Foliar Application of Equisetum arvense Extract Enhances Growth, Alleviates Lipid Peroxidation and Reduces Proline Accumulation in Tomato Plants Under Salt Stress
Abstract
1. Introduction
2. Results
2.1. Optimization of Equisetum arvense Extraction Method
2.2. Effect of Equisetum arvense Extract on Tomato Plants Growth, Mineral Analysis, Photosynthetic Pigments and Residual Transpiration Rates
2.3. Effect of Equisetum arvense Extract on Oxidative Stress of Tomato Plants Growth
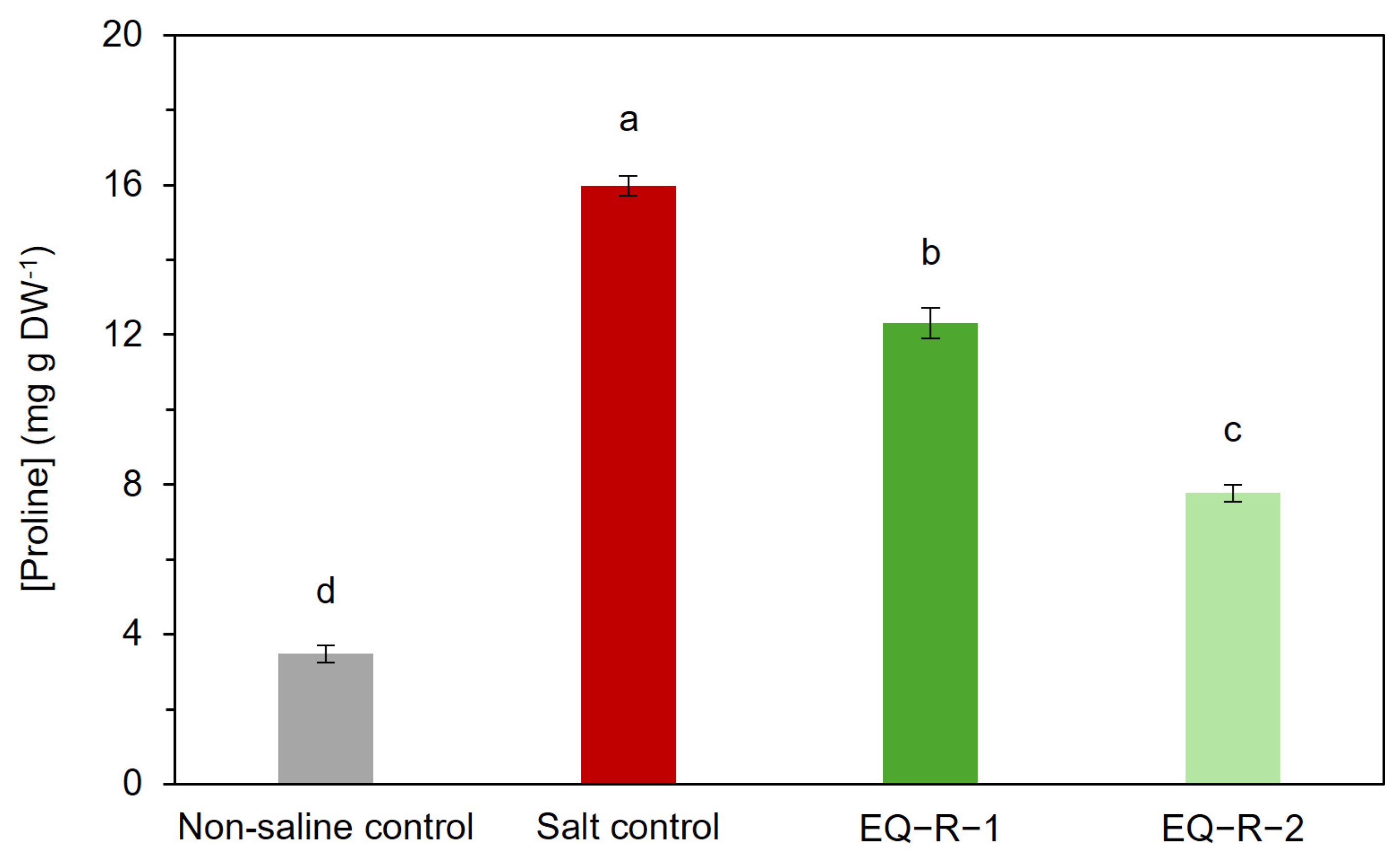
2.4. Effect of Equisetum arvense Extract on Proline Content of Tomato Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Equisetum arvense Extracts and Characterization
4.3. Cultural Conditions and Treatment
4.4. Determination of Plant Growth, Macro- and Micronutrient Concentrations, Photosynthetic Pigment Content and Transpiration Intensity
4.5. Malondialdehyde Concentration, H2O2 Content and Root Electrolyte Leakage
4.6. Proline Osmolyte Concentration
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ibi, A.; Du, M.; Beuerle, T.; Melchert, D.; Solnier, J.; Chang, C. A Multi-pronged technique for identifying Equisetum palustre and Equisetum arvense—Combining HPTLC, HPLC-ESI-MS/MS and optimized DNA barcoding techniques. Plants 2022, 11, 2562. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Yu, H.-S.; Ra, M.-J.; Jung, S.-M.; Yu, J.-N.; Kim, J.-C.; Kim, K.H. Phytochemical investigation of equisetum arvense and evaluation of their anti-inflammatory potential in TNFα/INFγ-Stimulated keratinocytes. Pharmaceuticals 2023, 16, 1478. [Google Scholar] [CrossRef]
- Peyghambarzadeh, S.; Babaeinejad, T.; Hadian, J.; Fallah, A.; Ghanavati, N. Growth and Phytochemical Properties of Horsetail Plant Affected by Organic and Mineral Fertilization. Silicon 2023, 15, 4751–4759. [Google Scholar] [CrossRef]
- Pratt, M.K.; Westbrook, A.S.; DiTommaso, A. Reproductive strategy, management, and medicinal uses of field horsetail (Equisetum arvense). Weed Technol. 2024, 38, e47. [Google Scholar] [CrossRef]
- Al-Snafi, P.D.A.E. The pharmacology of Equisetum arvense—A review. IOSR J. Pharm. 2017, 7, 31–42. [Google Scholar] [CrossRef]
- Nosrati Gazafroudi, K.; Mailänder, L.K.; Daniels, R.; Kammerer, D.R.; Stintzing, F.C. From Stem to spectrum: Phytochemical characterization of five Equisetum species and evaluation of their antioxidant potential. Molecules 2024, 29, 2821. [Google Scholar] [CrossRef]
- Patova, O.A.; Smirnov, V.V.; Golovchenko, V.V.; Vityazev, F.V.; Shashkov, A.S.; Popov, S.V. Structural, rheological and antioxidant properties of pectins from Equisetum arvense L. and Equisetum sylvaticum L. Carbohydr. Polym. 2019, 209, 239–249. [Google Scholar] [CrossRef]
- Alberti, A.; Zielinski, A.A.F.; Zardo, D.M.; Demiate, I.M.; Nogueira, A.; Mafra, L.I. Optimisation of the extraction of phenolic compounds from apples using response surface methodology. Food Chem. 2014, 149, 151–158. [Google Scholar] [CrossRef]
- Eghlima, G.; Esmaeili, H.; Frzaneh, M.; Mirjalili, M.H. Multivariate analysis of Equisetum arvense L. ecotypes based on silicon content, phytochemical and morphological characterization. Silicon 2024, 16, 115–122. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, Y.; Hu, T.; Li, W.; Deng, Y.; Wang, X.; Tang, H.; Zhao, L.; Yan, G. Optimization of cellulase-assisted extraction of total flavonoids from Equisetum via response surface methodology based on antioxidant activity. Processes 2023, 11, 1978. [Google Scholar] [CrossRef]
- Pallag, A.; Filip, G.A.; Olteanu, D.; Clichici, S.; Baldea, I.; Jurca, T.; Micle, O.; Vicaş, L.; Marian, E.; Soriţău, O.; et al. Equisetum arvense L. Extract induces antibacterial activity and modulates oxidative stress, inflammation, and apoptosis in endothelial vascular cells exposed to hyperosmotic stress. Oxidative Med. Cell. Loongev. 2018, 2018, 3060525. [Google Scholar] [CrossRef] [PubMed]
- Saeed Abadi, B.; Eghlima, G.; Mirjalili, M.H.; Ghorbanpour, M. Optimizing the ultrasound-assisted phenolic extraction from Equisetum arvense L. and its antioxidant activity using response surface methodology. Biomass Convers. Biorefinery 2024, 15, 2713–2726. [Google Scholar] [CrossRef]
- Saeed-Abadi, B.; Eghlima, G.; Mirjalili, M.H.; Hadian, J.; Ghorbanpour, M. Effect of extraction solvent on silicon, isoquercitroside content, and antioxidant activity of common horsetail (Equisetum arvense L.) extract. Biomass Convers. Biorefinery 2024. [Google Scholar] [CrossRef]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef] [PubMed]
- Vivancos, J.; Deshmukh, R.; Grégoire, C.; Rémus-Borel, W.; Belzile, F.; Bélanger, R.R. Identification and characterization of silicon efflux transporters in horsetail (Equisetum arvense). J. Plant Physiol. 2016, 200, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Eghlima, G.; Chegini, K.G.; Farzaneh, M.; Aghamir, F. Effect of common horsetail extract on growth characteristics, essential oil yield and chemical compositions of basil (Ocimum basilicum L.). Sci. Rep. 2024, 14, 11082. [Google Scholar] [CrossRef] [PubMed]
- Greger, M.; Landberg, T. Equisetum arvense as a silica fertilizer. Plant Physiol. Biochem. 2024, 210, 108606. [Google Scholar] [CrossRef]
- Jang, S.; Sadiq, N.B.; Hamayun, M.; Jung, J.; Lee, T.; Yang, J.-S.; Lee, B.; Kim, H.-Y. Silicon foliage spraying improves growth characteristics, morphological traits, and root quality of Panax ginseng C.A.Mey. Ind. Crops Prod. 2020, 156, 112848. [Google Scholar] [CrossRef]
- Liang, Y.; Sun, W.; Zhu, Y.-G.; Christie, P. Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environ. Pollut. 2007, 147, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Mitani, N.; Yamaji, N.; Ma, J.F. Identification of maize silicon influx transporters. Plant Cell Physiol. 2009, 50, 5–12. [Google Scholar] [CrossRef]
- Alhousari, F.; Greger, M. Silicon and mechanisms of plant resistance to insect pests. Plants 2018, 7, 33. [Google Scholar] [CrossRef]
- Ferrández-Gómez, B.; Jordá, J.D.; Cerdán, M.; Sánchez-Sánchez, A. Enhancing salt stress tolerance in tomato (Solanum lycopersicum L.) through silicon application in roots. Plants 2024, 13, 1415. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, R.; Mur, L.A.J.; Ruan, J.; Shen, Q.; Guo, S. Functions of silicon in plant drought stress responses. Hortic. Res. 2021, 8, 254. [Google Scholar] [CrossRef]
- Korndörfer, G.H.; Lepsch, I. Chapter 7 Effect of silicon on plant growth and crop yield. In Studies in Plant Science; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 133–147. ISBN 978-0-444-50262-9. [Google Scholar]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon influences soil availability and accumulation of mineral nutrients in various plant species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Guo, Z.; Di, S.; Lu, Y.; Rehmani Muhammad, I.A.; Rong, C.; Ding, Y.; Li, W.; Ding, C. OsMFT1 inhibits seed germination by modulating abscisic acid signaling and gibberellin biosynthesis under salt stress in rice. Plant Cell Physiol. 2023, 64, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, M.; Motesharezadeh, B.; Mousavi, S.M.; Ma, Q.; Ahmadabadi, Z. Silicon (Si): A regulator nutrient for optimum growth of wheat under salinity and drought stresses- A review. J. Plant Growth Regul. 2023, 42, 5354–5378. [Google Scholar] [CrossRef]
- Zahra, N.; Al Hinai, M.S.; Hafeez, M.B.; Rehman, A.; Wahid, A.; Siddique, K.H.M.; Farooq, M. Regulation of photosynthesis under salt stress and associated tolerance mechanisms. Plant Physiol. Biochem. 2022, 178, 55–69. [Google Scholar] [CrossRef]
- Rady, M.M.; Salama, M.M.M.; Kuşvuran, S.; Kuşvuran, A.; Ahmed, A.F.; Ali, E.F.; Farouk, H.A.; Osman, A.S.; Selim, K.A.; Mahmoud, A.E.M. Exploring the role of novel biostimulators in suppressing oxidative stress and reinforcing the antioxidant defense systems in cucurbita pepo plants exposed to cadmium and lead toxicity. Agronomy 2023, 13, 1916. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Garcia, D.; Garcia-Cela, E.; Ramos, A.J.; Sanchis, V.; Marín, S. Mould growth and mycotoxin production as affected by Equisetum arvense and Stevia rebaudiana extracts. Food Control 2011, 22, 1378–1384. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Farghaly, F.A.; Salam, H.K.; Hamada, A.M.; Radi, A.A. The Role of Benzoic Acid, Gallic Acid and Salicylic Acid in Protecting Tomato Callus Cells from Excessive Boron Stress. Sci. Hortic. 2021, 278, 109867. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Garrido, I.; Casimiro, I.D.J.; Casero, P.J.; Espinosa, F.; García-Romera, I.; Aranda, E. Defence response of tomato seedlings to oxidative stress induced by phenolic compounds from dry olive mill residue. Chemosphere 2012, 89, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Kumar, V.; Burritt, D.J.; Fujita, M.; Mäkelä, P.S.A. Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-27422-1. [Google Scholar]
- Liang, Y.; Nikolic, M.; Bélanger, R.; Gong, H.; Song, A. Silicon in Agriculture: From Theory to Practice; Springer Netherlands: Dordrecht, The Netherlands, 2015; ISBN 978-94-017-9977-5. [Google Scholar]
- Luan, S.; Lan, W.; Chul Lee, S. Potassium nutrition, sodium toxicity, and calcium signaling: Connections through the CBL–CIPK network. Curr. Opin. Plant Biol. 2009, 12, 339–346. [Google Scholar] [CrossRef]
- Yavuz, D.; Rashid, B.A.R.; Seymen, M. The Influence of NaCl Salinity on evapotranspiration, yield traits, antioxidant status, and mineral composition of lettuce grown under deficit irrigation. Sci. Hortic. 2023, 310, 111776. [Google Scholar] [CrossRef]
- Haghighi, M.; Pessarakli, M. Influence of silicon and nano-silicon on salinity tolerance of cherry tomatoes (Solanum lycopersicum L.) at early growth stage. Sci. Hortic. 2013, 161, 111–117. [Google Scholar] [CrossRef]
- Sivanesan, I.; Jeong, B.R. Silicon promotes adventitious shoot regeneration and enhances salinity tolerance of Ajuga multiflora bunge by altering activity of antioxidant enzyme. Sci. World J. 2014, 2014, 521703. [Google Scholar] [CrossRef]
- Muneer, S.; Park, Y.; Manivannan, A.; Soundararajan, P.; Jeong, B. Physiological and proteomic analysis in chloroplasts of Solanum lycopersicum L. under silicon efficiency and salinity stress. Int. J. Mol. Sci. 2014, 15, 21803–21824. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209–212. [Google Scholar] [CrossRef]
- Rady, M.M.; Elrys, A.S.; Abo El-Maati, M.F.; Desoky, E.-S.M. Interplaying roles of silicon and proline effectively improve salt and cadmium stress tolerance in Phaseolus vulgaris Plant. Plant Physiol. Biochem. 2019, 139, 558–568. [Google Scholar] [CrossRef]
- Khodary, S.E.A. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int. J. Agri. Biol. 2004, 6, 5–8. [Google Scholar]
- Khan, N.; Syeed, S.; Masood, A.; Nazar, R.; Iqbal, N. Application of salicylic acid increases contents of nutrients and antioxidative metabolism in mungbean and alleviates adverse effects of salinity stress. Int. J. Plant Biol. 2010, 1, e1. [Google Scholar] [CrossRef]
- Soylemezoglu, G.; Demir, K.; Inal, A.; Gunes, A. Effect of silicon on antioxidant and stomatal response of two grapevine (Vitis vinifera L.) rootstocks grown in boron toxic, saline and boron toxic-saline soil. Sci. Hortic. 2009, 123, 240–246. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Q.; Liu, Q.; Zhang, W.; Ding, R. Exogenous silicon (Si) increases antioxidant enzyme activity and reduces lipid peroxidation in roots of salt-stressed barley (Hordeum vulgare L.). J. Plant Physiol. 2003, 160, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Manquián-Cerda, K.; Cruces, E.; Escudey, M.; Zúñiga, G.; Calderón, R. Interactive effects of aluminum and cadmium on phenolic compounds, antioxidant enzyme activity and oxidative stress in blueberry (Vaccinium corymbosum L.) plantlets cultivated in vitro. Ecotoxicol. Environ. Saf. 2018, 150, 320–326. [Google Scholar] [CrossRef]
- Antonić, D.; Milošević, S.; Cingel, A.; Lojić, M.; Trifunović-Momčilov, M.; Petrić, M.; Subotić, A.; Simonović, A. Effects of exogenous salicylic acid on Impatiens walleriana L. grown in vitro under polyethylene glycol-imposed drought. S. Afr. J. Bot. 2016, 105, 226–233. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Vishwakarma, K.; Singh, V.P.; Prakash, V.; Sharma, S.; Muneer, S.; Nikolic, M.; Deshmukh, R.; Vaculík, M.; Corpas, F.J. Silicon Crosstalk with Reactive Oxygen Species, Phytohormones and Other Signaling Molecules. J. Hazard. Mat. 2021, 408, 124820. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kumar, V.; Sharma, J.; Saini, S.; Sharma, P.; Kumar, S.; Sinhmar, Y.; Kumar, D.; Sharma, A. Silicon supplementation alleviates the salinity stress in wheat plants by enhancing the plant water status, photosynthetic pigments, proline content and antioxidant enzyme activities. Plants 2022, 11, 2525. [Google Scholar] [CrossRef]
- Blokhina, O. Antioxidants, oxidative damage and oxygen deprivation stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Konuk, H.B.; Ergüden, B. Phenolic –OH group is crucial for the antifungal activity of terpenoids via disruption of cell membrane integrity. Folia Microbiol. 2020, 65, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2005, 167, 645–663. [Google Scholar] [CrossRef]
- Ferrández-Gómez, B.; Jordá, J.D.; Cerdán, M.; Sánchez, A. Valorization of Posidonia oceanica biomass: Role on germination of cucumber and tomato seeds. Waste Manag. 2023, 171, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Khorasaninejad, S.; Zare, F.; Hemmati, K. Effects of silicon on some phytochemical traits of purple coneflower (Echinacea purpurea L.) under salinity. Sci. Hortic. 2020, 264, 108954. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Cerdán, M.; Sánchez-Sánchez, A.; Jordá, J.D.; Juárez, M.; Sánchez-Andreu, J. Effect of commercial amino acids on iron nutrition of tomato plants grown under lime-induced iron deficiency. J. Plant Nutr. Soil Sci. 2013, 176, 859–866. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; García-Jiménez, A.; Escobar-Sepúlveda, H.F.; Ramírez-Olvera, S.M.; Bello-Bello, J.J.; Gómez-Merino, F.C. Silicon induces hormetic dose-response effects on growth and concentrations of chlorophylls, amino acids and sugars in pepper plants during the early developmental stage. PeerJ 2020, 8, e9224. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhou, G.; He, Q.; Zhou, L.; Ji, Y.; Lv, X. Capability of leaf water content and its threshold values in reflection of soil–plant water status in maize during prolonged drought. Ecol. Indic. 2021, 124, 107395. [Google Scholar] [CrossRef]
- Abadía, J.; Monge, E.; Montañés, L.; Heras, L. Extraction of iron from plant leaves by Fe (II) chelators. J. Plant Nutr. 1984, 7, 777–784. [Google Scholar] [CrossRef]
- Pallas, J.R., Jr.; Michel, B.E.; Harris, D.G. Photosynthesis, transpiration, leaf temperature, and stomatal activity of cotton plants under varying water potentials. Plant Physiol. 1967, 42, 76–88. [Google Scholar] [CrossRef]
- Abd El Rahman, A.A.; Bierhuizen, J.F.; Kuiper, P.J.C. Growth and transpiration of tomato in relation to night temperature under controlled conditions. Meded. Landbouwhogeschool Wageningen 1959, 59, 1–6. [Google Scholar]
- Shu, X.; Yin, L.; Zhang, Q.; Wang, W. Effect of Pb toxicity on leaf growth, antioxidant enzyme activities, and photosynthesis in cuttings and seedlings of Jatropha curcas L. Environ. Sci. Pollut. Res. 2012, 19, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms: Effect of heavy metals. Aquat. Bot. 1981, 11, 67–77. [Google Scholar] [CrossRef]
- Magné, C.; Larher, F. High sugar content of extracts interferes with colorimetric determination of amino acid and free proline. Anal. Biochem. 1992, 200, 115–118. [Google Scholar] [CrossRef]

| Parameter | EQ-M | EQ-R |
|---|---|---|
| Nutrient (mg·L−1) | ||
| Na | 29 ± 3 | 35 ± 3 |
| K | 860 ± 78 | 931 ± 47 |
| Ca | 192 ± 16 | 170 ± 4 |
| Mg | 95 ± 7 | 91 ± 2 |
| Fe | 0.069 ± 0.004 | 0.15 ± 0.02 |
| Cu | 0.062 ± 0.002 | 0.19 ± 0.03 |
| Zn | 0.22 ± 0.03 | 0.29 ± 0.03 |
| Mn | 0.40 ± 0.04 | 0.36 ± 0.02 |
| Si (mg·L−1) | 5.9 ± 0.6 | 118 ± 22 |
| Phenolic compounds (mM) | 1.1 ± 0.3 | 2.6 ± 0.2 |
| Parameter | Non-Saline Control | Salt Control | EQ-R-1 | EQ-R-2 | Sig. 1 |
|---|---|---|---|---|---|
| AFW (g) | 23.3 ± 0.3a | 12.9 ± 0.3d | 16.9 ± 0.4c | 21.3 ± 0.4b | *** |
| ADW (g) | 2.40 ± 0.05a | 1.38 ± 0.17c | 1.79 ± 0.14b | 2.00 ± 0.16b | *** |
| RFW (g) | 7.3 ± 0.2a | 3.81 ± 0.09c | 3.95 ± 0.11bc | 4.3 ± 0.3b | *** |
| RDW (g) | 0.51 ± 0.04a | 0.25 ± 0.03b | 0.28 ± 0.03b | 0.32 ± 0.07b | *** |
| LWC (%) | 87.0 ± 0.2b | 83.5 ± 0.2d | 84.4 ± 0.3c | 96.5 ± 0.3a | ** |
| Ca (%) | 2.69 ± 0.03a | 1.82 ± 0.09b | 1.68 ± 0.10b | 1.76 ± 0.11b | *** |
| K (%) | 3.82 ± 0.08a | 2.36 ± 0.11c | 1.93 ± 0.07d | 2.61 ± 0.06b | *** |
| Mg (%) | 0.480 ± 0.019a | 0.345 ± 0.005b | 0.33 ± 0.02b | 0.37 ± 0.04b | *** |
| Na (%) | 0.33 ± 0.04c | 1.79 ± 0.09a | 1.53 ± 0.05b | 1.48 ± 0.09b | *** |
| Fe (mg·kg−1) | 122 ± 4a | 110 ± 7b | 111 ± 5b | 120 ± 4ab | * |
| Cu (mg·kg−1) | 18 ± 3 | 16 ± 4 | 13 ± 2 | 18 ± 2 | ns |
| Mn (mg·kg−1) | 116 ± 2a | 105 ± 3b | 88 ± 4c | 102 ± 3b | *** |
| Zn (mg·kg−1) | 65 ± 3a | 68 ± 4ab | 55 ± 3c | 56 ± 8bc | * |
| Si (mg·g−1) | 664 ± 33d | 995 ± 29c | 1276 ± 58a | 1115 ± 55b | *** |
| Chl total (mg·g AFW−1) | 1.5 ± 0.2a | 1.00 ± 0.15b | 1.4 ± 0.2ab | 1.5 ± 0.3a | * |
| Carotenoids (mg·gAFW−1) | 0.35 ± 0.06ab | 0.28 ± 0.03b | 0.35 ± 0.06ab | 0.42 ± 0.4a | * |
| TI (g·dm2·h−1) | 1.67 ± 0.12a | 0.55 ± 0.15c | 1.42 ± 0.17a | 1.04 ± 0.03b | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukhari, M.; Asencio-Vicedo, R.; Cerdán, M.; Sánchez-Sánchez, A.; Jordá, J.D.; Ferrández-Gómez, B. Foliar Application of Equisetum arvense Extract Enhances Growth, Alleviates Lipid Peroxidation and Reduces Proline Accumulation in Tomato Plants Under Salt Stress. Plants 2025, 14, 488. https://doi.org/10.3390/plants14030488
Boukhari M, Asencio-Vicedo R, Cerdán M, Sánchez-Sánchez A, Jordá JD, Ferrández-Gómez B. Foliar Application of Equisetum arvense Extract Enhances Growth, Alleviates Lipid Peroxidation and Reduces Proline Accumulation in Tomato Plants Under Salt Stress. Plants. 2025; 14(3):488. https://doi.org/10.3390/plants14030488
Chicago/Turabian StyleBoukhari, Messaouda, Rocío Asencio-Vicedo, Mar Cerdán, Antonio Sánchez-Sánchez, Juana D. Jordá, and Borja Ferrández-Gómez. 2025. "Foliar Application of Equisetum arvense Extract Enhances Growth, Alleviates Lipid Peroxidation and Reduces Proline Accumulation in Tomato Plants Under Salt Stress" Plants 14, no. 3: 488. https://doi.org/10.3390/plants14030488
APA StyleBoukhari, M., Asencio-Vicedo, R., Cerdán, M., Sánchez-Sánchez, A., Jordá, J. D., & Ferrández-Gómez, B. (2025). Foliar Application of Equisetum arvense Extract Enhances Growth, Alleviates Lipid Peroxidation and Reduces Proline Accumulation in Tomato Plants Under Salt Stress. Plants, 14(3), 488. https://doi.org/10.3390/plants14030488







