-K. (2025). ABA and Pre-Harvest Sprouting Differences in Knockout Lines of OsPHS3 Encoding Carotenoid Isomerase via CRISPR/Cas9 in Rice. Plants, 14(3), 345. https://doi.org/10.3390/plants14030345









