Phytochemical Analysis and Biological Activities of Flavonoids and Anthraquinones from Cassia alata (Linnaeus) Roxburgh and Their Implications for Atopic Dermatitis Management
Abstract
:1. Introduction
2. Results
2.1. HPLC–DAD Identification and Quantification of Important Bioactive Constituents in CA Extracts
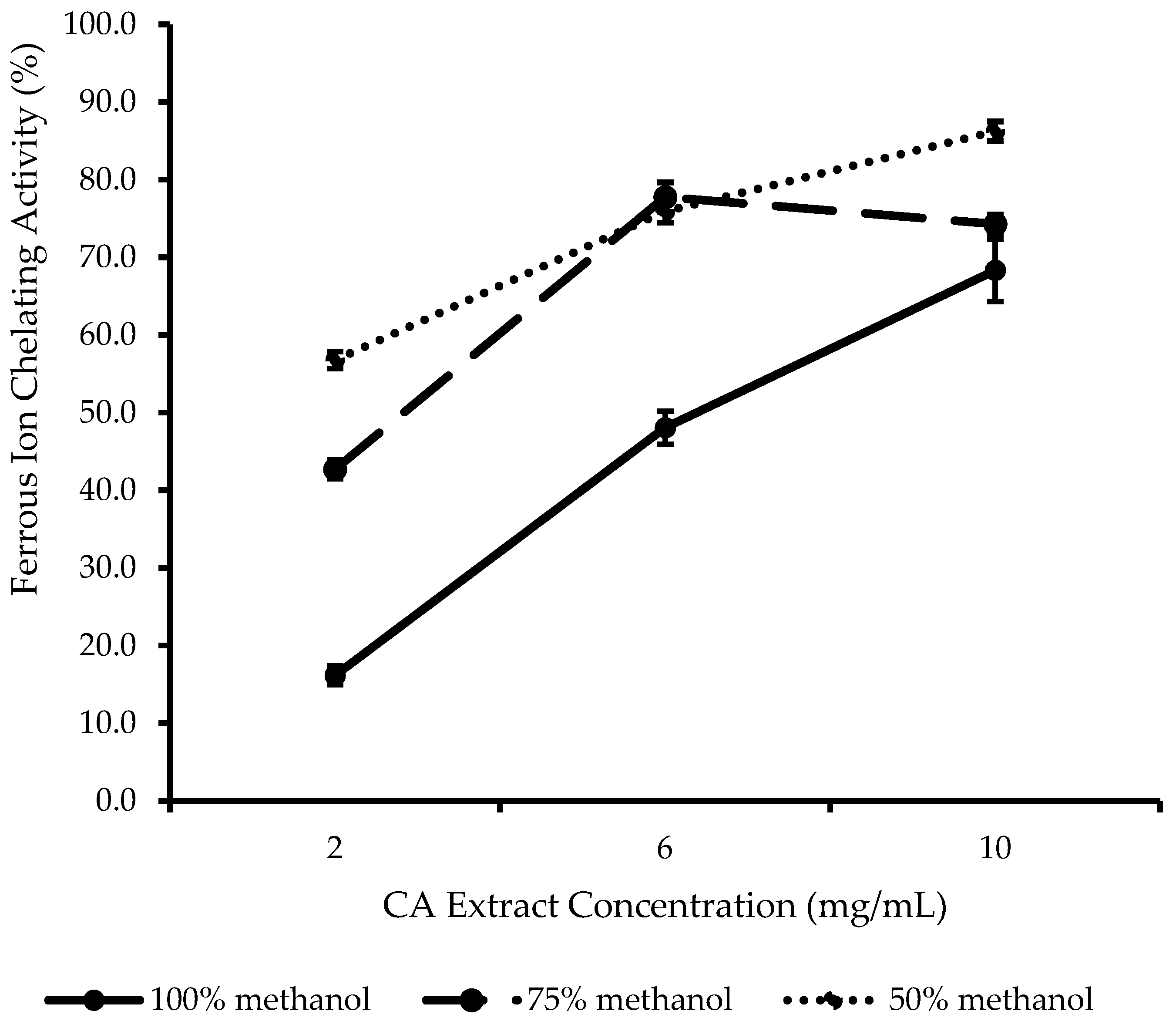
2.2. In Vitro Antioxidant Evaluation of CA Extracts
2.3. Evaluation of Antimicrobial Efficacy of CA Extracts Against S. aureus ATCC 25923
2.4. Selection of Best Solvent for C. alata Leaf Extraction
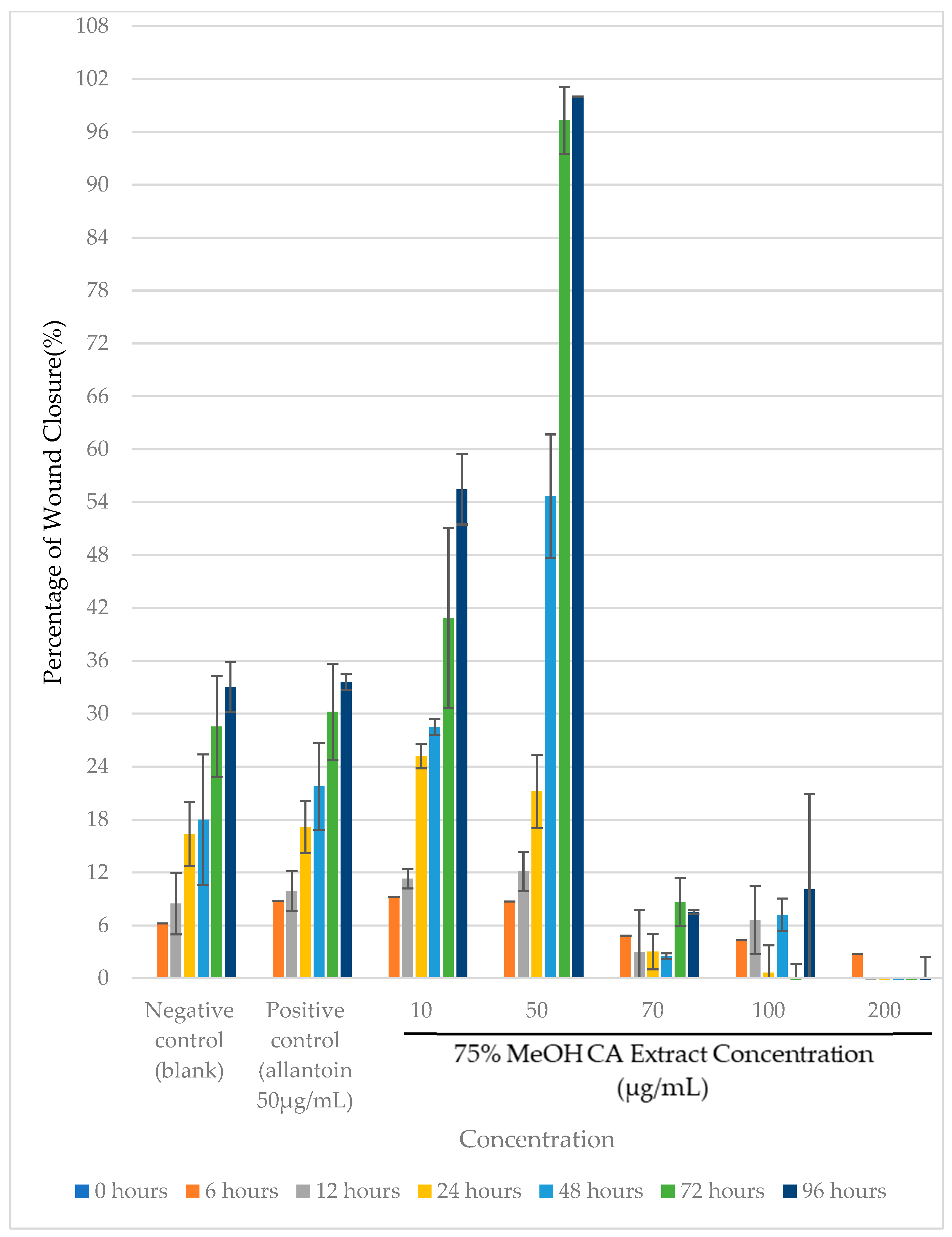
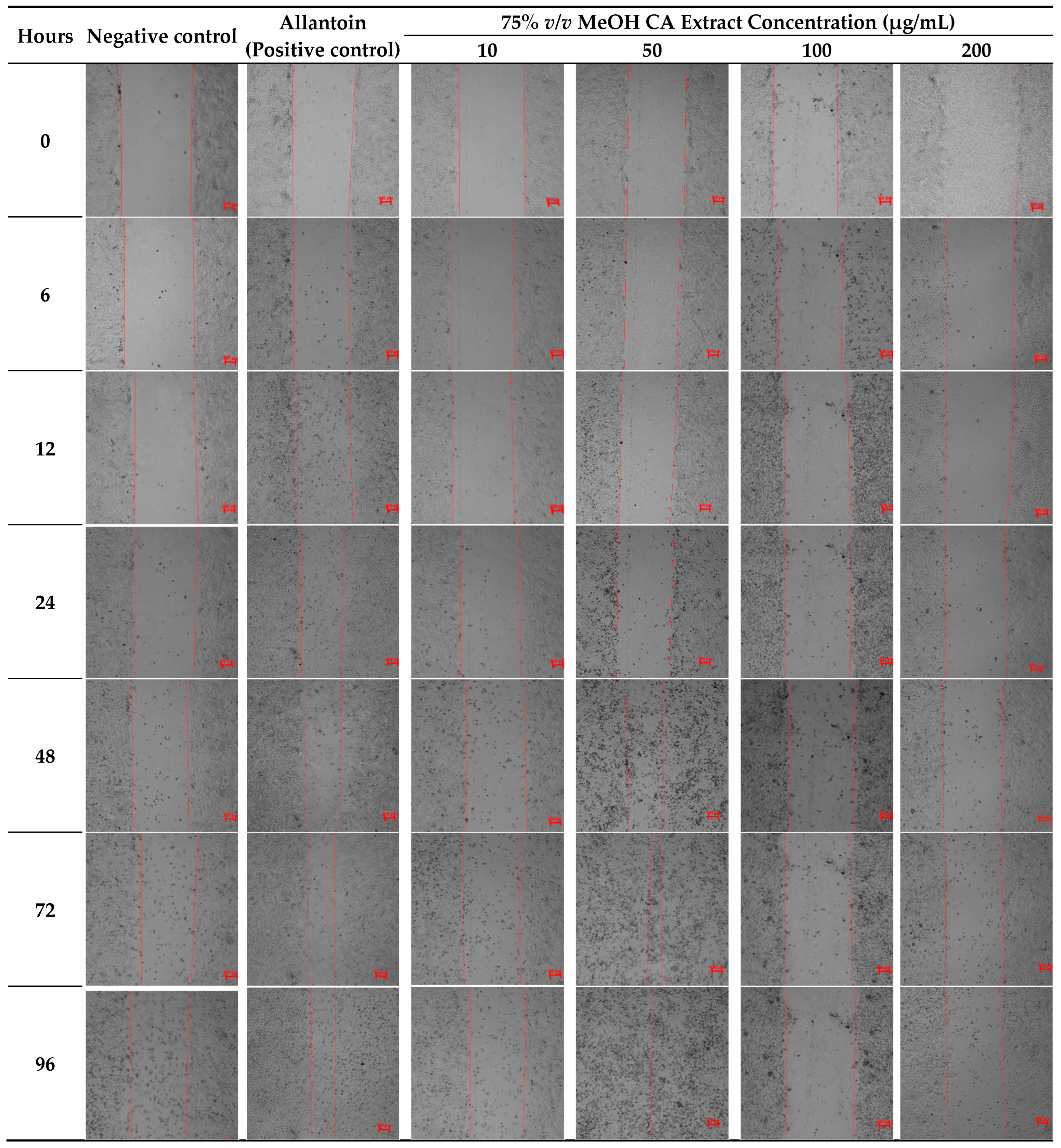
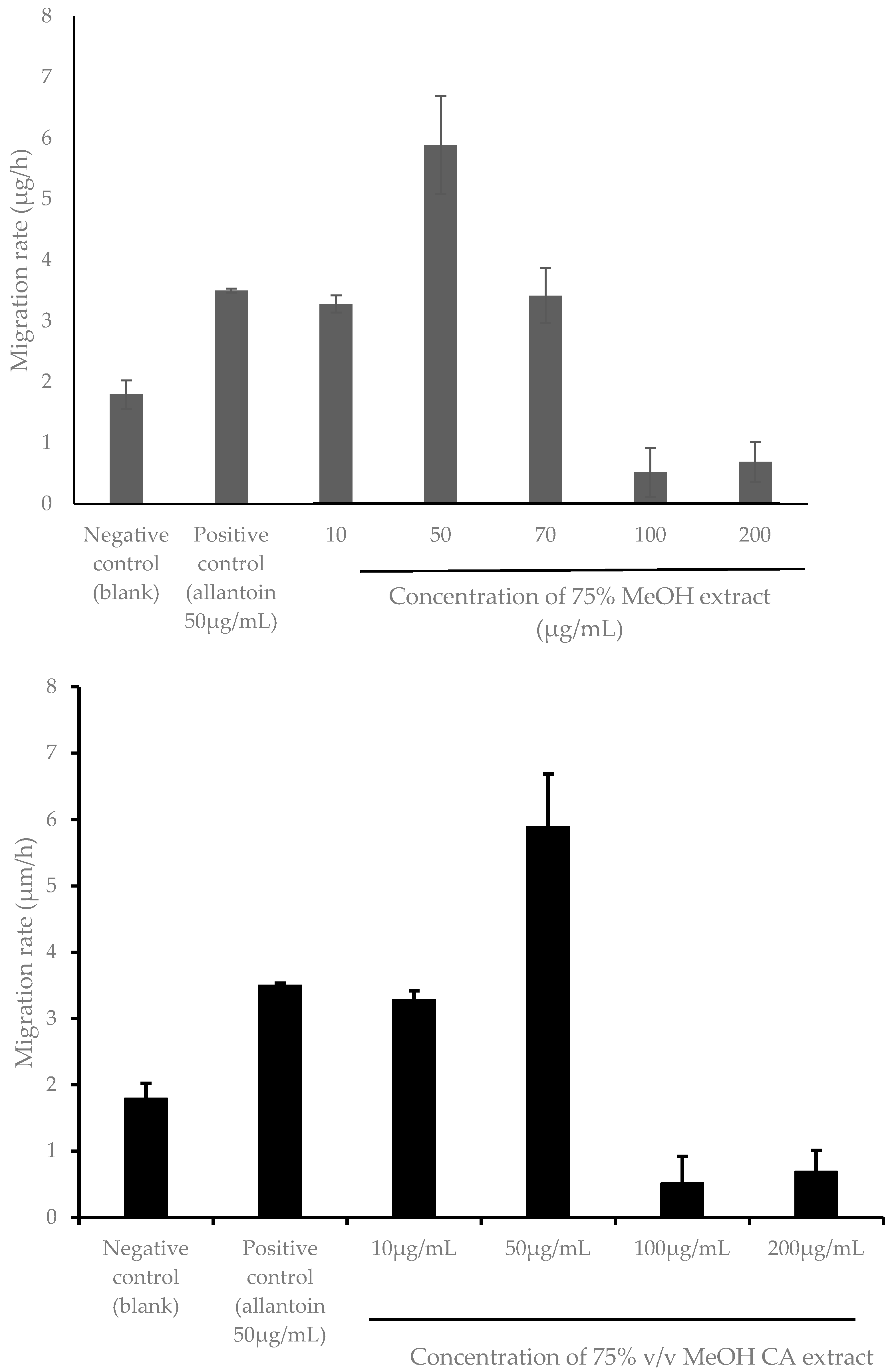
2.5. Evaluation of Cytotoxicity and Wound Healing Efficacy of 75% v/v MeOH CA Extract in the Immortalized Human Keratinocytes (HaCaT) Cell Line
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Chemicals
3.3. Plant Material and Extraction
3.4. HPLC–DAD Identification and Quantification of Major Constituents in Cassia alata Leaf Extracts
3.5. In Vitro Antioxidant Evaluation of Cassia alata Leaf Extracts
3.5.1. Total Phenolic Content (TPC)
3.5.2. Total Flavonoid Content (TFC)
3.5.3. 2,2-Diphenyl-1-picrylhydrazyl-hydrate (DPPH) Radical Scavenging Activity
3.5.4. Ferric Reducing Antioxidant Power (FRAP)
3.5.5. Ferrous Ion Chelating (FIC) Ability
3.6. Evaluation of Antimicrobial Efficacy of Cassia alata Leaf Extracts Against Staphylococcus aureus ATCC 25923
3.7. Cell Culture
3.8. Evaluation of Cell Cytotoxicity
3.9. In Vitro Wound-Healing Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yew, Y.W.; Thyssen, J.P.; Silverberg, J.I. A systematic review and meta-analysis of the regional and age-related differences in atopic dermatitis clinical characteristics. J. Am. Acad. Dermatol. 2019, 80, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.; Boguniewicz, J.; Boguniewicz, M.; Ong, P.Y. The infectious complications of atopic dermatitis. Ann. Allergy Asthma Immunol. 2021, 126, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Bao, L.; Chan, L.S.; DiPietro, L.A.; Chen, L. Aberrant wound healing in an epidermal interleukin-4 transgenic mouse model of atopic dermatitis. PLoS ONE 2016, 11, e0146451. [Google Scholar] [CrossRef]
- Simonetti, O.; Bacchetti, T.; Ferretti, G.; Molinelli, E.; Rizzetto, G.; Bellachioma, L.; Offidani, A. Oxidative stress and alterations of paraoxonases in atopic dermatitis. Antioxidants 2021, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Heinemann, N.; Rademacher, F.; Darvin, M.E.; Raab, C.; Keck, C.M.; Vollert, H.; Fluhr, J.W.; Glaser, R.; Harder, J.; et al. Skin care product rich in antioxidants and anti-inflammatory natural compounds reduces itching and inflammation in the skin of atopic dermatitis patients. Antioxidants 2022, 11, 1071. [Google Scholar] [CrossRef] [PubMed]
- Chew, Y.-L.; Al-Nema, M.; Ong, V.W.-M. Management and treatment of atopic dermatitis with modern therapies, complementary and alternative medicines: A review. Orient. Pharm. Exp. Med. 2018, 18, 67–76. [Google Scholar] [CrossRef]
- Koo, K.; Nagayah, R.; Begum, S.; Mahmood, T.M.T.; Shah, N.M. The use of complementary and alternative medicine in children with atopic eczema at a tertiary care centre in Malaysia. Complement. Ther. Med. 2020, 49, 102355. [Google Scholar] [CrossRef] [PubMed]
- Globinmed. Senna alata (L.) Roxb. Available online: https://globinmed.com/medicinal_herbs/senna-alata-l-roxb-104893/ (accessed on 2 August 2023).
- Yon, J.-A.-L.; Lee, S.-K.; Keng, J.-W.; Chow, S.-C.; Liew, K.-B.; Teo, S.-S.; Shaik Mossadeq, W.M.; Marriott, P.J.; Akowuah, G.A.; Ming, L.C.; et al. Cassia alata (Linnaeus) Roxburgh for skin: Natural remedies for atopic dermatitis in Asia and their pharmacological activities. Cosmetics 2022, 10, 5. [Google Scholar] [CrossRef]
- Oladeji, O.S.; Adelowo, F.E.; Oluyori, A.P.; Bankole, D.T. Ethnobotanical Description and Biological Activities of Senna alata. Evid.-Based Complement. Altern. Med. 2020, 2020, 2580259. [Google Scholar] [CrossRef]
- Chew, Y.L.; Khor, M.A.; Lim, Y.Y. Choices of chromatographic methods as stability indicating assays for pharmaceutical products: A review. Heliyon 2021, 7, e06553. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Nadkarni, J.R.; Vishwakarma, R.A.; Bharate, S.B.; Nivsarkar, M.; Anandjiwala, S. The hydroalcoholic extract of Cassia alata (Linn.) leaves and its major compound rhein exhibits antiallergic activity via mast cell stabilization and lipoxygenase inhibition. J. Ethnopharmacol. 2012, 141, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef] [PubMed]
- Wadkhien, K.; Chinpaisal, C.; Satiraphan, M.; Wetwitayaklung, P.; Pongnimitprasert, N. Anti-inflammatory effects of rhein and crude extracts from Cassia alata L. in HaCaT cells. Sci. Eng. Health Stud. 2018, 12, 19–32. [Google Scholar] [CrossRef]
- Gritsanapan, W.; Mangmeesri, P. Standardized Senna alata leaf extract. J. Health Res. 2009, 23, 59–64. [Google Scholar]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Hazni, H.; Ahmad, N.; Hitotsuyanagi, Y.; Takeya, K.; Choo, C.Y. Phytochemical constituents from Cassia alata with inhibition against methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2008, 74, 1802–1805. [Google Scholar] [CrossRef]
- Dong, X.; Zeng, Y.; Liu, Y.; You, L.; Yin, X.; Fu, J.; Ni, J. Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics. Phytother. Res. 2020, 34, 270–281. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, S.; Dey, K.K.; Dutta, S. Study of antioxidant activity and immune stimulating potency of the ethnomedicinal plant, Cassia alata (L.) Roxb. Med. Aromat. Plants 2013, 2, 131. [Google Scholar] [CrossRef]
- Zhao, S.; Xiong, F.; Wang, L.; Wang, B.; Chen, K.; Chen, C.; Zhou, G. Study on the quality characteristics and geographical origin authentication of wild Rheum tanguticum in three authentic regions. J. Food Compost. Anal. 2023, 123, 105463. [Google Scholar] [CrossRef]
- Shen, N.; Chen, Y.; Guo, F.; Cui, Y.; Wei, M.; Cheng, Z. Influence of different drying methods coupled with different process modes on physicochemical qualities and anthraquinones contents of Rheum palmatum L. LWT 2022, 170, 114021. [Google Scholar] [CrossRef]
- Aung, W.W.; Krongrawa, W.; Ponphaiboon, J.; Kulpicheswanich, P.; Limmatvapirat, C. Yields, phytochemicals, and biological activities of different solvent extracts of Senna alata leaves. Key Eng. Mater. 2022, 914, 135–140. [Google Scholar] [CrossRef]
- Barchan, A.; Bakkali, M.; Arakrak, A.; Pagán, R.; Laglaoui, A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 399–412. [Google Scholar]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.K.; Wood, L.G.; Garg, M.L. Methodology for the determination of biological antioxidant capacity in vitro: A review. J. Sci. Food Agric. 2006, 86, 2046–2056. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Chew, Y.-L.; Goh, J.-K.; Lim, Y.-Y. Assessment of in vitro antioxidant capacity and polyphenolic composition of selected medicinal herbs from Leguminosae family in Peninsular Malaysia. Food Chem. 2009, 116, 13–18. [Google Scholar] [CrossRef]
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT-Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- Vargas, F.; Díaz, Y.; Carbonell, K. Antioxidant and scavenging activity of emodin, aloe-emodin, and rhein on free-radical and reactive oxygen species. Pharm. Biol. 2004, 42, 342–348. [Google Scholar] [CrossRef]
- Park, S.N.; Kim, S.Y.; Lim, G.N.; Jo, N.R.; Lee, M.H. In vitro skin permeation and cellular protective effects of flavonoids isolated from Suaeda asparagoides extracts. J. Ind. Eng. Chem. 2012, 18, 680–683. [Google Scholar] [CrossRef]
- Pinchuk, I.; Shoval, H.; Dotan, Y.; Lichtenberg, D. Evaluation of antioxidants: Scope, limitations and relevance of assays. Chem. Phys. Lipids 2012, 165, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Bertino, L.; Guarneri, F.; Cannavo, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative stress and atopic dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Loh, Z.H.; Lim, Y.Y. Drying effects on antioxidant activity, enzyme activity, and phytochemicals of avocado (Persea americana) leaves. J. Food Process. Preserv. 2018, 42, e13667. [Google Scholar] [CrossRef]
- Li, Q.; Huang, Y.; Liu, X.; Gan, J.; Chen, H.; Yang, C.G. Rhein inhibits AlkB repair enzymes and sensitizes cells to methylated DNA damage. J. Biol. Chem. 2016, 291, 11083–11093. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; You, Q.; Zhang, X. Medicinal chemistry of metal chelating fragments in metalloenzyme active sites: A perspective. Eur. J. Med. Chem. 2019, 165, 172–197. [Google Scholar] [CrossRef] [PubMed]
- Yen, G. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Jiang, Q.; Li, X.; Tian, Y.; Lin, Q.; Xie, H.; Lu, W.; Chi, Y.; Chen, D. Lyophilized aqueous extracts of Mori fructus and Mori ramulus protect mesenchymal stem cells from *OH-treated damage: Bioassay and antioxidant mechanism. BMC Complement. Altern. Med. 2017, 17, 242. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-N.; Kim, E.-S.; Kwon, Y.-I.; Jang, H.-D. Potential mechanism of kaempferol against Cu2+-induced oxidative stress through chelating activity and regulation of nuclear factorerythroid-2-related factor 2 signaling. Food Sci. Biotechnol. 2012, 21, 1469–1475. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus aureus and atopic dermatitis: A complex and evolving relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef]
- Somchit, M.N.; Reezal, I.; Nur, I.E.; Mutalib, A.R. In vitro antimicrobial activity of ethanol and water extracts of Cassia alata. J. Ethnopharmacol. 2003, 84, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Awal, M.; Nahar, A.; Hossain, M.S.; Bari, M.; Rahman, M.; Haque, M. Brine shrimp toxicity of leaf and seed extracts of Cassia alata Linn. and their antibacterial potency. J. Med. Sci. 2004, 4, 188–193. [Google Scholar] [CrossRef]
- El-Mahmood, A.; Doughari, J. Phytochemical screening and antibacterial evaluation of the leaf and root extracts of Cassia alata Linn. Afr. J. Pharm. Pharmacol. 2008, 2, 124–129. [Google Scholar]
- Doughari, J.; Okafor, B. Antimicrobial activity of Senna alata Linn. East. Central Afr. J. Pharm. Sci. 2007, 10, 17–21. [Google Scholar] [CrossRef]
- Angelina, M.; Mardhiyah, A.; Dewi, R.T.; Fajriah, S.; Muthiah, N.; Ekapratiwi, Y.; Dewijanti, I.D.; Sukirno, S.; Jamilah, J.; Hartati, S. Physicochemical and phytochemical standardization, and antibacterial evaluation of Cassia alata leaves from different locations in Indonesia. Pharmacia 2021, 68, 947–956. [Google Scholar] [CrossRef]
- Saito, S.; Silva, G.; Santos, R.X.; Gosmann, G.; Pungartnik, C.; Brendel, M. Astragalin from Cassia alata induces DNA adducts in vitro and repairable DNA damage in the yeast Saccharomyces cerevisiae. Int. J. Mol. Sci. 2012, 13, 2846–2862. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lu, Y.; Zhang, H.; Wang, L.; Beier, R.C.; Jin, Y.; Wang, W.; Li, H.; Hou, X. Antibacterial activity and membrane-targeting mechanism of aloe-emodin against Staphylococcus epidermidis. Front. Microbiol. 2021, 12, 621866. [Google Scholar] [CrossRef] [PubMed]
- Tatsimo, S.J.N.; Tsague, V.T.; Lamshoft, M.; Sarkar, P.; Bag, P.K.; Spiteller, M. Antibacterial-guided isolation of constituents from Senna alata leaves with a particular reference against multi-drug-resistant Vibrio cholerae and Shigella flexneri. Int. J. Biol. Chem. Sci. 2017, 11, 46–53. [Google Scholar] [CrossRef]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Folliero, V.; Palma, F.; Crudele, V.; Finamore, E.; Sanna, G.; Manzin, A.; De Filippis, A.; Galdiero, M.; Franci, G. Anthraquinone rhein exhibits antibacterial activity against Staphylococcus aureus. Appl. Sci. 2022, 12, 8691. [Google Scholar] [CrossRef]
- Chew, Y.-L.; Arasi, C.; Goh, J.-K. Pyrogallol induces antimicrobial effect and cell membrane disruption on methicillin-resistant Staphylococcus aureus (MRSA). Curr. Bioact. Compd. 2022, 18, 38–46. [Google Scholar] [CrossRef]
- Agampodi, V.A.; Collet, T. Anti-inflammatory effects and keratinocyte regenerative potential of Cassia alata (Linn) leaf extracts and their implications for wound healing. J. Appl. Biol. Sci. 2022, 16, 503–526. [Google Scholar]
- Kittiwattanokhun, A.; Samosorn, S.; Innajak, S.; Watanapokasin, R. Inhibitory effects on chondrosarcoma cell metastasis by Senna alata extract. Biomed. Pharmacother. 2021, 137, 111337. [Google Scholar] [CrossRef]
- Akinyemi, E.B.; Babatunde, S.; Feyisayo, A.K.; Michael, O.W. Membrane stabilization and inhibition of protein denaturation as mechanisms of the anti-inflammatory activity of some plant species. Trends Pharm. Sci. 2021, 7, 269–278. [Google Scholar]
- Patrick-Iwuanyanwu, K.C.; Onyeike, E.N.; Dar, A. Anti-inflammatory effect of crude methanolic extract and fractions of ring worm plant Senna alata (L. Roxb) leaves from Nigeria. Pharm. Sin. 2011, 2, 9–16. [Google Scholar]
- Midawa, S.; Ali, B.; Mshelia, B.; Johnson, J. Cutaneous wound healing activity of the ethanolic extracts of the leaf of Senna alata L. (Fabaceae). J. Biol. Sci. Bioconserv. 2010, 2, 63–68. [Google Scholar]
- Chew, Y.-L.; Khor, M.-A.; Xu, Z.; Lee, S.-K.; Keng, J.-W.; Sang, S.-H.; Akowuah, G.A.; Goh, K.W.; Liew, K.B.; Ming, L.C. Cassia alata, Coriandrum sativum, Curcuma longa and Azadirachta indica: Food ingredients as complementary and alternative therapies for atopic dermatitis—A comprehensive review. Molecules 2022, 27, 5475. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin improves inflammation, oxidative stress, and impaired wound healing in atopic dermatitis model of human keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Palanichamy, S.; Bhaskar, E.A.; Bakthavathsalam, R.; Nagarajan, S. Wound healing activity of Cassia alata. Fitoterapia 1991, 62, 153–156. [Google Scholar]
- Saidin, S.H.; Ali, N.A.M.; Mohd, N.; Hirmizi, N.Y.; Abdullah, Z.; Markandan, S.; Khoo, M.; Pisar, M.; Jamil, M.; Lee, T.A. Skin care active ingredients from Senna alata (L.) Roxb extracts. Asian J. Pharmacogn. 2019, 3, 23–31. [Google Scholar]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Raja; Sivamani, K.; Garcia, M.S.; Isseroff, R.R. Wound re-epithelialization: Modulating keratinocyte migration in wound healing. Front. Biosci. 2007, 12, 2849–2868. [Google Scholar] [CrossRef] [PubMed]
- Karuppannan, S.K.; Bushion, J.; Ramalingam, R.; Swaminathan, S.; Arunachalam, K.D.; Kadam, A.A.; Rajagopal, R.; Sathya, R.; Chinnappan, S. Fabrication, characterization and in vitro evaluation of Melia dubia extract infused nanofibers for wound dressing. J. King Saud. Univ.-Sci. 2022, 34, 101931. [Google Scholar] [CrossRef]
- Wedler, J.; Daubitz, T.; Schlotterbeck, G.; Butterweck, V. In vitro anti-inflammatory and wound-healing potential of a Phyllostachys edulis leaf extract--identification of isoorientin as an active compound. Planta Med. 2014, 80, 1678–1684. [Google Scholar] [CrossRef]
- Hu, W.H.; Wang, H.Y.; Xia, Y.T.; Dai, D.K.; Xiong, Q.P.; Dong, T.T.; Duan, R.; Chan, G.K.; Qin, Q.W.; Tsim, K.W. Kaempferol, a major flavonoid in Ginkgo folium, potentiates angiogenic functions in cultured endothelial cells by binding to vascular endothelial growth factor. Front. Pharmacol. 2020, 11, 526. [Google Scholar] [CrossRef]
- Ozay, Y.; Guzel, S.; Yumrutas, O.; Pehlivanoglu, B.; Erdogdu, I.H.; Yildirim, Z.; Turk, B.A.; Darcan, S. Wound healing effect of kaempferol in diabetic and nondiabetic rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef]
- Li, H.; Yang, L.; Zhang, Y.; Gao, Z. Kaempferol inhibits fibroblast collagen synthesis, proliferation and activation in hypertrophic scar via targeting TGF-beta receptor type I. Biomed. Pharmacother. 2016, 83, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Chen, Y.; Guo, D.; Deng, Y.; Guo, W.; Liu, X.; Wang, Y.; Lu, H.; Liu, A.; Zhu, J.; et al. Rhein promotes the proliferation of keratinocytes by targeting oestrogen receptors for skin ulcer treatment. BMC Comp. Med. Ther. 2022, 22, 209. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, X.; Zhang, R.; Zhang, K.; Li, Y.; Xu, F.J. Self-assembled herbal medicine encapsulated by an oxidation-sensitive supramolecular hydrogel for chronic wound treatment. ACS Appl. Mater. Interfaces 2020, 12, 56898–56907. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Han, X.; Liu, S.; Gao, X.; Guo, C.; Wu, X. Aramid nanofibers-reinforced rhein fibrous hydrogels as antibacterial and anti-Inflammatory burn wound dressings. ACS Appl. Mater. Interfaces 2022, 14, 45167–45177. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.X.; Wang, P.; Wang, Y.T.; Huang, Y.; Jiang, L.; Wang, X.M. Aloe vera and Vitis vinifera improve wound healing in an in vivo rat burn wound model. Mol. Med. Rep. 2016, 13, 1070–1076. [Google Scholar] [CrossRef]
- National Parks Board. Senna alata (Linnaeus) Roxburgh. Available online: https://www.nparks.gov.sg/florafaunaweb/flora/2/4/2450 (accessed on 23 July 2023).
- Cujic, N.; Savikin, K.; Jankovic, T.; Pljevljakusic, D.; Zdunic, G.; Ibric, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ben Attia, I.; Zucca, P.; Cesare Marincola, F.; Piras, A.; Rosa, A.; Chaieb, M.; Rescigno, A. Chemical composition and antioxidant potential differences between Cynomorium coccineum L. growing in Italy and in Tunisia: Effect of environmental stress. Diversity 2018, 10, 53. [Google Scholar] [CrossRef]
- de A Cavalcante, M.; Oliveira, J.D.S.; da S Barreto, M.S.; Pinheiro, L.P.; de C Cantuária, P.; Borges, W.L.; da Silva, G.A.; de Souza, T.M. An HPLC method to determine phenolic compounds of plant extracts: Application to Byrsonima crassifolia and Senna alata leaves. Pharmacogn. Res. 2022, 14, 395–404. [Google Scholar] [CrossRef]
- Chong, K.; Lim, Y.Y. Effects of drying on the antioxidant properties of herbal tea from selected Vitex species. J. Food Qual. 2012, 35, 51–59. [Google Scholar] [CrossRef]
- Tan, J.B.; Lim, Y.Y.; Lee, S.M. Antioxidant and antibacterial activity of Rhoeo spathacea (Swartz) Stearn leaves. J. Food Sci. Technol. 2015, 52, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Rukayadi, Y.; Hwang, J.K. In vitro anti-Malassezia activity of xanthorrhizol isolated from Curcuma xanthorrhiza Roxb. Lett. Appl. Microbiol. 2007, 44, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Dusan, M.; Ruzica, A.; Jasna, I.; Irena, Z. Investigation of antibacterial activity of supercritical extracts of plants, as well as of extracts obtained by other technological processes on some bacteria isolated from animals. Acta Vet. Brno. 2009, 59, 557–568. [Google Scholar] [CrossRef]
- Thongrakard, V.; Tencomnao, T. Modulatory effects of Thai medicinal plant extract on proinflammatory cytokines-induced apoptosis in human keratinocyte HaCaT cells. Afr. J. Biotechnol. 2010, 9, 4999–5003. [Google Scholar]
- Martinotti, S.; Ranzato, E. Scratch wound healing assay. In Epidermal Cells: Methods in Molecular Biology; Humana: New York, NY, USA, 2020; pp. 225–229. [Google Scholar] [CrossRef]
- Yuksel, S.; Dikmen, M.; Canturk, Z. Evaluation of real time cell proliferation, anti-inflammatory and wound healing potential of helenalin on HaCaT keratinocytes treated with lipopolysaccharide-stimulated monocytes. Indian J. Pharm. Sci. 2021, 83, 219–229. [Google Scholar] [CrossRef]
- Sonkar, K.S.; Pachauri, M.; Kumar, A.; Shukla, A.; Patel, M.; Jagannadham, M.V. Heme-peroxidase from medicinal plant Artocarpus lakoocha: Purification, characterization and wound healing studies. Biocatal. Agric. Biotechnol. 2015, 4, 180–190. [Google Scholar] [CrossRef]

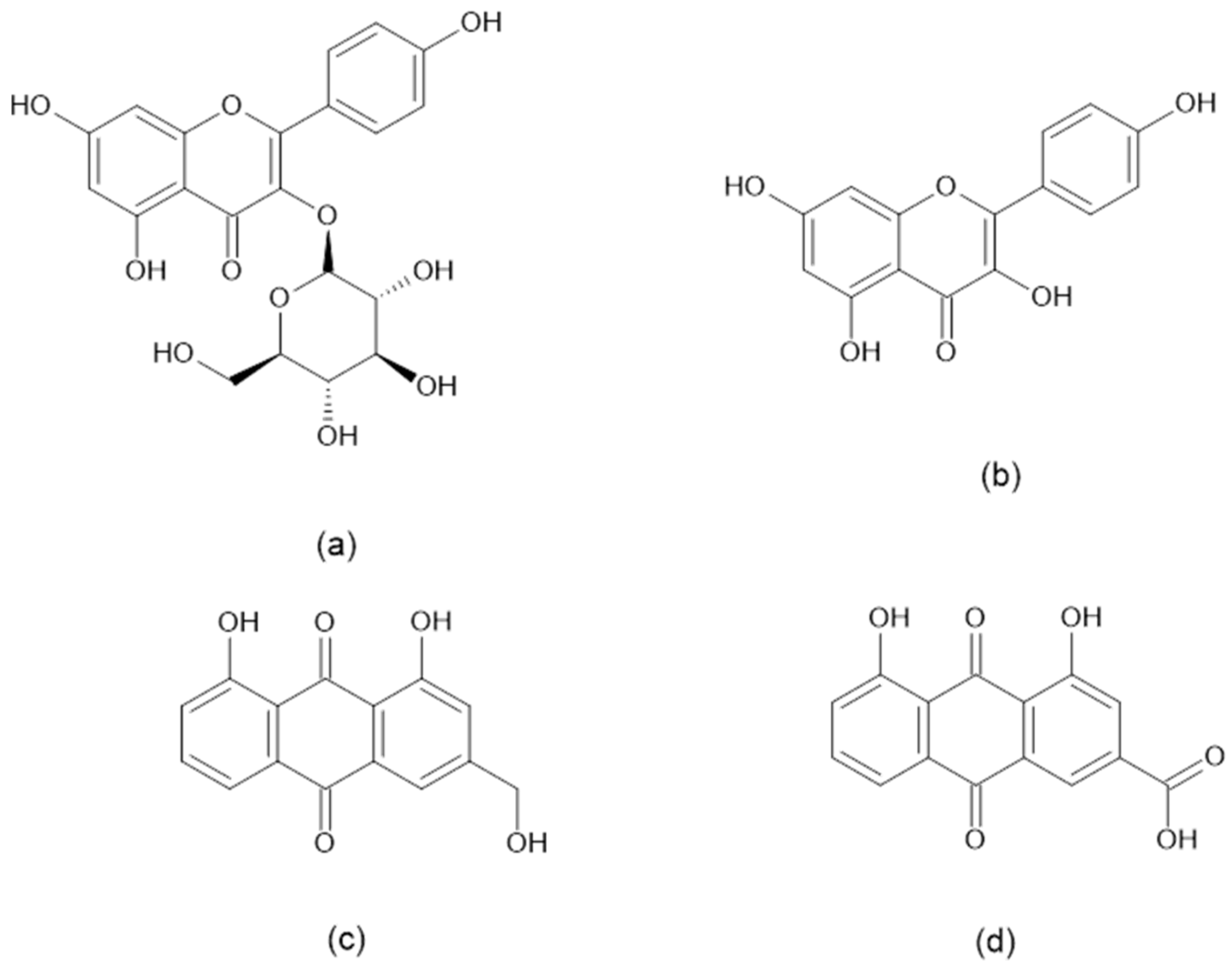
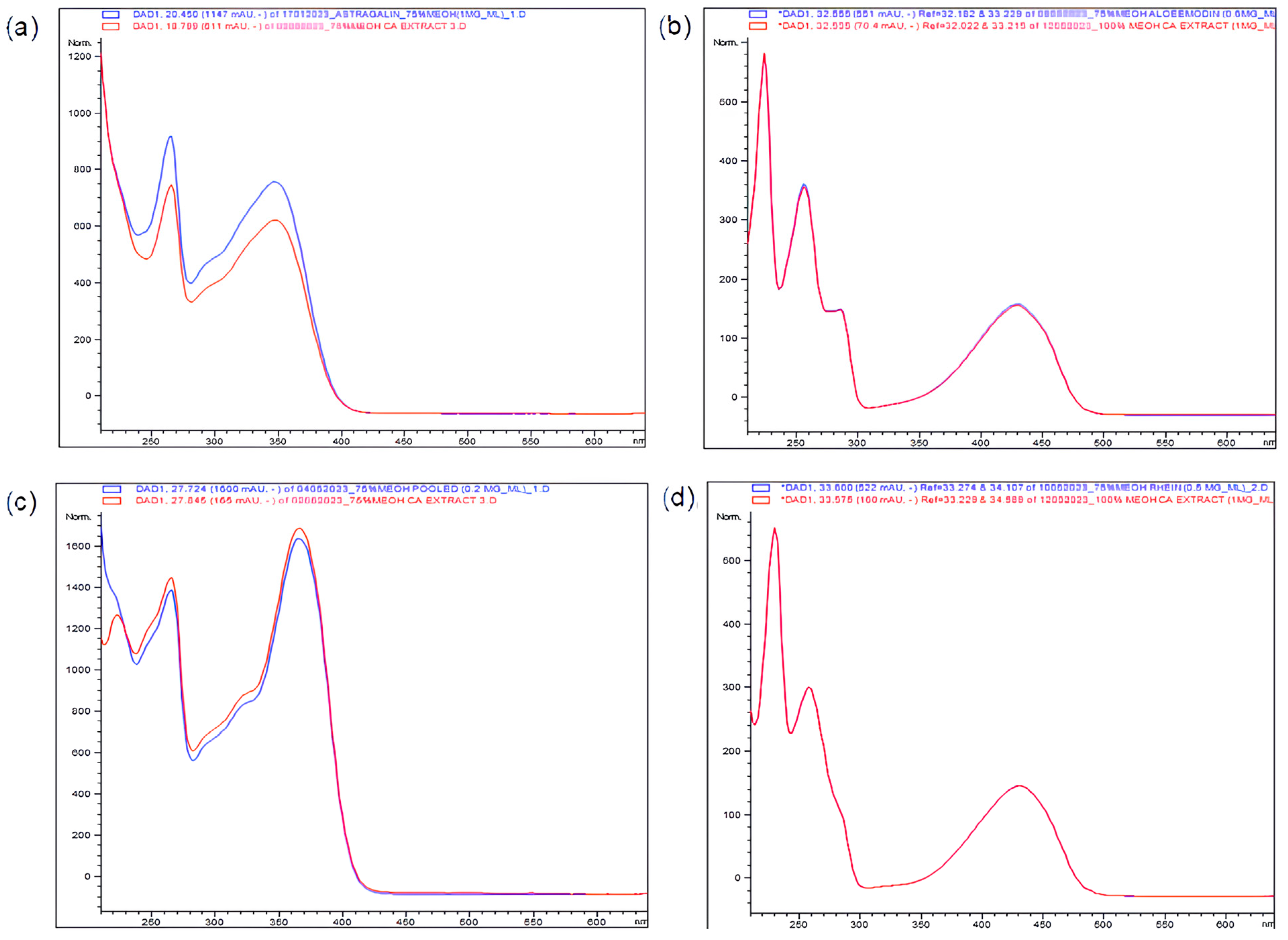

| Chemical Constituent | Concentration (µg/mg) | ||
|---|---|---|---|
| 100% v/v MeOH CA Extract | 75% v/v MeOH CA Extract | 50% v/v MeOH CA Extract | |
| Astragalin | 54.0 ± 1.92 a | 66.0 ± 0.357 b | 60.7 ± 0.315 c |
| Kaempferol | 16.4 ± 0.100 a | 25.2 ± 0.100 b | 22.4 ± 0.050 c |
| Aloe-emodin | 77.2 ± 0.342 a | 121 ± 0.388 b | 101 ± 0.246 c |
| Rhein | 157 ± 0.217 a | 174 ± 0.407 b | 163 ± 0.235 c |
| Samples | TPC (mg GAE/100 g CA Extract) | TFC (mg RE/100 g CA Extract) | Antioxidant Activity | ||
|---|---|---|---|---|---|
| IC50 (mg/mL) | AEAC (mg AA/100 g CA Extract) | FRAP (mg GAE/g CA Extract) | |||
| 100% v/v MeOH CA Extract | 7440 ± 15.7 a | 1680 ± 34.8 a | 0.521 ± 0.025 a | 784 ± 36.7 a | 23.9 ± 0.60 a |
| 75% v/v MeOH CA Extract | 8190 ± 83.7 b | 1710 ± 16.2 a | 0.446 ± 0.009 b | 915 ± 19.2 b | 22.1 ± 0.47 b |
| 50% v/v MeOH CA Extract | 7160 ± 47.2 c | 922 ± 27.7 b | 0.555 ± 0.024 a | 736 ± 31.2 a | 19.8 ± 0.29 c |
| Sample | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| 100% v/v MeOH CA Extract | 1.25 | 5.00 |
| 75% v/v MeOH CA Extract | 0.625 | 1.25 |
| 50% v/v MeOH CA Extract | 1.25 | >5.00 |
| Ampicillin (Positive Control) | 7.81 × 10−5 | 7.81 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-K.; Keng, J.-W.; Yon, J.-A.-L.; Mai, C.-W.; Lim, H.-C.; Chow, S.-C.; Akowuah, G.A.; Liew, K.B.; Lee, S.-K.; Marriott, P.J.; et al. Phytochemical Analysis and Biological Activities of Flavonoids and Anthraquinones from Cassia alata (Linnaeus) Roxburgh and Their Implications for Atopic Dermatitis Management. Plants 2025, 14, 362. https://doi.org/10.3390/plants14030362
Lee S-K, Keng J-W, Yon J-A-L, Mai C-W, Lim H-C, Chow S-C, Akowuah GA, Liew KB, Lee S-K, Marriott PJ, et al. Phytochemical Analysis and Biological Activities of Flavonoids and Anthraquinones from Cassia alata (Linnaeus) Roxburgh and Their Implications for Atopic Dermatitis Management. Plants. 2025; 14(3):362. https://doi.org/10.3390/plants14030362
Chicago/Turabian StyleLee, Sue-Kei, Jing-Wen Keng, Jessica-Ai-Lyn Yon, Chun-Wai Mai, Heng-Chee Lim, Sek-Chuen Chow, Gabriel Akyirem Akowuah, Kai Bin Liew, Siew-Keah Lee, Philip J. Marriott, and et al. 2025. "Phytochemical Analysis and Biological Activities of Flavonoids and Anthraquinones from Cassia alata (Linnaeus) Roxburgh and Their Implications for Atopic Dermatitis Management" Plants 14, no. 3: 362. https://doi.org/10.3390/plants14030362
APA StyleLee, S.-K., Keng, J.-W., Yon, J.-A.-L., Mai, C.-W., Lim, H.-C., Chow, S.-C., Akowuah, G. A., Liew, K. B., Lee, S.-K., Marriott, P. J., & Chew, Y.-L. (2025). Phytochemical Analysis and Biological Activities of Flavonoids and Anthraquinones from Cassia alata (Linnaeus) Roxburgh and Their Implications for Atopic Dermatitis Management. Plants, 14(3), 362. https://doi.org/10.3390/plants14030362






