Identification of Ethylene Response Factors in Wheat Reveals That TaERF16-B Contributes to Salt Tolerance
Abstract
1. Introduction
2. Results
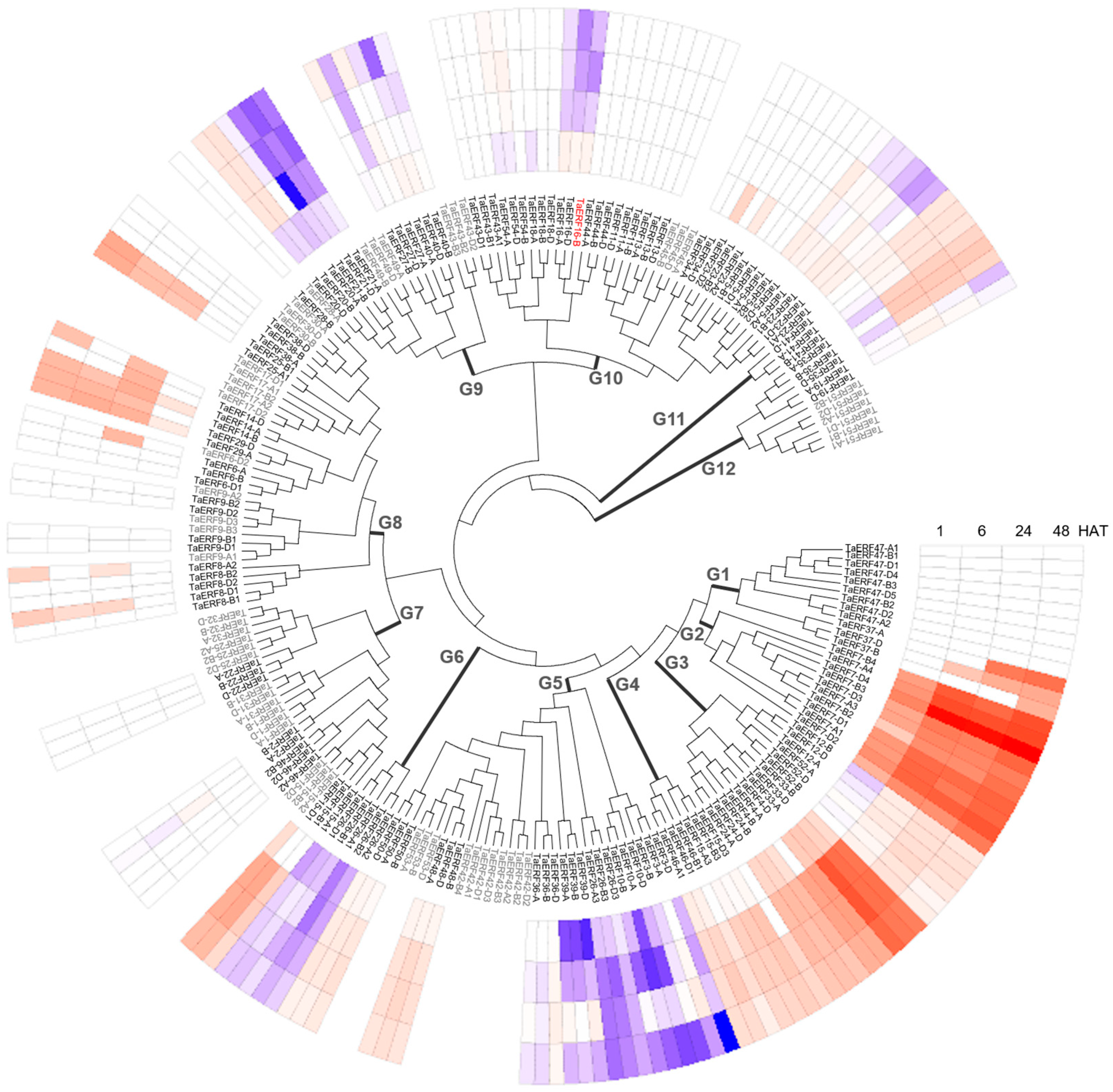
2.1. Transcriptional Response to Salt Stress of TaERFs
2.2. Association Analysis Between TaERFs and Leaf Salt Injury Index
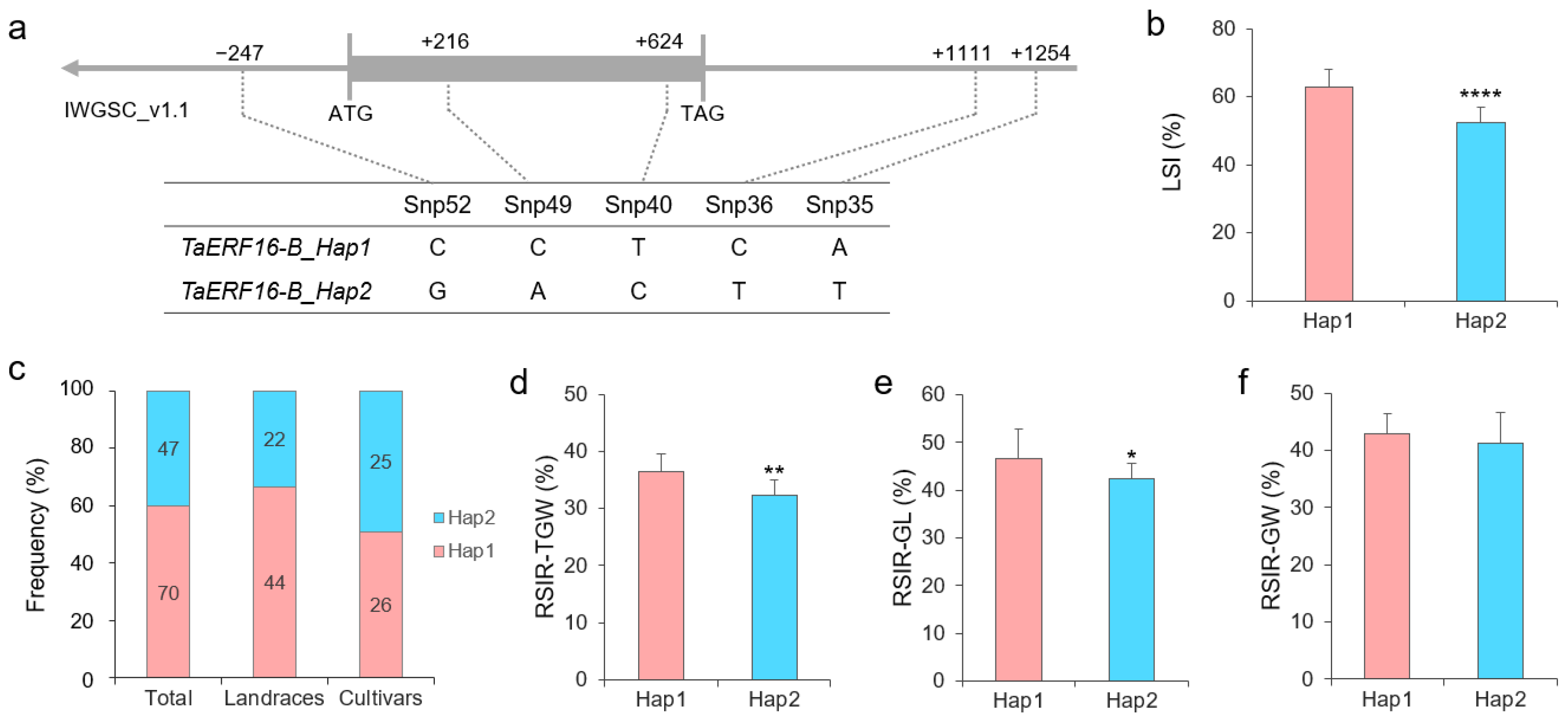
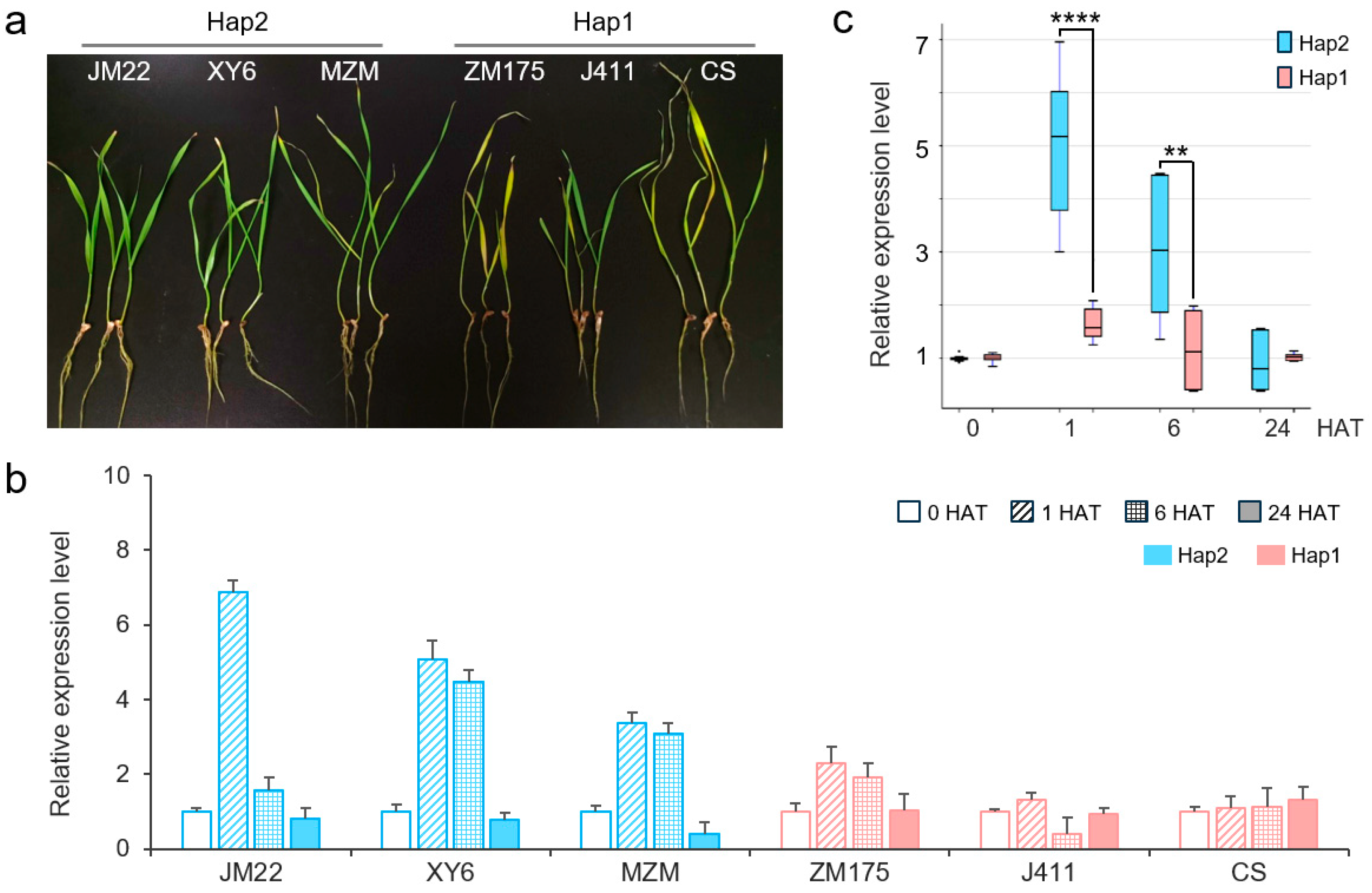
2.3. Haplotypes of Salt Tolerance-Related TaERF16-B
2.4. Expression Patterns of TaERF16-B_Hap1 and _Hap2
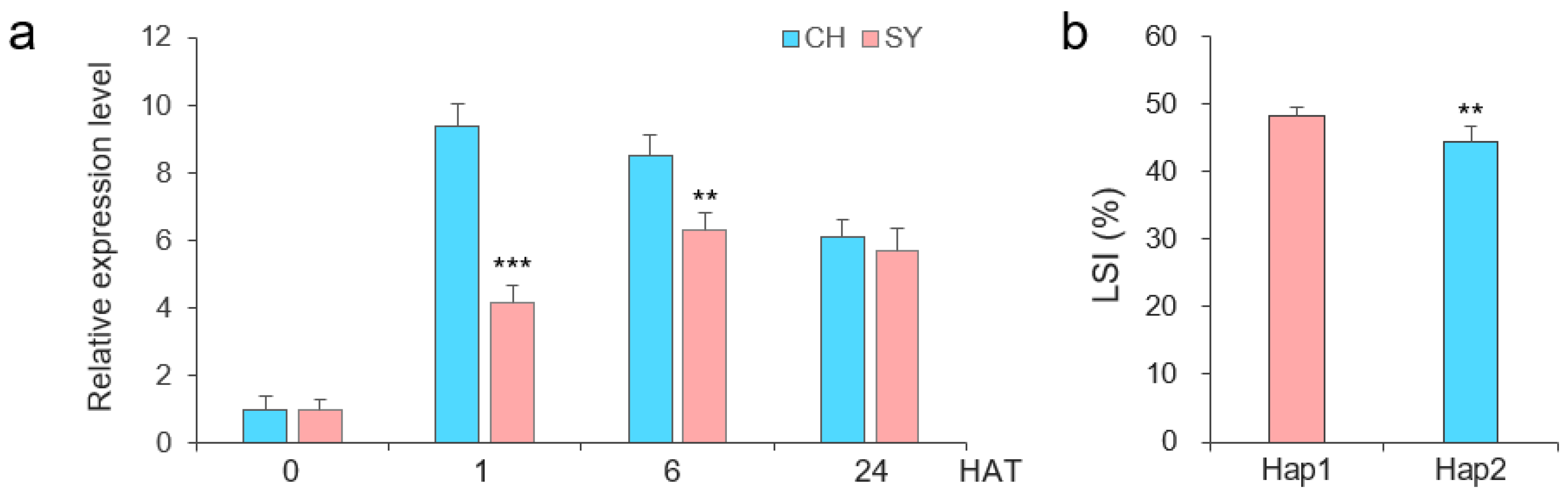
2.5. Confirmation of the Salt Tolerance of TaERF16-B in an RIL Population
2.6. Co-Expression Network of TaERF16-B
3. Discussion
3.1. Response of Wheat ERF Family Genes to Salt Stress
3.2. TaERF16-B Is Related to Salt Tolerance
3.3. Predicted Target Genes of TaERF16-B Protein
4. Materials and Methods
4.1. Plant Materials
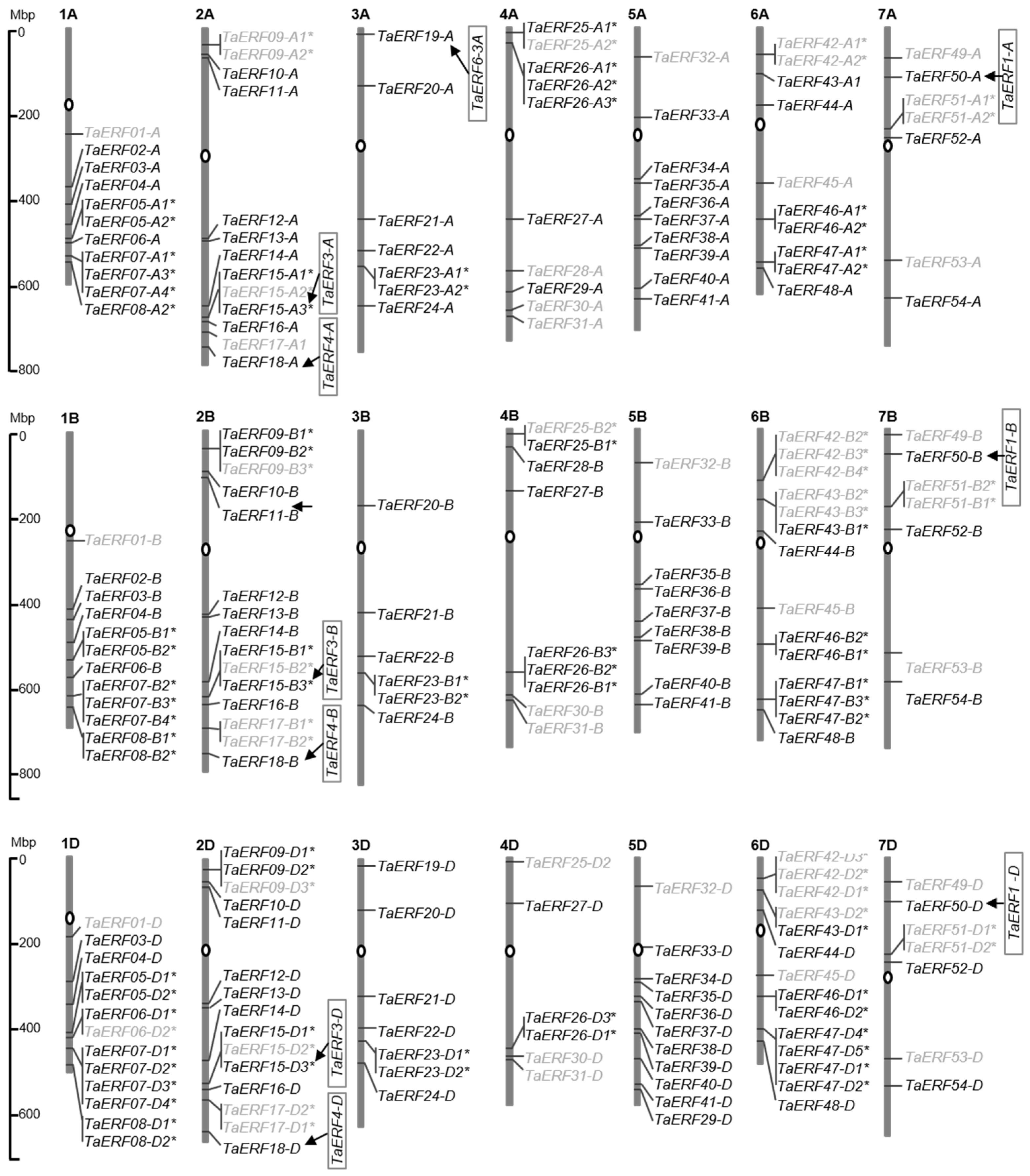
4.2. Isolation of ERF Sequences from the Wheat Genome
4.3. Transcriptional Data of TaERF Genes After Salt Treatment
4.4. Association Analysis of TaERF Genes
4.5. Association Analysis of TaERF16-B
4.6. RT-qPCR
4.7. Co-Expression Network Construction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Butcher, K.; Wick, A.F.; Desutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Srivastava, R.; Kumar, R. The Expanding Roles of APETALA2/Ethylene Responsive Factors and Their Potential Applications in Crop Improvement. Brief Funct. Genom. 2019, 18, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Wei, S.; Zheng, X.; Tu, P.; Tao, F. APETALA2/Ethylene-Responsive Factors in Higher Plant and Their Roles in Regulation of Plant Stress Response. Crit. Rev. Biotechnol. 2024, 44, 1533–1551. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.W. Class VIIIb APETALA2 Ethylene Response Factors in Plant Development. Trends Plant Sci. 2018, 23, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Nolan, T.M.; Jiang, H.; Yin, Y. AP2/ERF Transcription Factor Regulatory Networks in Hormone and Abiotic Stress Responses in Arabidopsis. Front. Plant Sci. 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Hou, X.L.; Xing, G.M.; Liu, J.X.; Duan, A.Q.; Xu, Z.S.; Li, M.Y.; Zhuang, J.; Xiong, A.S. Advances in AP2/ERF Super-Family Transcription Factors in Plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef] [PubMed]
- Sharoni, A.M.; Nuruzzaman, M.; Satoh, K.; Shimizu, T.; Kondoh, H.; Sasaya, T.; Choi, I.R.; Omura, T.; Kikuchi, S. Gene Structures, Classification and Expression Models of the AP2/EREBP Transcription Factor Family in Rice. Plant Cell Physiol. 2011, 52, 344–360. [Google Scholar] [CrossRef]
- Debbarma, J.; Sarki, Y.N.; Saikia, B.; Boruah, H.P.D.; Singha, D.L.; Chikkaputtaiah, C. Ethylene Response Factor (ERF) Family Proteins in Abiotic Stresses and CRISPR–Cas9 Genome Editing of ERFs for Multiple Abiotic Stress Tolerance in Crop Plants: A Review. Mol. Biotechnol. 2019, 61, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Zhang, J.; Zhao, H.; Tan, S.; Xu, W.; Pan, J.; Yang, F.; Pi, E. ERF Subfamily Transcription Factors and Their Function in Plant Responses to Abiotic Stresses. Front. Plant Sci. 2022, 13, 1042084. [Google Scholar] [CrossRef]
- Khoudi, H. SHINE Clade of ERF Transcription Factors: A Significant Player in Abiotic and Biotic Stress Tolerance in Plants. Plant Physiol. Biochem. 2023, 195, 77–88. [Google Scholar] [CrossRef]
- Shi, Q.; Dong, Y.; Zhou, Q.; Qiao, D.; Ma, Z.; Zhang, L.; Li, Y. Characterization of a Maize ERF Gene, ZmERF1, in Hormone and Stress Responses. Acta Physiol. Plant 2016, 38, 126. [Google Scholar] [CrossRef]
- Chen, H.C.; Chien, T.C.; Chen, T.Y.; Chiang, M.H.; Lai, M.H.; Chang, M.C. Overexpression of a Novel ERF-X-Type Transcription Factor, OsERF106MZ, Reduces Shoot Growth and Tolerance to Salinity Stress in Rice. Rice 2021, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Chen, X.; Liu, J.; Ye, J.; Guo, Z. The Rice ERF Transcription Factor OsERF922 Negatively Regulates Resistance to Magnaporthe Oryzae and Salt Tolerance. J. Exp. Bot. 2012, 63, 3899–3911. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.J.; Yin, L.J.; Liu, Y.; Ma, J.; Zheng, J.C.; Lan, J.H.; Fu, J.D.; Chen, M.; Xu, Z.S.; Ma, Y.Z. The ABA-Induced Soybean ERF Transcription Factor Gene GmERF75 Plays a Role in Enhancing Osmotic Stress Tolerance in Arabidopsis and Soybean. BMC Plant Biol. 2019, 19, 506. [Google Scholar] [CrossRef]
- Li, L.; Zhu, Z.; Liu, J.; Zhang, Y.; Lu, Y.; Zhao, J.; Xing, H.; Guo, N. Transcription Factor GmERF105 Negatively Regulates Salt Stress Tolerance in Arabidopsis thaliana. Plants 2023, 12, 3007. [Google Scholar] [CrossRef]
- Zhao, M.J.; Yin, L.J.; Ma, J.; Zheng, J.C.; Wang, Y.X.; Lan, J.H.; Fu, J.D.; Chen, M.; Xu, Z.S.; Ma, Y.Z. The Roles of GmERF135 in Improving Salt Tolerance and Decreasing ABA Sensitivity in Soybean. Front. Plant Sci. 2019, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Qanmber, G.; Li, J.; Pu, M.; Chen, G.; Li, S.; Liu, L.; Qin, W.; Ma, S.; Wang, Y.; et al. Identification and Characterization of the ERF Subfamily B3 Group Revealed GhERF13.12 Improves Salt Tolerance in Upland Cotton. Front. Plant Sci. 2021, 12, 705883. [Google Scholar] [CrossRef]
- Xu, Z.S.; Xia, L.Q.; Chen, M.; Cheng, X.G.; Zhang, R.Y.; Li, L.C.; Zhao, Y.X.; Lu, Y.; Ni, Z.Y.; Liu, L.; et al. Isolation and Molecular Characterization of the Triticum aestivum L. Ethylene-Responsive Factor 1 (TaERF1) That Increases Multiple Stress Tolerance. Plant Mol. Biol. 2007, 65, 719–732. [Google Scholar] [CrossRef]
- Rong, W.; Qi, L.; Wang, A.; Ye, X.; Du, L.; Liang, H.; Xin, Z.; Zhang, Z. The ERF Transcription Factor TaERF3 Promotes Tolerance to Salt and Drought Stresses in Wheat. Plant Biotechnol. J. 2014, 12, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ai, X.; Xu, F.; Quan, T.; Liu, S.; Xia, G. Isolation and Characterization of a Bread Wheat Salinity Responsive ERF Transcription Factor. Gene 2012, 511, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, M.; Zhang, S.; Song, T.; Zhang, M.; Zhou, H.; Wang, Y.; Xiang, J.; Zhang, X. Transcriptomic Identification of Wheat AP2/ERF Transcription Factors and Functional Characterization of TaERF-6-3A in Response to Drought and Salinity Stresses. Int. J. Mol. Sci. 2022, 23, 3272. [Google Scholar] [CrossRef] [PubMed]
- Makhloufi, E.; Yousfi, F.E.; Marande, W.; Mila, I.; Hanana, M.; Berg s, H.; Mzid, R.; Bouzayen, M. Isolation and Molecular Characterization of ERF1, an Ethylene Response Factor Gene from Durum Wheat (Triticum turgidum L. Subsp. Durum), Potentially Involved in Salt-Stress Responses. J. Exp. Bot. 2014, 65, 6359–6371. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, L.; Bai, J.; Wang, P.; Duan, W.; Yuan, S.; Yuan, G.; Zhang, F.; Zhang, L.; Zhao, C. Genome-Wide Characterization of JASMONATE-ZIM DOMAIN Transcription Repressors in Wheat (Triticum aestivum L.). BMC Genom. 2017, 18, 152. [Google Scholar] [CrossRef] [PubMed]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Yuan, L. ERF Gene Clusters: Working Together to Regulate Metabolism. Trends Plant Sci. 2021, 26, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, M.; Sierro, N.; Kawaguchi, H.; Bakaher, N.; Ivanov, N.V.; Hashimoto, T.; Shoji, T. Genomic Insights into the Evolution of the Nicotine Biosynthesis Pathway in Tobacco. Plant Physiol. 2017, 174, 999–1011. [Google Scholar] [CrossRef]
- Shoji, T.; Hashimoto, T. Stress-Induced Expression of NICOTINE2-Locus Genes and Their Homologs Encoding Ethylene Response Factor Transcription Factors in Tobacco. Phytochemistry 2015, 113, 41–49. [Google Scholar] [CrossRef]
- Wang, X.; Han, H.; Yan, J.; Chen, F.; Wei, W. A New AP2/ERF Transcription Factor from the Oil Plant Jatropha curcas Confers Salt and Drought Tolerance to Transgenic Tobacco. Appl. Biochem. Biotechnol. 2015, 176, 582–597. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Park, C.J.; Lee, S.B.; Ham, B.K.; Shin, R.; Paek, K.H. Overexpression of the Tobacco Tsi1 Gene Encoding an EREBP/AP2-Type Transcription Factor Enhances Resistance against Pathogen Attack and Osmotic Stress in Tobacco. Plant Cell 2001, 13, 1035–1046. [Google Scholar] [PubMed]
- Kim, B.K.; Im, J.Y.; Han, G.; Lee, W.J.; Won, K.J.; Chung, K.S.; Lee, K.; Ban, H.S.; Song, K.B.; Won, M. P300 Cooperates with C-Jun and PARP-1 at the P300 Binding Site to Activate RhoB Transcription in NSC126188-Mediated Apoptosis. Biochim. Biophys. Acta Gene Regul. Mech. 2014, 1839, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Lin, J.; Liu, X.; Chu, W.; Li, J.; Gao, Y.; An, K.; Song, W.; Xin, M.; Yao, Y.; et al. Histone Acetyltransferase TaHAG1 Acts as a Crucial Regulator to Strengthen Salt Tolerance of Hexaploid Wheat. Plant Physiol. 2021, 186, 1951–1969. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Huang, X.; Ding, L.; Wang, Z.; Tang, D.; Chen, B.; Ao, L.; Liu, Y.; Kang, Z.; Mao, H. TaERF87 and TaAKS1 Synergistically Regulate TaP5CS1/TaP5CR1-Mediated Proline Biosynthesis to Enhance Drought Tolerance in Wheat. N. Phytol. 2023, 237, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Liu, L.; Hu, Z.; Wang, X.; Zhu, Y.; Zhang, J.; Li, X.; Kang, Z.; Lin, Y.; Yin, C. Salt-Induced Inhibition of Rice Seminal Root Growth Is Mediated by Ethylene-Jasmonate Interaction. J. Exp. Bot. 2021, 72, 5656–5672. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, L.E.; Sotolongo, M.; Menendez, C.; Ochogavia, M.E.; Coll, Y.; Hernandez, I.; Borrás-Hidalgo, O.; Thomma, B.P.; Vera, P.; Hernandez, L. SodERF3, a Novel Sugarcane Ethylene Responsive Factor (ERF), Enhances Salt and Drought Tolerance when Overexpressed in Tobacco Plants. Plant Cell Physiol. 2008, 49, 512–525. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, J.; Li, Z.; Qiao, J.; Quan, R.; Wang, J.; Huang, R.; Qin, H. SALT AND ABA RESPONSE ERF1 Improves Seed Germination and Salt Tolerance by Repressing ABA Signaling in Rice. Plant Physiol. 2022, 189, 1110–1127. [Google Scholar] [CrossRef] [PubMed]
- Peethambaran, P.K.; Glenz, R.; Höninger, S.; Shahinul Islam, S.M.; Hummel, S.; Harter, K.; Kolukisaoglu, Ü.; Meynard, D.; Guiderdoni, E.; Nick, P.; et al. Salt-Inducible Expression of OsJAZ8 Improves Resilience against Salt-Stress. BMC Plant Biol. 2018, 18, 311. [Google Scholar] [CrossRef]
- Wu, H.; Ye, H.; Yao, R.; Zhang, T.; Xiong, L. OsJAZ9 Acts as a Transcriptional Regulator in Jasmonate Signaling and Modulates Salt Stress Tolerance in Rice. Plant Sci. 2015, 232, 1–12. [Google Scholar] [CrossRef]
- Ebel, C.; BenFeki, A.; Hanin, M.; Solano, R.; Chini, A. Characterization of Wheat (Triticum aestivum) TIFY Family and Role of Triticum durum TdTIFY11a in Salt Stress Tolerance. PLoS ONE 2018, 13, e0200566. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yao, Y.; Ma, J.; Wang, S.; Li, S.; Wang, W.; Yu, X.; Sun, F.; Zhang, C.; Xi, Y. Wheat TaTIFY3B and TaTIFY10A Play Roles in Seed Germination and Abiotic Stress Responses in Transgenic Arabidopsis and Rice. BMC Plant Biol. 2024, 24, 951. [Google Scholar] [CrossRef]
- Sen, S.; Kundu, S.; Dutta, S.K. Proteomic Analysis of JAZ Interacting Proteins under Methyl Jasmonate Treatment in Finger Millet. Plant Physiol. Biochem. 2016, 108, 79–89. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, H.; Han, Z.; Wang, S.; Wang, T.; Li, Q.; Tian, J.; Wang, Y.; Zhang, X.; Xu, X.; et al. ERF4 Affects Fruit Ripening by Acting as a JAZ Interactor between Ethylene and Jasmonic Acid Hormone Signaling Pathways. Hortic. Plant J. 2022, 8, 689–699. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, G.; Zhou, H.; Yin, S.; Li, Y.; Ma, C.; Chen, P.; Sun, L.; Hao, F. GhPYL9-5D and GhPYR1-3A Positively Regulate Arabidopsis and Cotton Responses to ABA, Drought, High Salinity and Osmotic Stress. BMC Plant Biol. 2023, 23, 310. [Google Scholar]
- Kim, H.; Lee, K.; Hwang, H.; Bhatnagar, N.; Kim, D.Y.; Yoon, I.S.; Byun, M.O.; Kim, S.T.; Jung, K.H.; Kim, B.G. Overexpression of PYL5 in Rice Enhances Drought Tolerance, Inhibits Growth, and Modulates Gene Expression. J. Exp. Bot. 2014, 65, 453–464. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Li, T.; Ni, C.; Han, L.; Du, P.; Xiao, K. TaPYL4, an ABA Receptor Gene of Wheat, Positively Regulates Plant Drought Adaptation through Modulating the Osmotic Stress-Associated Processes. BMC Plant Biol. 2022, 22, 423. [Google Scholar] [CrossRef] [PubMed]
- Korasick, D.A.; Končitíková, R.; Kopečná, M.; Hájková, E.; Vigouroux, A.; Moréra, S.; Becker, D.F.; Šebela, M.; Tanner, J.J.; Kopečný, D. Structural and Biochemical Characterization of Aldehyde Dehydrogenase 12, the Last Enzyme of Proline Catabolism in Plants. J. Mol. Biol. 2019, 431, 576–592. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ma, X.; Wang, Q.; Gao, Y.; Xue, Y.; Niu, X.; Yu, G.; Liu, Y. Significant Improvement of Stress Tolerance in Tobacco Plants by Overexpressing a Stress-Responsive Aldehyde Dehydrogenase Gene from Maize (Zea mays). Plant Mol. Biol. 2008, 68, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Zhao, S.; Fang, Q.; Ruan, J.; Li, S.; Liu, T.; Qi, Y.; Zhang, L.; Zhang, X.; et al. Overexpression of the Aldehyde Dehydrogenase AhALDH3H1 from Arachis hypogaea in Soybean Increases Saline-Alkali Stress Tolerance. Front. Plant Sci. 2023, 14, 1165384. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, B.; Li, G.; Fan, R.; Zhong, Y.; Wang, X.; Zhang, X. TraeALDH7B1-5A, Encoding Aldehyde Dehydrogenase 7 in Wheat, Confers Improved Drought Tolerance in Arabidopsis. Planta 2015, 242, 137–151. [Google Scholar] [CrossRef]
- Shamloo-Dashtpagerdi, R.; Aliakbari, M.; Lindlöf, A.; Tahmasebi, S. A Systems Biology Study Unveils the Association between a Melatonin Biosynthesis Gene, O-Methyl Transferase 1 (OMT1) and Wheat (Triticum aestivum L.) Combined Drought and Salinity Stress Tolerance. Planta 2022, 255, 99. [Google Scholar] [CrossRef]
- Zhang, X.W.; Li, S.J.; Zhang, X.J.; Li, X.; Yang, Z.J.; Zhang, S.W.; Chen, F.; Chang, L.F.; Guo, H.J.; Chang, Z.J.; et al. QTL Mapping for Salt Tolerance in Wheat Line CH7034. Acta Agro. Sin. 2022, 48, 2654–2662. [Google Scholar]
- Chen, J.; Zhang, L.; Liu, Y.; Shen, X.; Guo, Y.; Ma, X.; Zhang, X.; Li, X.; Cheng, T.; Wen, H.; et al. RNA-Seq-Based WGCNA and Association Analysis Reveal the Key Regulatory Module and Genes Responding to Salt Stress in Wheat Roots. Plants 2024, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Li, Y.; Wang, L.; Gu, C.; Luo, S.; Li, X.; Yan, J.; Lu, C.; Chang, Z.; Gao, W.; et al. Identification of Salt-Stress-Responding Genes by Weighted Gene Correlation Network Analysis and Association Analysis in Wheat Leaves. Plants 2024, 13, 2642. [Google Scholar] [CrossRef]
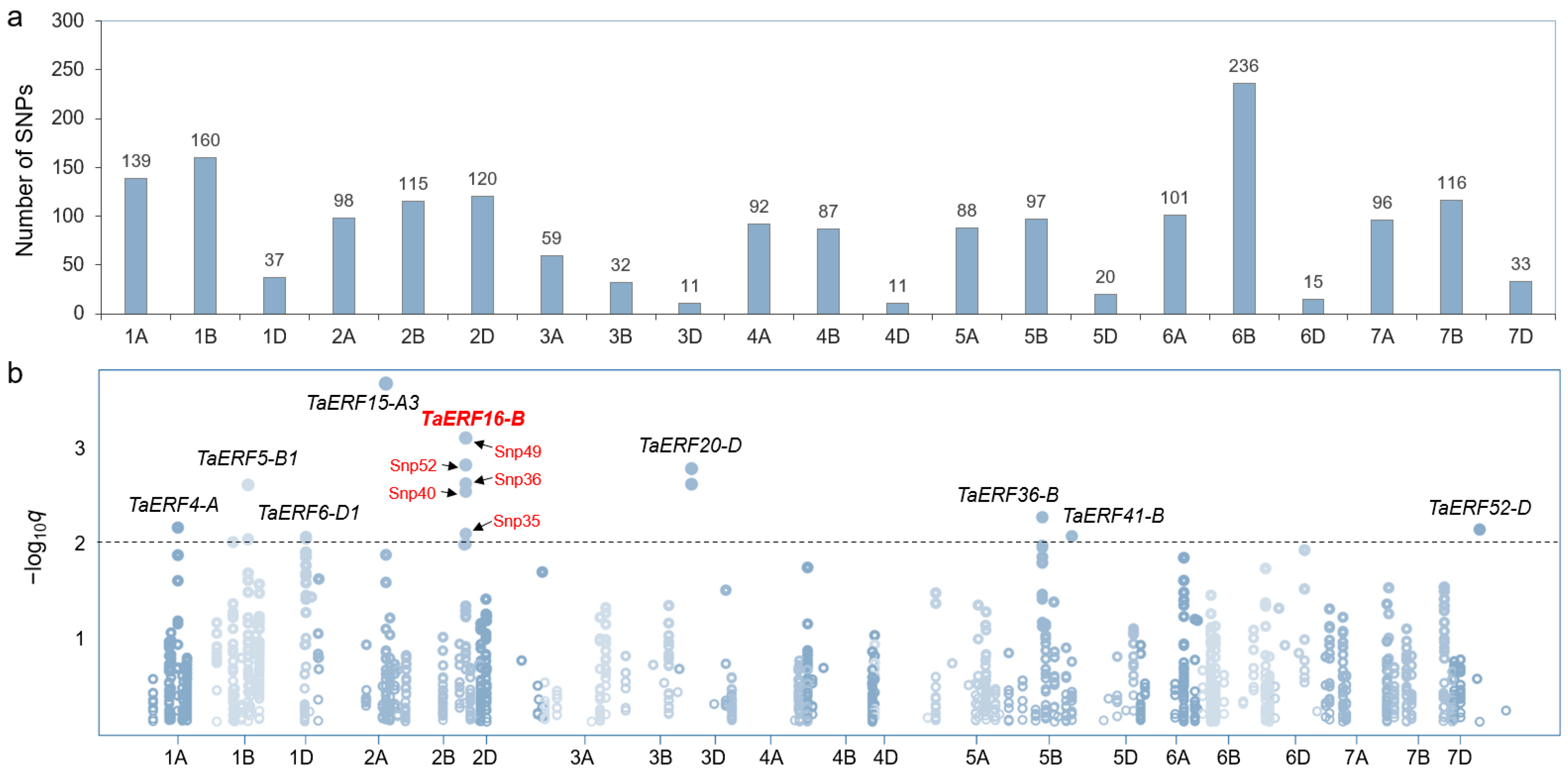


| TaERF Genes | SNP | Chromosome | Position (IWGSC v1.0) | q Value |
|---|---|---|---|---|
| TaERF04-A | Snp22[A/C] | 1A | 465,495,549 | 0.0071 |
| TaERF05-B1 | Snp13[C/G] | 1B | 551,869,884 | 0.0095 |
| TaERF05-B1 | Snp14[C/T] | 1B | 551,869,911 | 0.0024 |
| TaERF06-D1 | Snp08[T/C] | 1D | 421,224,882 | 0.0089 |
| TaERF06-D1 | Snp24[C/T] | 1D | 421,227,176 | 0.0093 |
| TaERF15-A3 | Snp36[G/A] | 2A | 673,328,463 | 0.0002 |
| TaERF16-B | Snp35[A/T] | 2B | 640,764,124 | 0.0083 |
| TaERF16-B | Snp36[C/T] | 2B | 640,764,267 | 0.0023 |
| TaERF16-B | Snp40[T/C] | 2B | 640,764,754 | 0.0028 |
| TaERF16-B | Snp49[C/A] | 2B | 640,765,162 | 0.0007 |
| TaERF16-B | Snp52[C/G] | 2B | 640,765,624 | 0.0014 |
| TaERF20-D | Snp02[G/A] | 3D | 124,865,746 | 0.0023 |
| TaERF20-D | Snp03[T/C] | 3D | 124,866,660 | 0.0016 |
| TaERF36-B | Snp82[C/G] | 5B | 387,177,561 | 0.0054 |
| TaERF41-B | Snp03[A/G] | 5B | 653,804,805 | 0.0088 |
| TaERF52-D | Snp01[A/G] | 7D | 246,072,894 | 0.0074 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wei, A.; Chen, J.; Wu, L.; Li, T.; Qiao, L. Identification of Ethylene Response Factors in Wheat Reveals That TaERF16-B Contributes to Salt Tolerance. Plants 2025, 14, 621. https://doi.org/10.3390/plants14040621
Zhang L, Wei A, Chen J, Wu L, Li T, Qiao L. Identification of Ethylene Response Factors in Wheat Reveals That TaERF16-B Contributes to Salt Tolerance. Plants. 2025; 14(4):621. https://doi.org/10.3390/plants14040621
Chicago/Turabian StyleZhang, Lei, Aili Wei, Jiating Chen, Lijuan Wu, Tian Li, and Linyi Qiao. 2025. "Identification of Ethylene Response Factors in Wheat Reveals That TaERF16-B Contributes to Salt Tolerance" Plants 14, no. 4: 621. https://doi.org/10.3390/plants14040621
APA StyleZhang, L., Wei, A., Chen, J., Wu, L., Li, T., & Qiao, L. (2025). Identification of Ethylene Response Factors in Wheat Reveals That TaERF16-B Contributes to Salt Tolerance. Plants, 14(4), 621. https://doi.org/10.3390/plants14040621







