Unveiling Drought Tolerant Cotton Genotypes: Insights from Morpho-Physiological and Biochemical Markers at Flowering
Abstract
:1. Introduction
2. Results
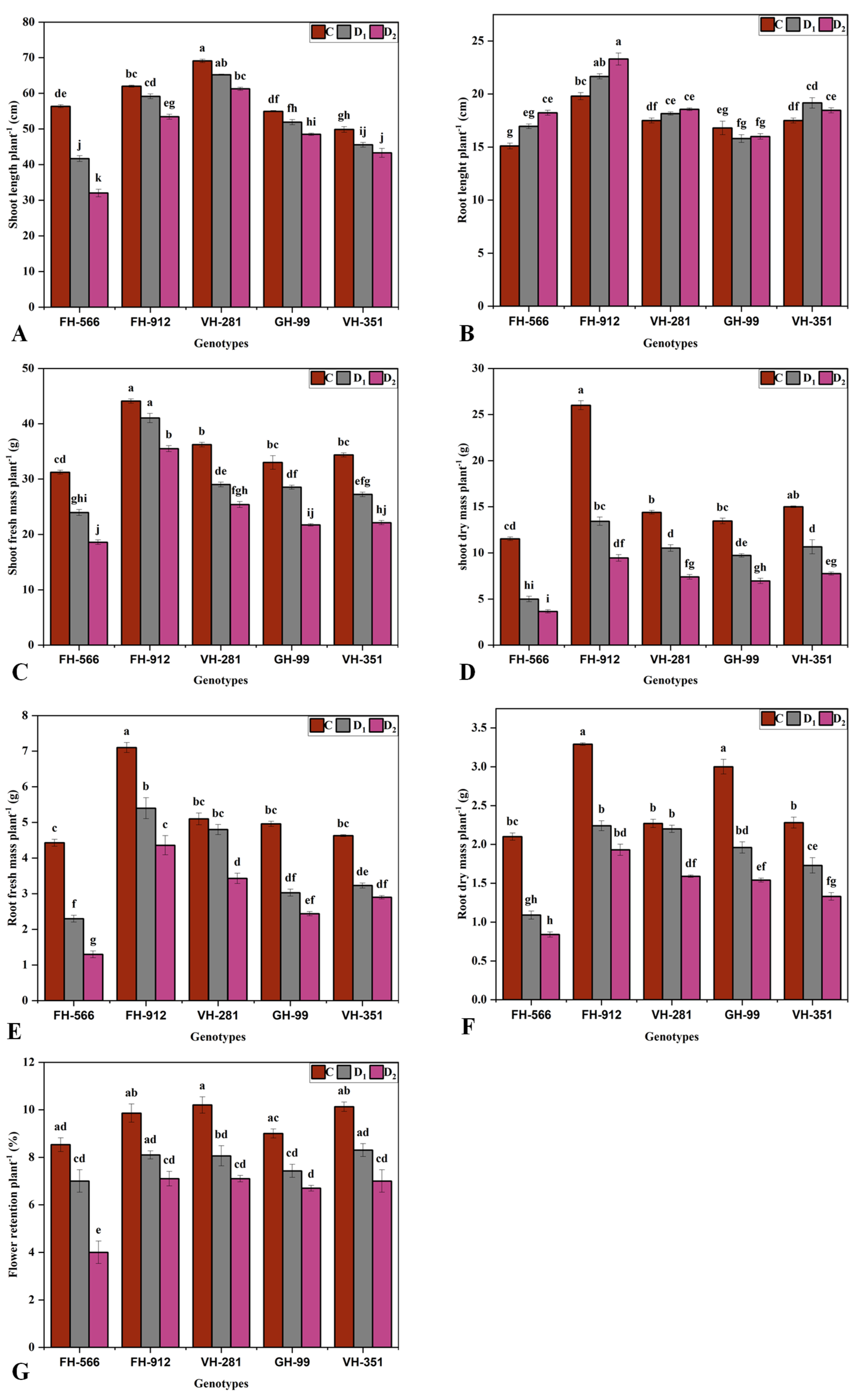
2.1. Growth Parameters
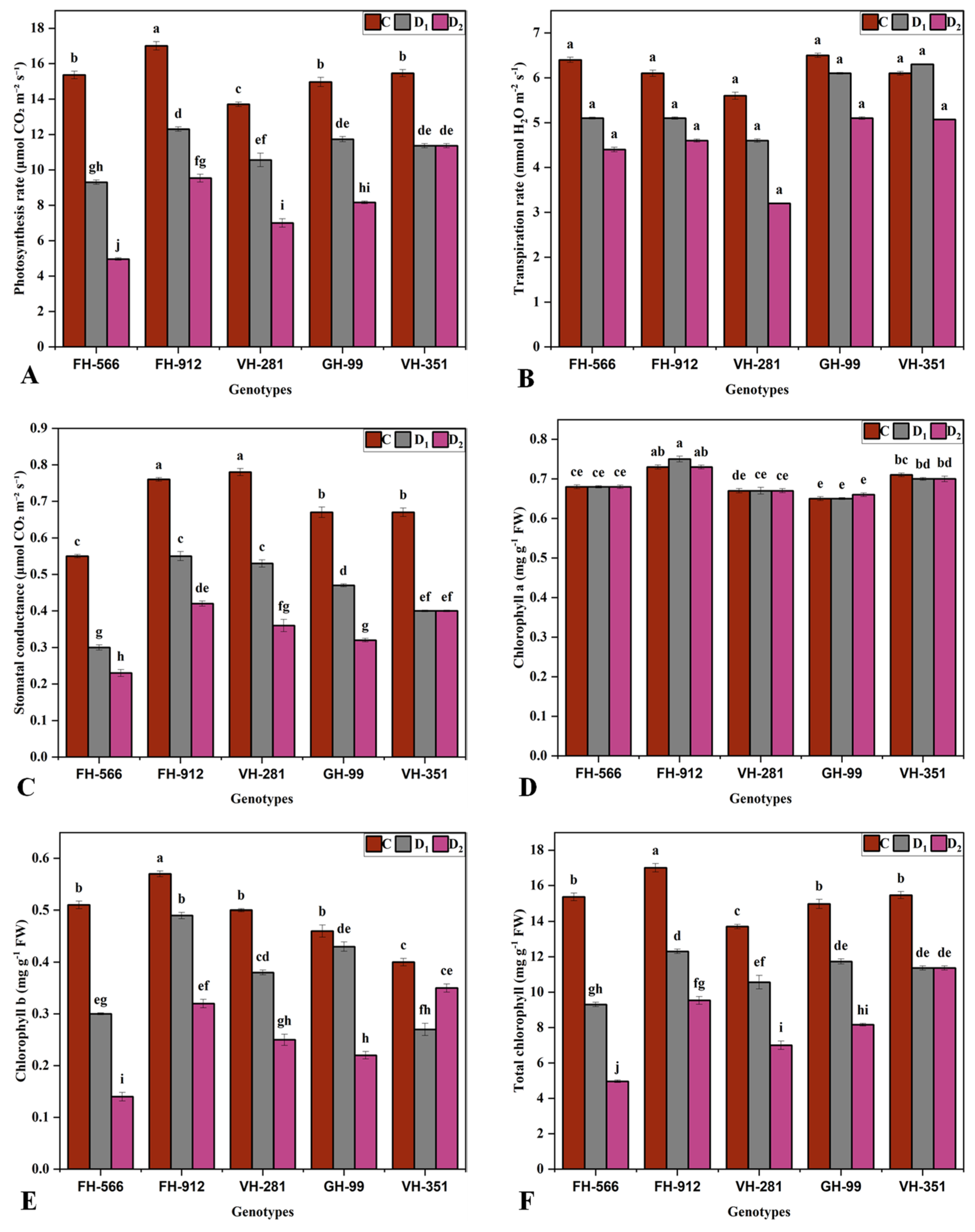
2.2. Physio-Biochemical Parameters
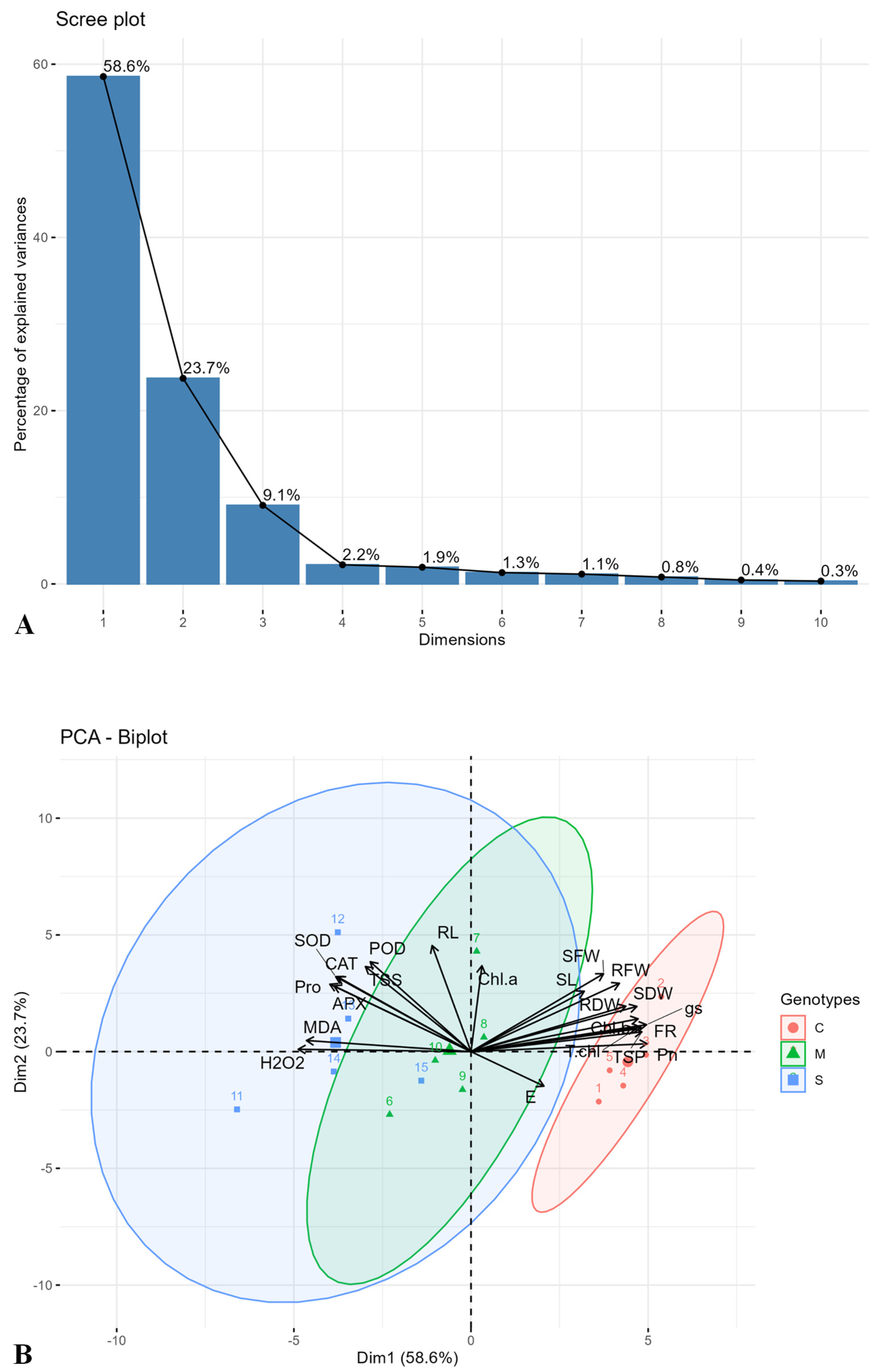
2.3. Principal Component and Heatmap Analysis
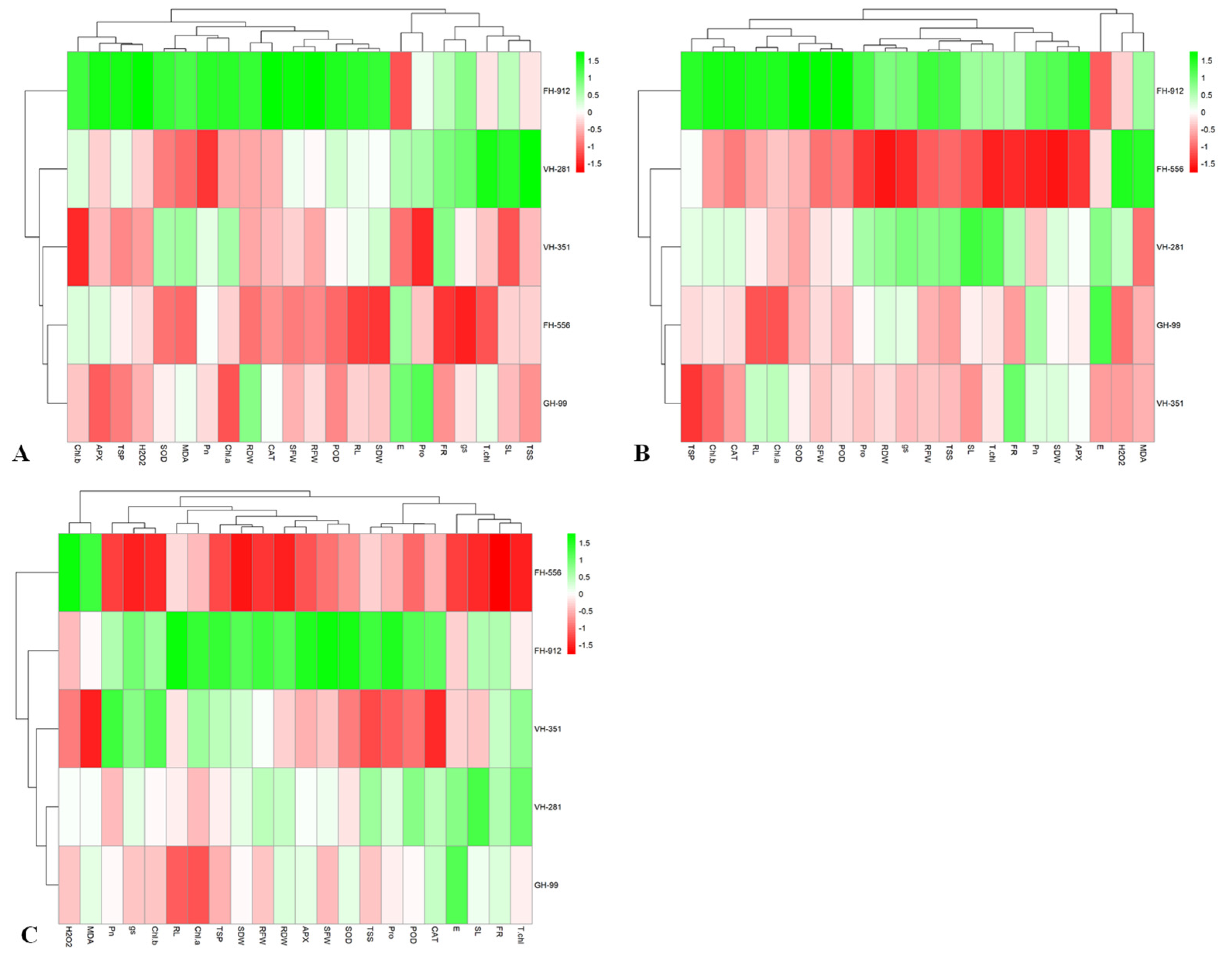
2.4. Heatmap Analysis
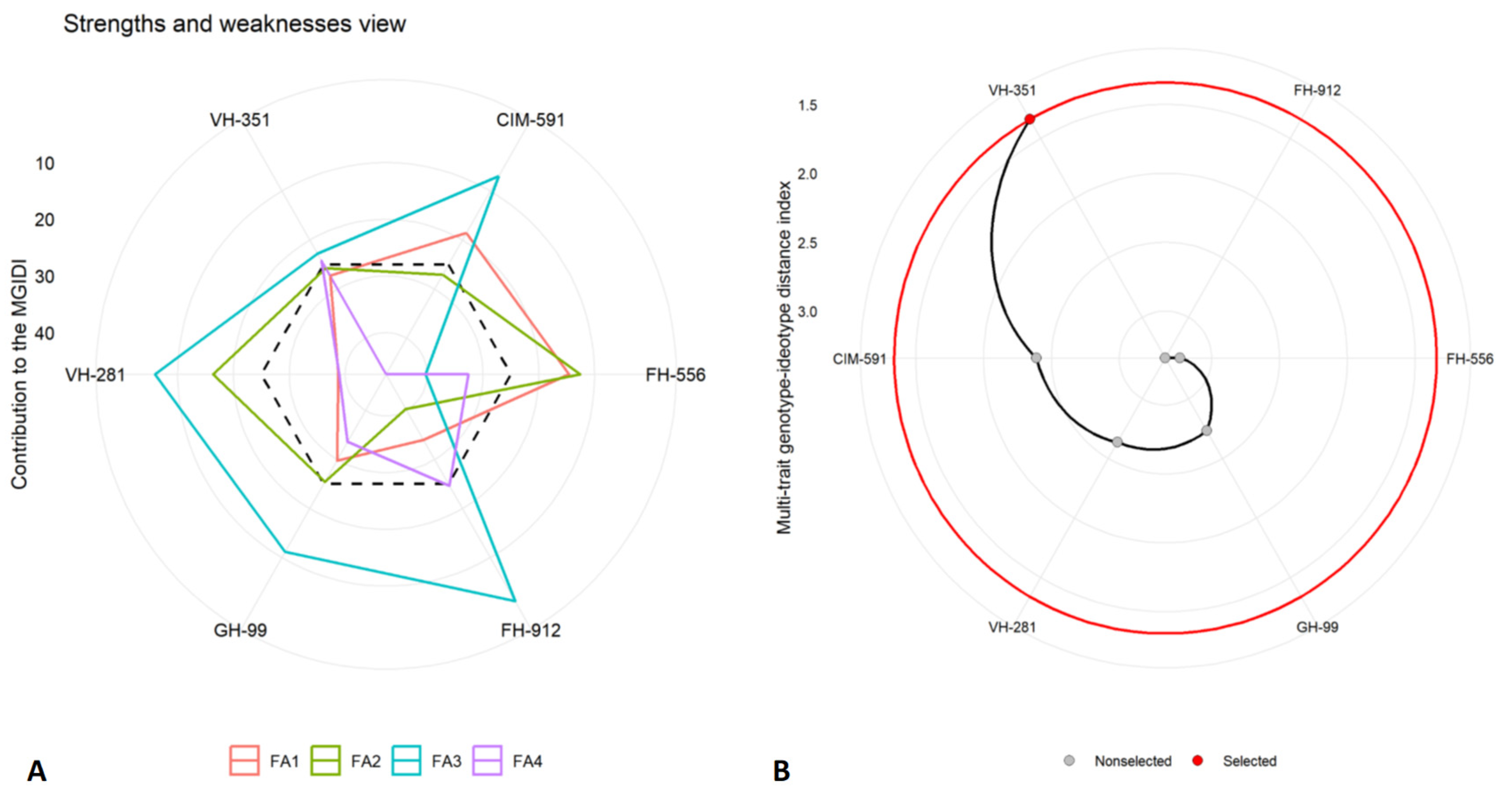
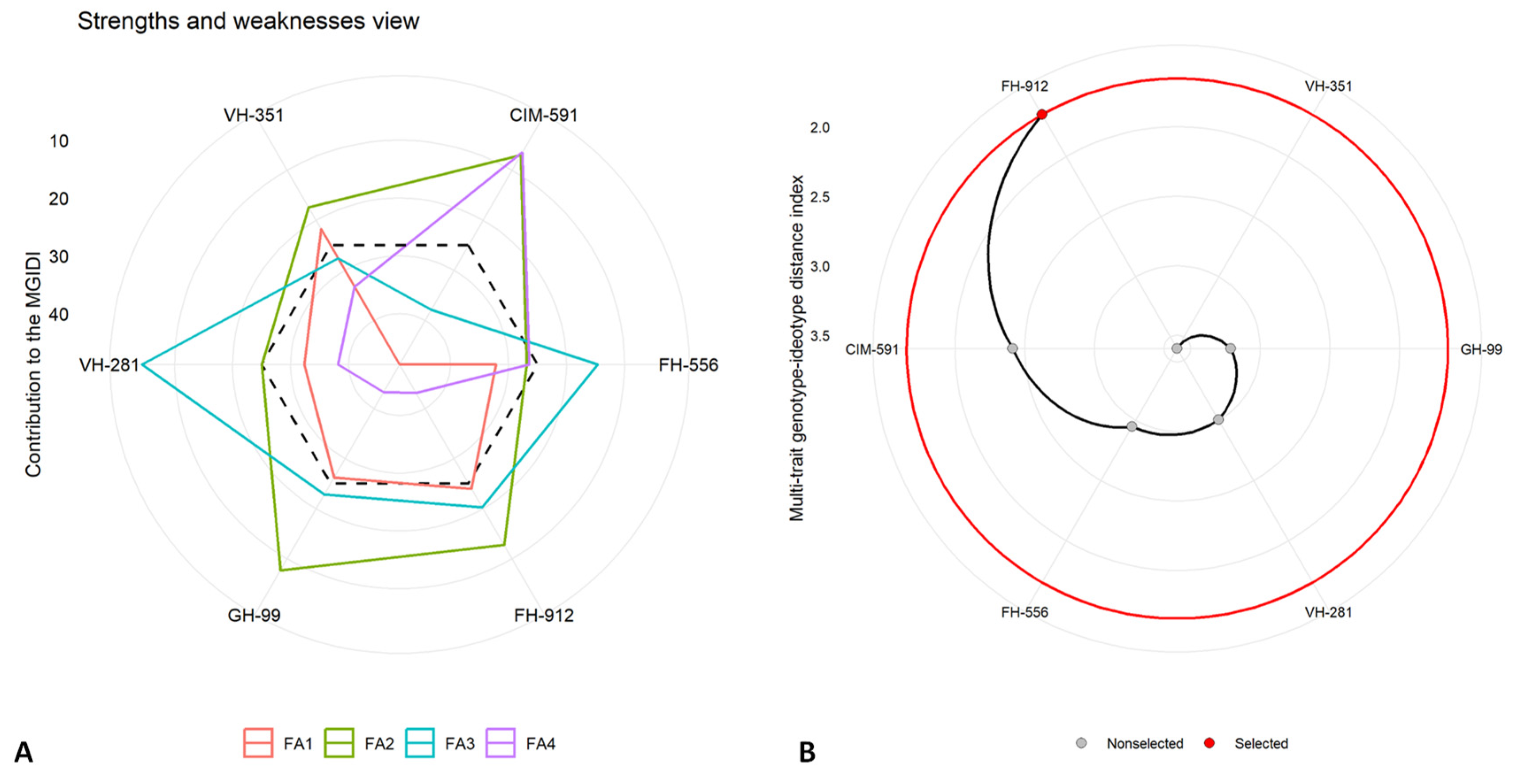
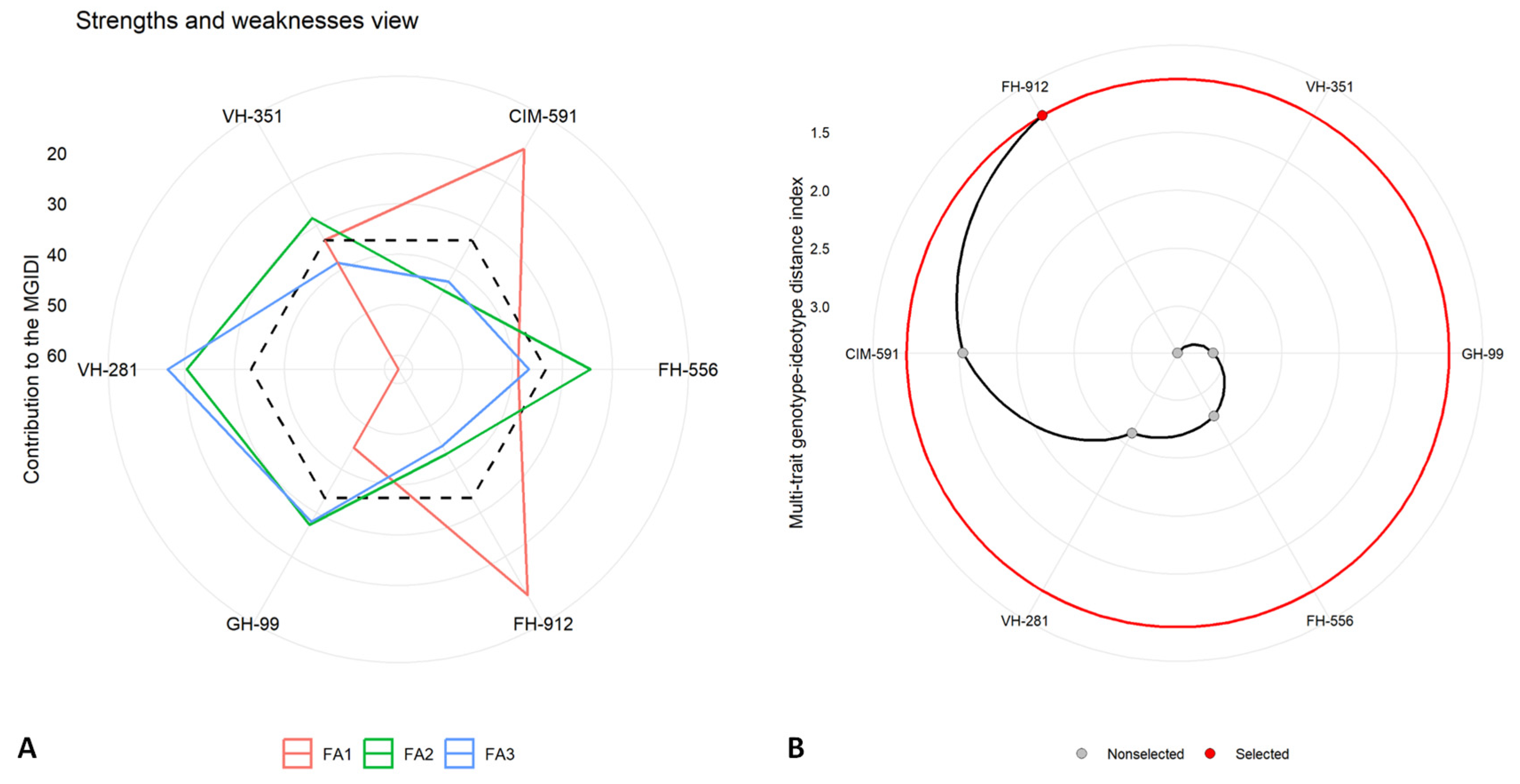
2.5. Multi-Trait Genotype–Idiotype Distance Index (MGIDI)
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Selection of Drought-Tolerant Cotton Genotypes
5.2. Experimental Setup
5.3. Morphological Parameters
5.4. Physiological Parameters
5.5. Biochemical Analysis
5.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, D.P.; Mattoo, A.K. Sustainable agriculture—Enhancing environmental benefits, food nutritional quality and building crop resilience to abiotic and biotic stresses. Agriculture 2018, 8, 8. [Google Scholar] [CrossRef]
- Khilchevskyi, V.; Karamushka, V. Global water resources: Distribution and demand. In Clean Water and Sanitation; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–11. [Google Scholar]
- Ahluwalia, O.; Singh, P.C.; Bhatia, R. A review on drought stress in plants: Implications, mitigation and the role of plant growth promoting rhizobacteria. Resour. Environ. Sustain. 2021, 5, 100032. [Google Scholar] [CrossRef]
- Ashraf, S.; Ch, K.M.; Ashraf, I.; Akbar, N. A phenomenological inquiry into farmers’ experiences growing cotton in Punjab, Pakistan. Sci. Rep. 2024, 14, 13846. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmad, G. Water supply and demand: National and regional trends. In Water Policy in Pakistan: Issues and Options; Springer: Berlin/Heidelberg, Germany, 2023; pp. 91–121. [Google Scholar]
- Aslam, S.; Khan, S.H.; Ahmed, A.; Dandekar, A.M. The tale of cotton plant: From wild type to domestication, leading to its improvement by genetic transformation. Am. J. Mol. Biol. 2020, 10, 91–127. [Google Scholar] [CrossRef]
- Shuli, F.; Jarwar, A.H.; Wang, X.; Wang, L.; Ma, Q. Overview of the cotton in Pakistan and its future prospects. Pak. J. Agric. Res. 2018, 31, 396. [Google Scholar] [CrossRef]
- Muhammad, A.; Smith, S.A.; Yu, T.H.E. COVID-19 and cotton import demand in China. Agribusiness 2021, 37, 3–24. [Google Scholar] [CrossRef]
- Ali, M.A.; Farooq, J.; Batool, A.; Zahoor, A.; Azeem, F.; Mahmood, A.; Jabran, K. Cotton production in Pakistan. In Cotton production; Wiley Online Library: Hoboken, NJ, USA, 2019; pp. 249–276. [Google Scholar]
- Farooq, M.A.; Chattha, W.S.; Shafique, M.S.; Karamat, U.; Tabusam, J.; Zulfiqar, S.; Shakeel, A. Transgenerational impact of climatic changes on cotton production. Front. Plant Sci. 2023, 14, 987514. [Google Scholar] [CrossRef]
- Zahid, Z.; Khan, M.K.R.; Hameed, A.; Akhtar, M.; Ditta, A.; Hassan, H.M.; Farid, G. Dissection of drought tolerance in upland cotton through morpho-physiological and biochemical traits at seedling stage. Front. Plant Sci. 2021, 12, 627107. [Google Scholar] [CrossRef] [PubMed]
- Viot, C.R.; Wendel, J.F. Evolution of the cotton genus, Gossypium, and its domestication in the Americas. Crit. Rev. Plant Sci. 2023, 42, 1–33. [Google Scholar] [CrossRef]
- Rashid, M.E.; Islam, M.A.; Kanon, T.A.; Khan, M.R.; Uddin, M.B.; Haque, R.U.; Tonmoy, M.W.; Hasan, M.R.; Haque, M.M. Valorization of dyed brush fiber waste through production of upcycled mélange yarn: A sustainable approach. J. Clean. Prod. 2024, 447, 141464. [Google Scholar] [CrossRef]
- Conaty, W.C.; Broughton, K.J.; Egan, L.M.; Li, X.; Li, Z.; Liu, S.; Llewellyn, D.J.; MacMillan, C.P.; Moncuquet, P.; Rolland, V. Cotton breeding in Australia: Meeting the challenges of the 21st century. Front. Plant Sci. 2022, 13, 904131. [Google Scholar] [CrossRef]
- Arshad, A.; Raza, M.A.; Zhang, Y.; Zhang, L.; Wang, X.; Ahmed, M.; Habib-ur-Rehman, M. Impact of climate warming on cotton growth and yields in China and Pakistan: A regional perspective. Agriculture 2021, 11, 97. [Google Scholar] [CrossRef]
- Mubarik, M.S.; Ma, C.; Majeed, S.; Du, X.; Azhar, M.T. Revamping of cotton breeding programs for efficient use of genetic resources under changing climate. Agronomy 2020, 10, 1190. [Google Scholar] [CrossRef]
- Sinha, R.; Fritschi, F.B.; Zandalinas, S.I.; Mittler, R. The impact of stress combination on reproductive processes in crops. Plant Sci. 2021, 311, 111007. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef]
- Khadka, K.; Earl, H.J.; Raizada, M.N.; Navabi, A. A physio-morphological trait-based approach for breeding drought tolerant wheat. Front. Plant Sci. 2020, 11, 715. [Google Scholar] [CrossRef] [PubMed]
- Shelke, D.B.; Pandey, M.; Nikalje, G.C.; Zaware, B.N.; Suprasanna, P.; Nikam, T.D. Salt responsive physiological, photosynthetic and biochemical attributes at early seedling stage for screening soybean genotypes. Plant Physiol. Biochem. 2017, 118, 519–528. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.; Ansari, M.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Bhattacharya, A. Effect of soil water deficit on growth and development of plants: A review. In Soil Water Deficit and Physiological Issues in Plants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 393–488. [Google Scholar]
- Hasanuzzaman, M.; Raihan, M.R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Zulfiqar, B.; Raza, M.A.S.; Saleem, M.F.; Ali, B.; Aslam, M.U.; Al-Ghamdi, A.A.; Elshikh, M.S.; Hassan, M.U.; Toleikienė, M.; Ahmed, J. Abscisic acid improves drought resilience, growth, physio-biochemical and quality attributes in wheat (Triticum aestivum L.) at critical growth stages. Sci. Rep. 2024, 14, 20411. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Bheemanahalli, R.; Saha, S.; Singh, K.; Lokhande, S.B.; Gajanayake, B.; Read, J.J.; Jenkins, J.N.; Raska, D.A.; Santiago, L.M.D. High-temperature and drought-resilience traits among interspecific chromosome substitution lines for genetic improvement of upland cotton. Plants 2020, 9, 1747. [Google Scholar] [CrossRef]
- Galeano, E.; Vasconcelos, T.S.; Novais de Oliveira, P.; Carrer, H. Physiological and molecular responses to drought stress in teak (Tectona grandis Lf). PLoS ONE 2019, 14, e0221571. [Google Scholar] [CrossRef] [PubMed]
- Vadez, V.; Grondin, A.; Chenu, K.; Henry, A.; Laplaze, L.; Millet, E.J.; Carminati, A. Crop traits and production under drought. Nat. Rev. Earth Environ. 2024, 5, 211–225. [Google Scholar] [CrossRef]
- Wang, F.; Li, S.; Kong, F.; Lin, X.; Lu, S. Altered regulation of flowering expands growth ranges and maximizes yields in major crops. Front. Plant Sci. 2023, 14, 1094411. [Google Scholar] [CrossRef]
- Kumar, A.; Sheoran, P.; Mann, A.; Yadav, D.; Kumar, A.; Devi, S.; Kumar, N.; Dhansu, P.; Sharma, D.K. Deciphering trait associated morpho-physiological responses in pearlmillet hybrids and inbred lines under salt stress. Front. Plant Sci. 2023, 14, 1121805. [Google Scholar] [CrossRef]
- Meshram, J.H.; Singh, S.B.; Raghavendra, K.P.; Waghmare, V.N. Drought stress tolerance in cotton: Progress and perspectives. In Climate Change and Crop Stress; Elsevier: Amsterdam, The Netherlands, 2022; pp. 135–169. [Google Scholar]
- Croce, R.; Carmo-Silva, E.; Cho, Y.B.; Ermakova, M.; Harbinson, J.; Lawson, T.; McCormick, A.J.; Niyogi, K.K.; Ort, D.R.; Patel-Tupper, D. Perspectives on improving photosynthesis to increase crop yield. Plant Cell 2024, 36, koae132. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.-j.; Luo, M.-Z.; Zhang, S.; Jia, G.-Q.; Sha, T.; Jia, Y.-C.; Hui, Z.H.I.; Diao, X.-M. Variations in chlorophyll content, stomatal conductance, and photosynthesis in Setaria EMS mutants. J. Integr. Agric. 2023, 22, 1618–1630. [Google Scholar] [CrossRef]
- Simkin, A.J.; Kapoor, L.; Doss, C.G.P.; Hofmann, T.A.; Lawson, T.; Ramamoorthy, S. The role of photosynthesis related pigments in light harvesting, photoprotection and enhancement of photosynthetic yield in planta. Photosynth. Res. 2022, 152, 23–42. [Google Scholar] [CrossRef]
- Kaur, H.; Kohli, S.K.; Khanna, K.; Bhardwaj, R. Scrutinizing the impact of water deficit in plants: Transcriptional regulation, signaling, photosynthetic efficacy, and management. Physiol. Plant. 2021, 172, 935–962. [Google Scholar] [CrossRef] [PubMed]
- Al-Ashkar, I.; Alderfasi, A.; Ben Romdhane, W.; Seleiman, M.F.; El-Said, R.A.; Al-Doss, A. Morphological and genetic diversity within salt tolerance detection in eighteen wheat genotypes. Plants 2020, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Miglani, G.S.; Kaur, R.; Sharma, P.; Gupta, N. Leveraging photosynthetic efficiency toward improving crop yields. J. Crop Improv. 2021, 35, 361–402. [Google Scholar] [CrossRef]
- Zhuang, J.; Wang, Y.; Chi, Y.; Zhou, L.; Chen, J.; Zhou, W.; Song, J.; Zhao, N.; Ding, J. Drought stress strengthens the link between chlorophyll fluorescence parameters and photosynthetic traits. PeerJ 2020, 8, e10046. [Google Scholar] [CrossRef]
- Motallebinia, S.; Sofalian, O.; Asghari, A.; Rasoulzadeh, A.; Achachlouei, B.F. Genetic diversity and morpho-physiological assessment of drought tolerance in rapeseed (Brassica napus L.) cultivars. Plant Genet. Resour. 2024, 22, 181–190. [Google Scholar] [CrossRef]
- Iqbal, N.; Rahman, M.M.; Cawthray, G.R.; Zhou, Y.; Denton, M.D.; Sadras, V.O. Drought and Herbivory Differentially Modulate the Leaf Exudation of Organic Acids in Chickpea. J. Soil Sci. Plant Nutr. 2024, 24, 2722–2733. [Google Scholar] [CrossRef]
- Choudhury, S.; Moulick, D.; Ghosh, D.; Soliman, M.; Alkhedaide, A.; Gaber, A.; Hossain, A. Drought-induced oxidative stress in pearl millet (Cenchrus americanus L.) at seedling stage: Survival mechanisms through alteration of morphophysiological and antioxidants activity. Life 2022, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, G.; Dong, H.; Li, C. Waterlogging stress in cotton: Damage, adaptability, alleviation strategies, and mechanisms. Crop J. 2021, 9, 257–270. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-l.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S. Recent developments in enzymatic antioxidant defence mechanism in plants with special reference to abiotic stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Hickey, K.; Wood, M.; Sexton, T.; Sahin, Y.; Nazarov, T.; Fisher, J.; Sanguinet, K.A.; Cousins, A.; Kirchhoff, H.; Smertenko, A. Drought tolerance strategies and autophagy in resilient wheat genotypes. Cells 2022, 11, 1765. [Google Scholar] [CrossRef]
- Omar, A.A.; Heikal, Y.M.; Zayed, E.M.; Shamseldin, S.A.M.; Salama, Y.E.; Amer, K.E.; Basuoni, M.M.; Abd Ellatif, S.; Mohamed, A.H. Conferring of drought and heat stress tolerance in wheat (Triticum aestivum L.) genotypes and their response to selenium nanoparticles application. Nanomaterials 2023, 13, 998. [Google Scholar] [CrossRef] [PubMed]
- Nkonya, E.; Kato, E. Rethinking agro-food sector to combat land degradation and desertification. In Life on Land; Springer: Berlin/Heidelberg, Germany, 2020; pp. 851–863. [Google Scholar]
- Raza, A.; Mubarik, M.S.; Sharif, R.; Habib, M.; Jabeen, W.; Zhang, C.; Chen, H.; Chen, Z.H.; Siddique, K.H.M.; Zhuang, W. Developing drought-smart, ready-to-grow future crops. Plant Genome 2023, 16, e20279. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Cai, X.; Zhou, Z.; Agong, S.G.; Wang, K.; Liu, F. Identification of QTLs and candidate genes for physiological traits associated with drought tolerance in cotton. J. Cotton Res. 2020, 3, 3. [Google Scholar] [CrossRef]
- Flores-Saavedra, M.; Villanueva, G.; Gramazio, P.; Vilanova, S.; Mauceri, A.; Abenavoli, M.R.; Sunseri, F.; Prohens, J.; Plazas, M. Nitrogen use efficiency, growth and physiological parameters in different tomato genotypes under high and low N fertilisation conditions. Plant Physiol. Biochem. 2024, 208, 108447. [Google Scholar] [CrossRef]
- Gul, N.; Khan, Z.; Shani, M.Y.; Hafiza, B.S.; Saeed, A.; Khan, A.I.; Shakeel, A.; Rahimi, M. Identification of salt-resilient cotton genotypes using integrated morpho-physiological and biochemical markers at the seedling stage. Sci. Rep. 2025, 15, 5276. [Google Scholar] [CrossRef]
- Hussain, T.; Akram, Z.; Shabbir, G.; Manaf, A.; Ahmed, M. Identification of drought tolerant Chickpea genotypes through multi trait stability index. Saudi J. Biol. Sci. 2021, 28, 6818–6828. [Google Scholar] [CrossRef]
- Pandey, A.; Khobra, R.; Mamrutha, H.M.; Wadhwa, Z.; Krishnappa, G.; Singh, G.; Singh, G.P. Elucidating the drought responsiveness in wheat genotypes. Sustainability 2022, 14, 3957. [Google Scholar] [CrossRef]
- Yahaya, M.A.; Shimelis, H. Drought stress in sorghum: Mitigation strategies, breeding methods and technologies—A review. J. Agron. Crop Sci. 2022, 208, 127–142. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. [136] Assay of Catalases and Peroxidases; Elsevier: Amsterdam, The Netherlands, 1955. [Google Scholar]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Horst, W.J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol. Plant. 1991, 83, 463–468. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Riazi, A.; Matsuda, K.; Arslan, A. Water-stress induced changes in concentrations of proline and other solutes in growing regions of young barley leaves. J. Exp. Bot. 1985, 36, 1716–1725. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [PubMed]
- Irnawati, I.; Riswanto, F.D.O.; Riyanto, S.; Martono, S.; Rohman, A. The use of software packages of R factoextra and FactoMineR and their application in principal component analysis for authentication of oils. Indones. J. Chemom. Pharm. Anal. 2021, 1, 1–10. [Google Scholar] [CrossRef]
- Gu, Z.; Hübschmann, D. Make interactive complex heatmaps in R. Bioinformatics 2022, 38, 1460–1462. [Google Scholar] [CrossRef]
- Olivoto, T.; Nardino, M. MGIDI: Toward an effective multivariate selection in biological experiments. Bioinformatics 2021, 37, 1383–1389. [Google Scholar] [CrossRef]


| Source | DF | SL | RL | SFM | SDM | RFM | RDM | FR | Pn | E | Gs | Chl.a | Chl.b |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | 4 | 710.5 *** | 39.2 *** | 321.2 *** | 51.5 *** | 11.3 *** | 1.6 *** | 6.4 *** | 0.01 *** | 0.01 ns | 0.06 *** | 0.009 *** | 0.2 *** |
| T | 2 | 434.3 *** | 9.1 *** | 463.5 *** | 200.5 *** | 21.7 *** | 5.0 *** | 36.8 *** | 0.08 *** | 0.008 ns | 0.4 *** | 0.0001 ns | 1.8 *** |
| G × T | 8 | 45.9 *** | 2.7 *** | 3.7 * | 1.3 * | 0.5 ** | 0.1 *** | 0.8 ns | 0.003 *** | 0.006 ns | 0.004 *** | 0.0001 ns | 0.05 *** |
| Error | 28 | 2.01 | 0.4 | 1.41 | 0.53 | 0.08 | 0.01 | 0.4 | 0.0001 | 0.008 | 0.0003 | 0.00013 | 0.002 |
| Total | 44 |
| Source | DF | T.chl | WUE | TSP | TSS | Pro | SOD | POD | CAT | APX | H2O2 | MDA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | 4 | 0.2 *** | 6.7 *** | 1.9 *** | 3.3 *** | 0.01 *** | 9284 *** | 42,725 *** | 45,154 *** | 24,863 *** | 0.6 *** | 0.009 *** |
| T | 2 | 1.81 *** | 0.65 *** | 32.3 *** | 6.1 *** | 0.08 *** | 50,093 *** | 103,839 *** | 192,437 *** | 247,140 *** | 9.4 *** | 0.1 *** |
| G × T | 8 | 0.05 *** | 0.15 *** | 0.5 *** | 0.7 *** | 0.003 *** | 2387 *** | 9524 *** | 5806 *** | 4925 *** | 0.2 *** | 0.008 *** |
| Error | 28 | 0.002 | 0.008 | 0.02 | 0.01 | 0.00019 | 93 | 183 | 164 | 140 | 0.01 | 0.0001 |
| Total | 44 |
| Soil Attributes | Values |
|---|---|
| Soil texture | Clay loam |
| Saturation percentage (%) | 40.2–44.7 |
| EC (dS m−1) | 1.72–1.93 |
| pH | 7.53–7.81 |
| Organic matter (%) | 0.42–0.73 |
| Mg + Ca (meq L−1) | 2.63–4.84 |
| CO3 (meq L−1) | None |
| HCO3 (meq L−1) | 2.52–4.83 |
| NO3-N (mg kg−1) | 3.54–4.52 |
| Total nitrogen (g kg−1) | 0.43–0.52 |
| Available K (mg kg−1) | 75.5–80.5 |
| Available P (mg kg−1) | 1.52–2.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shani, M.Y.; Ashraf, M.Y.; Ramzan, M.; Khan, Z.; Batool, N.; Gul, N.; Bauerle, W.L. Unveiling Drought Tolerant Cotton Genotypes: Insights from Morpho-Physiological and Biochemical Markers at Flowering. Plants 2025, 14, 616. https://doi.org/10.3390/plants14040616
Shani MY, Ashraf MY, Ramzan M, Khan Z, Batool N, Gul N, Bauerle WL. Unveiling Drought Tolerant Cotton Genotypes: Insights from Morpho-Physiological and Biochemical Markers at Flowering. Plants. 2025; 14(4):616. https://doi.org/10.3390/plants14040616
Chicago/Turabian StyleShani, Muhammad Yousaf, Muhammad Yasin Ashraf, Muhammad Ramzan, Zafran Khan, Nimra Batool, Nimra Gul, and William L. Bauerle. 2025. "Unveiling Drought Tolerant Cotton Genotypes: Insights from Morpho-Physiological and Biochemical Markers at Flowering" Plants 14, no. 4: 616. https://doi.org/10.3390/plants14040616
APA StyleShani, M. Y., Ashraf, M. Y., Ramzan, M., Khan, Z., Batool, N., Gul, N., & Bauerle, W. L. (2025). Unveiling Drought Tolerant Cotton Genotypes: Insights from Morpho-Physiological and Biochemical Markers at Flowering. Plants, 14(4), 616. https://doi.org/10.3390/plants14040616






