Physiological and Transcriptomic Analysis of Two Types of Hami Melons in Low-Temperature Storage
Abstract
1. Introduction
2. Results
2.1. Physiological Characteristics of Cold-Sensitive and Cold-Tolerant Hami Melon Fruits
2.1.1. Chilling Injury (CI) Symptoms Under Cold Storage Stress
2.1.2. Weight Loss Rates Under Cold Storage Stress
2.1.3. Firmness Decreases Under Cold Storage Stress
2.1.4. Free Proline Under Cold Storage Stress
2.1.5. H2O2 and MDA Contents Under Cold Storage Stress
2.2. Enzymatic Activity in Hami Melon Fruits Under Cold Storage Stress
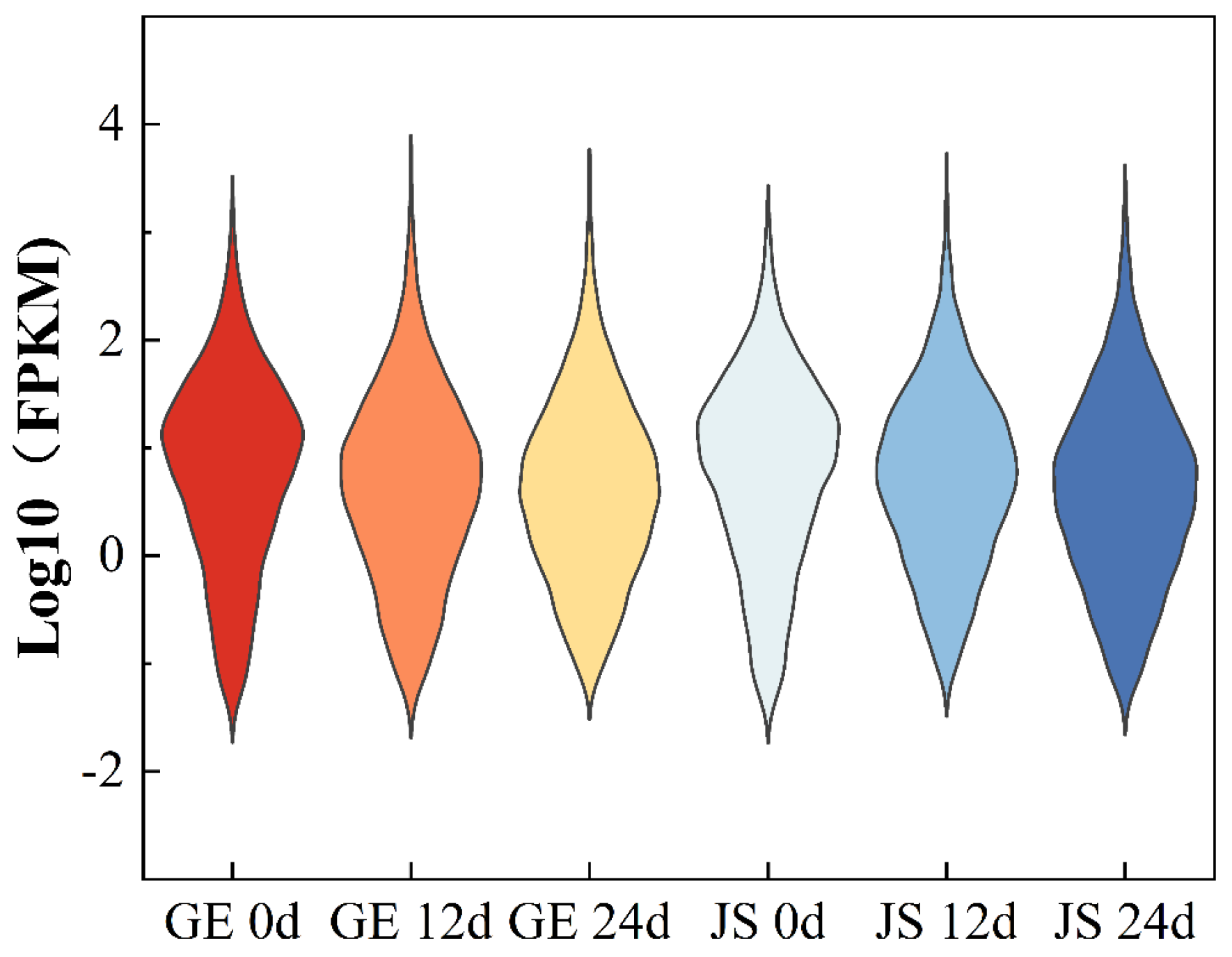
2.3. Transcriptional Analysis of Hami Melon Varieties Under Cold Storage Stress
2.4. Analysis of DEGs in Cold-Stored Hami Melons
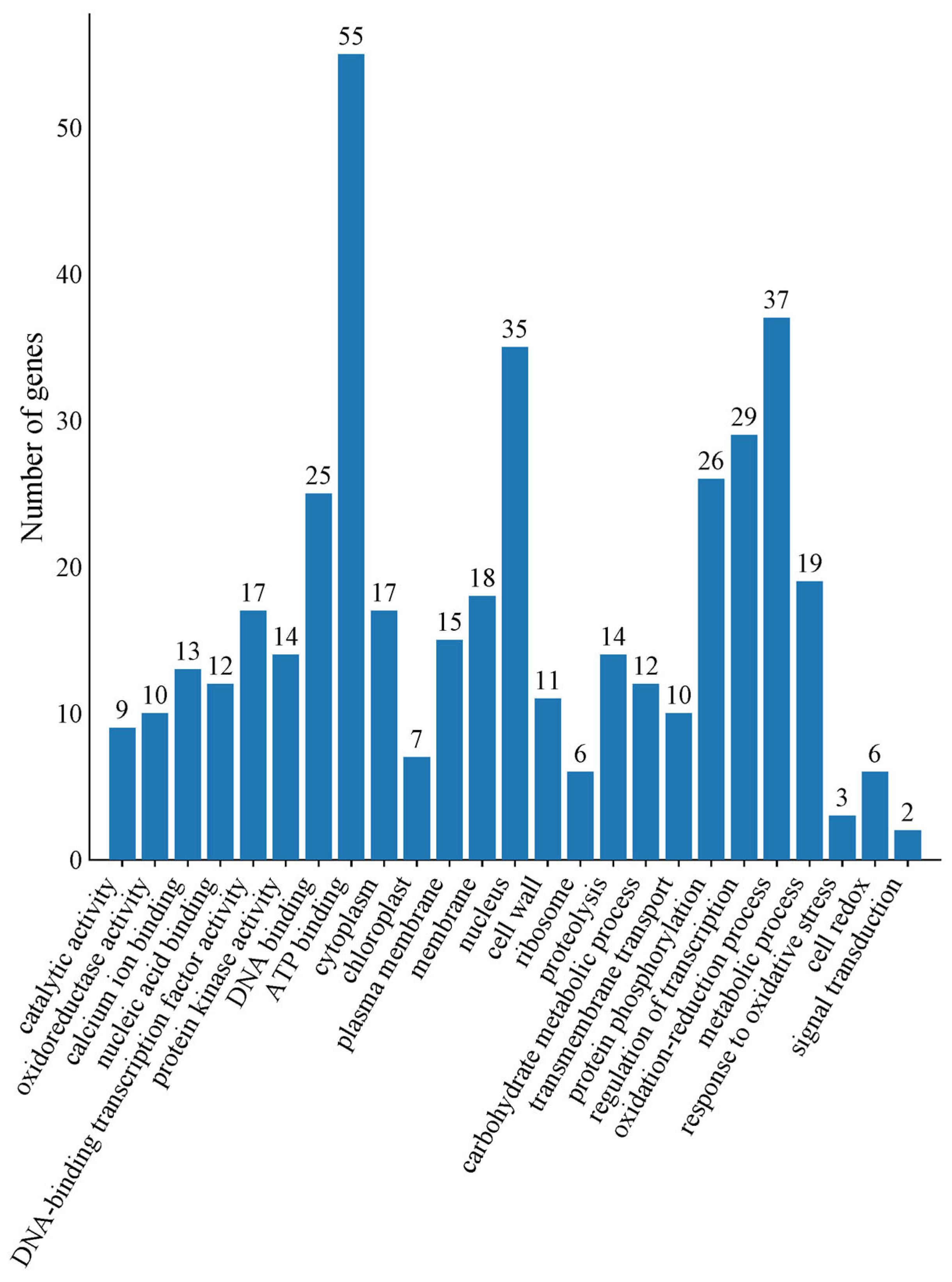
2.5. GO and KEGG Analyses of Co-Expressed DEGs in Hami Melons Under Cold Storage Stress
2.6. Analysis of Key Co-Expressed DEGs Related to the Cold Stress Response
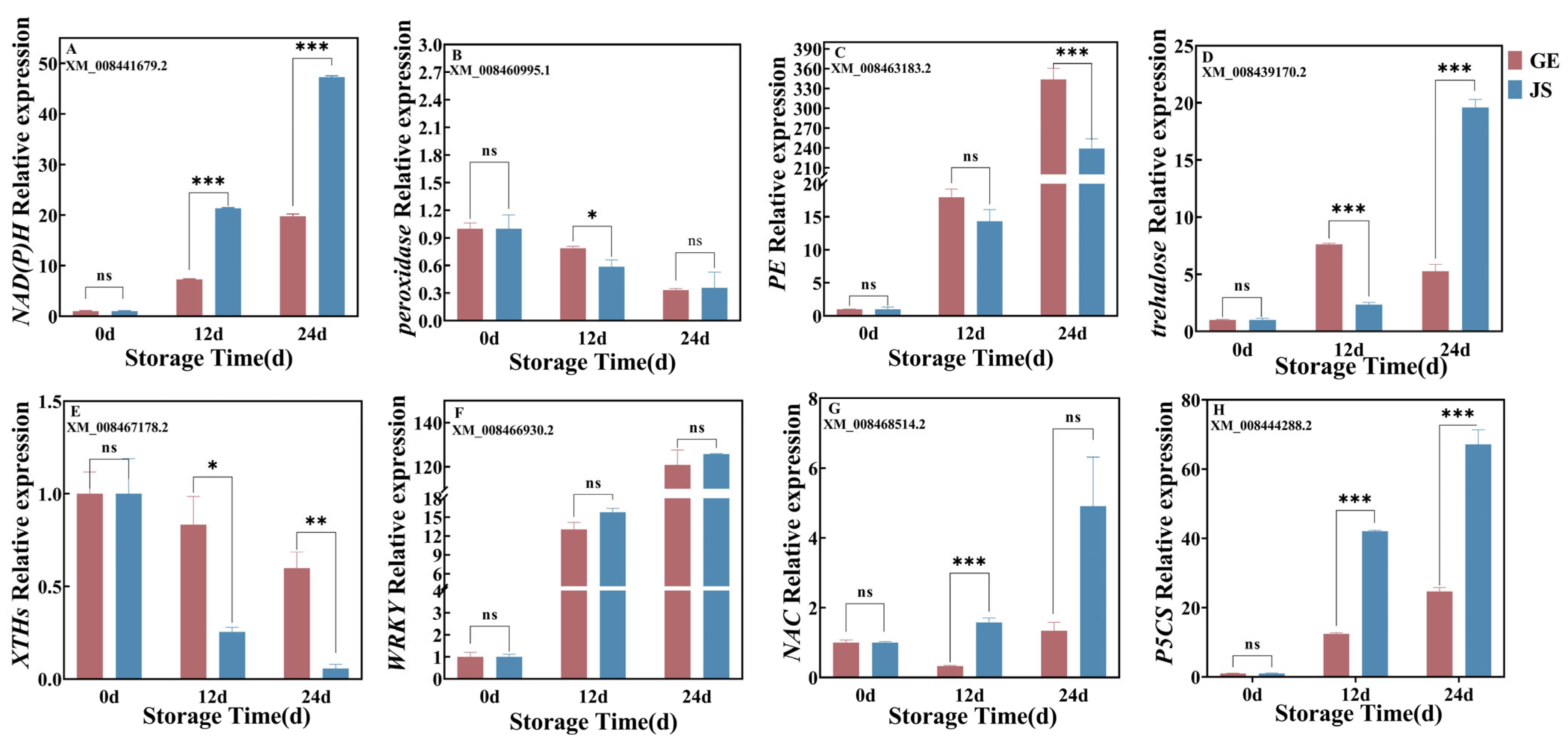
2.7. qRT-PCR Confirmation of DEGs
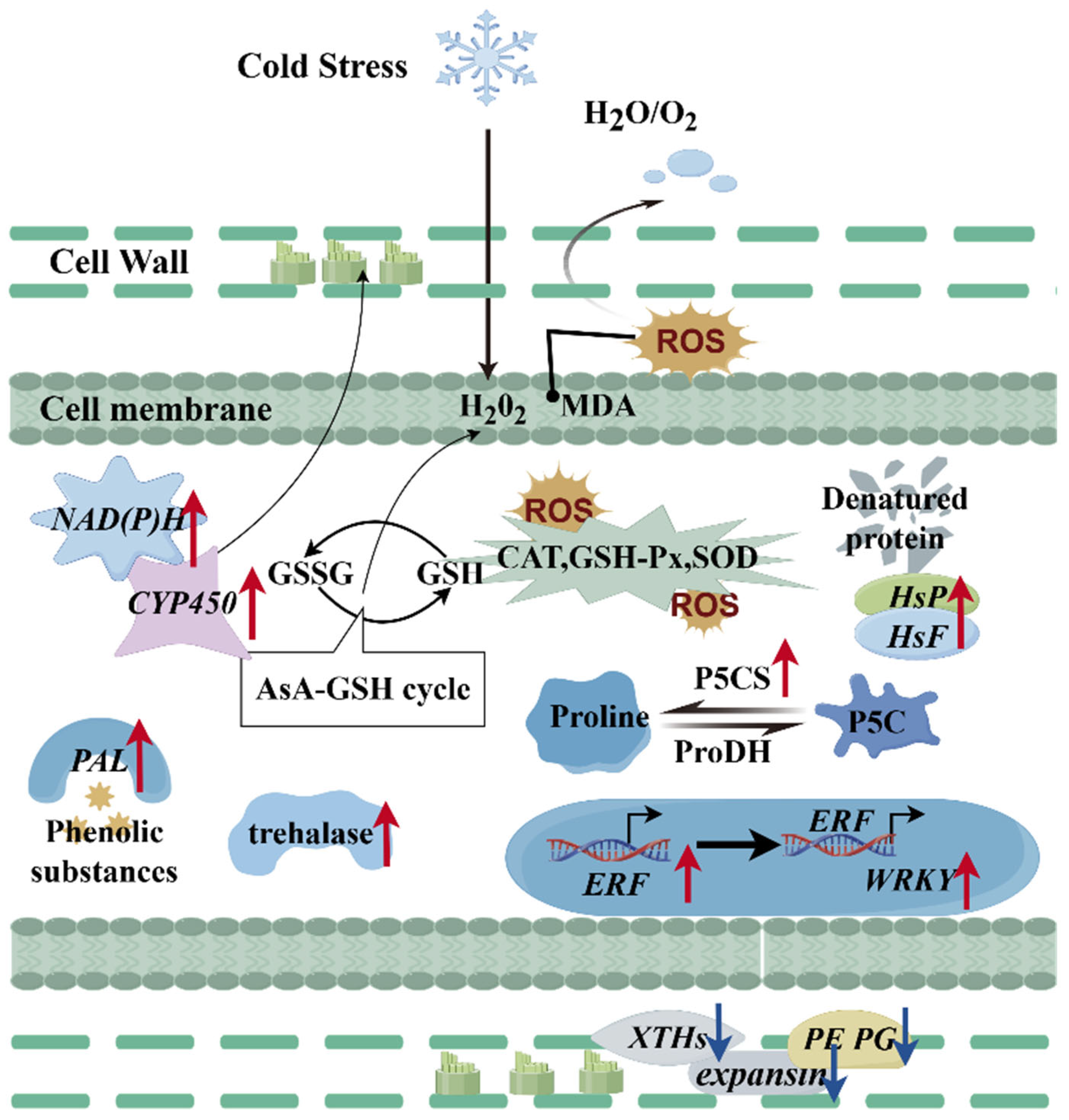
3. Discussion
3.1. Physiological Changes in Hami Melon Varieties Under Cold Storage Stress
3.2. Enzymatic Activity Changes in Hami Melons Under Cold Storage Stress
3.3. Impacts of Cold Storage Stress on Hami Melon Oxidoreductase Activity
3.4. Effects of Cold Storage Stress on Carbohydrate Metabolic Processes in Hami Melons
3.5. Effects of Cold Storage Stress on Cell Wall Metabolism in Hami Melon Fruits
3.6. Effects of Cold Storage Stress on Transcriptional Regulation in Hami Melons
4. Materials and Methods
4.1. Materials and Treatments
4.2. Quantification of Physiological Parameters
4.3. Measurement of Enzymatic Activity
4.4. DEG Annotation and Functional Analysis
4.5. Analysis via qRT-PCR
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GE | Gold Queen Melons |
| JS | Jia Shi Melons |
| ROS | Reactive oxygen species |
| CAT | Catalase |
| GSH-Px | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| ProDH | Proline dehydrogenase |
| P5CS | Pyrroline-5-carboxylic acid synthetase |
| DEGs | Differentially expressed genes |
| CI | Chilling injury |
| MDA | Malondialdehyde |
| GO | Gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| DEPs | Differentially expressed proteins |
| MF | Molecular function |
| CC | Cellular component |
| BP | Biological process |
| PAL | Phenylalanine ammonia-lyase |
| PE | Pectinesterase |
| PG | Polygalacturonase |
| GAUT | Galacturonosyl transferase |
| XTH | Xyloglucan endotransglycosylase/hydrolase |
| CYP450 | CytochromeP450 |
| HsF | Heat shock factor |
| HsP | Heat shock protein |
Appendix A
Appendix A.1
| Name | Accession Number | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| NAD(P)H-ubiquinone oxidoreductase | XM_008441679.2 | TGGCTCTGTCTTGAACCT | CTGTGGCGTTATACTTATTCC |
| peroxidase | XM_008460995.1 | CGAGAATGGTTAGAGAATACAG | ATTAACAACGCCACATTGC |
| PE | XM_008463183.2 | CGAGGACAAGGAGTAGCA | TCATAGAAGGATCTTCCATAGC |
| trehalase | XM_008439170.2 | CTTGAGCGTCTTCAGGTT | ACAGAGCCATTGGAGGAT |
| XTHs | XM_008467178.2 | ACCAGCCGTTCGTATCAAAGTACC | ACCCACTCCATTGCCTTGTATTGC |
| WRKY | XM_008466930.2 | AAGGTGAACACAATCATCCA | GCTTCCATAATCGGTTTCG |
| NAC | XM_008468514.2 | CGATGTCAGATGGCAATCA | CTCCGAACCGCTTGAATC |
| P5CS | XM_008444288.2 | CATACGAGGATTCTTCTGGTA | ACAAGCCTTCAACATCACTA |
Appendix A.2
| Sample | Raw Data Size (Mp) | Clean Data Size (Mp) | Clean Data Rate (%) | Clean Read Q20 (%) ≥ 90 | Clean Read Q30 (%) ≥ 90 |
|---|---|---|---|---|---|
| GE 0.5 °C-0 d | 1206.83 | 1205.51 | 99.89 | 97.9 (Y) | 91.14 (Y) |
| GE 0.5 °C-12 d | 1206.81 | 1205.52 | 99.89 | 97.8 (Y) | 90.98 (Y) |
| GE 0.5 °C-24 d | 1206.82 | 1204.60 | 99.81 | 97.6 (Y) | 91.01 (Y) |
| JS 0.5 °C-0 d | 1206.83 | 1204.93 | 99.84 | 97.9 (Y) | 91.54 (Y) |
| JS 0.5 °C-12 d | 1206.81 | 1205.48 | 99.88 | 97.9 (Y) | 91.21 (Y) |
| JS 0.5 °C-24 d | 1206.82 | 1204.82 | 99.83 | 97.9 (Y) | 92.02 (Y) |
Appendix A.3
References
- Liu, D.-K.; Xu, C.-C.; Guo, C.-X.; Zhang, X.-X. Sub-Zero temperature preservation of fruits and vegetables: A review. J. Food Eng. 2020, 275, 109881. [Google Scholar] [CrossRef]
- Rohloff, J.; Kopka, J.; Erban, A.; Winge, P.; Wilson, R.C.; Bones, A.M.; Davik, J.; Randall, S.K.; Alsheikh, M.K. Metabolite profiling reveals novel multi-level cold responses in the diploid model Fragaria vesca (woodland strawberry). Phytochemistry 2012, 77, 99–109. [Google Scholar] [CrossRef]
- Saltveit, M.E.; Hepler, P.K. Effect of heat shock on the chilling sensitivity of trichomes and petioles of African violet (Saintpaulia ionantha). Physiol. Plant. 2004, 121, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Zuo, J.-H.; Qing, W.; Dong, H.-Z.; Gao, L.-P. Salicylic acid alleviates postharvest chilling injury of sponge gourd (Luffa cylindrica). J. Integr. Agric. 2017, 16, 735–741. [Google Scholar] [CrossRef]
- Singh, S.; Singh, Z.; Swinny, E. Postharvest nitric oxide fumigation delays fruit ripening and alleviates chilling injury during cold storage of Japanese plums (Prunus salicina Lindell). Postharvest Biol. Technol. 2009, 53, 101–108. [Google Scholar] [CrossRef]
- Fernández-Trujillo, J.P.; Obando-Ulloa, J.M.; Martínez, J.A.; Moreno, E.; García-Mas, J.; Monforte, A.J. Climacteric and non-climacteric behavior in melon fruit: 2. Linking climacteric pattern and main postharvest disorders and decay in a set of near-isogenic lines. Postharvest Biol. Technol. 2008, 50, 125–134. [Google Scholar] [CrossRef]
- Saltveit, M.E. The rate of ion leakage from chilling-sensitive tissue does not immediately increase upon exposure to chilling temperatures. Postharvest Biol. Technol. 2002, 26, 295–304. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS homeostasis in abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Devi, D.D.; Shanker, A.K.; Sheeba, J.A.; Bangarusamy, U. Selenium—An antioxidative protectant in soybean during senescence. Plant Soil 2005, 272, 77–86. [Google Scholar] [CrossRef]
- Orabi, S.A.; Abou-Hussein, S. Antioxidant defense mechanisms enhance oxidative stress tolerance in plants. A review. Curr. Sci. Int. 2019, 8, 565–576. [Google Scholar]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef]
- Xin, Z.; Browse, J. Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant. Proc. Natl. Acad. Sci. USA 1998, 95, 7799–7804. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Wang, X.; Li, H.; Lei, Y.; Wang, X.; Wang, F.; Xu, W.; Wang, J. Proline Metabolism of Different Varieties of Hami Melon Fruits in Response to Low Temperature. Food Sci. 2024, 45, 216–224. [Google Scholar] [CrossRef]
- Bokhary, S.U.F.; Wang, L.; Zheng, Y.; Jin, P. Pre-Storage hot water treatment enhances chilling tolerance of zucchini (Cucurbita pepo L.) squash by regulating arginine metabolism. Postharvest Biol. Technol. 2020, 166, 111229. [Google Scholar] [CrossRef]
- Chen, G.; Kuang, X.; Fan, Z.; Chen, Y.; Lin, Y.; Wang, H.; Chen, Y.; Lin, H. Energy and proline metabolisms participate in the occurrence of chilling injury in cold-stored Chinese olive fruit. Postharvest Biol. Technol. 2024, 214, 112955. [Google Scholar] [CrossRef]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 1994, 6, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shan, C.; Song, W.; Cai, W.; Zhou, F.; Ning, M.; Tang, F. Transcriptome analysis of starch and sucrose metabolism change in Gold Queen Hami melons under different storage temperatures. Postharvest Biol. Technol. 2021, 174, 111445. [Google Scholar] [CrossRef]
- Carvajal, F.; Rosales, R.; Palma, F.; Manzano, S.; Cañizares, J.; Jamilena, M.; Garrido, D. Transcriptomic changes in Cucurbita pepo fruit after cold storage: Differential response between two cultivars contrasting in chilling sensitivity. BMC Genom. 2018, 19, 125. [Google Scholar] [CrossRef]
- Bai, C.; Wu, C.; Ma, L.; Fu, A.; Zheng, Y.; Han, J.; Li, C.; Yuan, S.; Zheng, S.; Gao, L.; et al. Transcriptomics and metabolomics analyses provide insights into postharvest ripening and senescence of tomato fruit under low temperature. Hortic. Plant J. 2023, 9, 109–121. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Jia, K.; Liao, K.; Liu, L.; Fan, G.; Zhang, S.; Wang, Y. Metabolomic and transcriptomice analyses of flavonoid biosynthesis in apricot fruits. Front. Plant Sci. 2023, 14, 1210309. [Google Scholar] [CrossRef]
- Feng, C.; Chen, M.; Xu, C.-J.; Bai, L.; Yin, X.-R.; Li, X.; Allan, A.C.; Ferguson, I.B.; Chen, K.-S. Transcriptomic analysis of Chinese bayberry (Myrica rubra) fruit development and ripening using RNA-Seq. BMC Genom. 2012, 13, 19. [Google Scholar] [CrossRef]
- Zhang, F.; Ji, S.; Wei, B.; Cheng, S.; Wang, Y.; Hao, J.; Wang, S.; Zhou, Q. Transcriptome analysis of postharvest blueberries (Vaccinium corymbosum ‘Duke’) in response to cold stress. BMC Plant Biol. 2020, 20, 80. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, K.; Chen, W.; Li, X.; Zhu, X. Transcriptomic and metabolomic analyses reveal key factors regulating chilling stress-induced softening disorder in papaya fruit. Postharvest Biol. Technol. 2023, 205, 112534. [Google Scholar] [CrossRef]
- Ning, M.; Tang, F.; Chen, J.; Song, W.; Cai, W.; Zhang, Q.; Zhao, X.; Yang, X.; Shan, C.; Hao, G. Low-Temperature adaptation and preservation revealed by changes in physiological–biochemical characteristics and proteome expression patterns in post-harvest Hami melon during cold storage. Planta 2022, 255, 91. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Cohen, E.; Shapiro, B.; Shalom, Y.; Klein, J. Water loss: A nondestructive indicator of enhanced cell membrane permeability of chilling-injured Citrus fruit. J. Am. Soc. Hortic. Sci. 1994, 119, 983–986. [Google Scholar] [CrossRef]
- Song, W.; Tang, F.; Cai, W.; Zhang, Q.; Zhou, F.; Ning, M.; Tian, H.; Shan, C. iTRAQ-based quantitative proteomics analysis of cantaloupe (Cucumis melo var. saccharinus) after cold storage. BMC Genom. 2020, 21, 390. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline biosynthesis and osmoregulation in plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Deuschle, K.; Funck, D.; Forlani, G.; Stransky, H.; Biehl, A.; Leister, D.; van der Graaff, E.; Kunze, R.; Frommer, W.B. The role of Δ1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell 2004, 16, 3413–3425. [Google Scholar] [CrossRef] [PubMed]
- Kishor, P.B.K.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Yang, S.-L.; Lan, S.-S.; Gong, M. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J. Plant Physiol. 2009, 166, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Z.; Du, R.; Liu, Y.; Ying, T.; Mao, L. Effect of nitric oxide on antioxidative response and proline metabolism in banana during cold storage. J. Agric. Food Chem. 2013, 61, 8880–8887. [Google Scholar] [CrossRef]
- Zuo, X.; Cao, S.; Zhang, M.; Cheng, Z.; Cao, T.; Jin, P.; Zheng, Y. High relative humidity (HRH) storage alleviates chilling injury of zucchini fruit by promoting the accumulation of proline and ABA. Postharvest Biol. Technol. 2021, 171, 111344. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Tran, G.-B.; Nguyen, C.T. Anti-Oxidative effects of superoxide dismutase 3 on inflammatory diseases. J. Mol. Med. 2020, 98, 59–69. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Li, M.; Fu, X.; Zhao, X.; Min, D.; Li, F.; Li, X.; Zhang, X. Hot air pretreatment alleviates browning of fresh-cut pitaya fruit by regulating phenylpropanoid pathway and ascorbate-glutathione cycle. Postharvest Biol. Technol. 2022, 190, 111954. [Google Scholar] [CrossRef]
- Li, Z.; Min, D.; Fu, X.; Zhao, X.; Wang, J.; Zhang, X.; Li, F.; Li, X. The roles of SlMYC2 in regulating ascorbate-glutathione cycle mediated by methyl jasmonate in postharvest tomato fruits under cold stress. Sci. Hortic. 2021, 288, 110406. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Li, B.; Chen, T.; Tian, S. Reactive oxygen species: A generalist in regulating development and pathogenicity of phytopathogenic fungi. Comput. Struct. Biotechnol. J. 2020, 18, 3344–3349. [Google Scholar] [CrossRef]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Wei, B.; Ji, S. Exogenous glutathione alleviates chilling injury in postharvest bell pepper by modulating the ascorbate-glutathione (AsA-GSH) cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef]
- Székelyg, G.; Ábrahám, E.; Cseplo, A.; Rigó, G.; Zsigmond, L.; Csiszar, J.; Ayaydin, F.; Strizhov, N.; Jasik, J.; Schmelzer, E. DuplicatedP5CSgenes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008, 53, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.D.; Kasaikina, O.T. Bio-Antioxidants—A chemical base of their antioxidant activity and beneficial effect on human health. Curr. Med. Chem. 2013, 20, 4784–4805. [Google Scholar] [CrossRef]
- Zhang, D.; Ren, L.; Chen, G.-Q.; Zhang, J.; Reed, B.M.; Shen, X.-H. ROS-Induced oxidative stress and apoptosis-like event directly affect the cell viability of cryopreserved embryogenic callus in Agapanthus praecox. Plant Cell Rep. 2015, 34, 1499–1513. [Google Scholar] [CrossRef]
- Martinez-Tellez, M.; Lafuente, M. Effect of high temperature conditioning on ethylene, phenylalanine ammonia-lyase, peroxidase and polyphenol oxidase activities in flavedo of chilled‹ Fortune› mandarin fruit. J. Plant Physiol. 1997, 150, 674–678. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Tsantili, E. Participation of phenylalanine ammonia-lyase (PAL) in increased phenolic compounds in fresh cold stressed walnut (Juglans regia L.) kernels. Postharvest Biol. Technol. 2015, 104, 17–25. [Google Scholar] [CrossRef]
- Chen, J.-Y.; He, L.-H.; Jiang, Y.-M.; Wang, Y.; Joyce, D.C.; Ji, Z.-L.; Lu, W.-J. Role of phenylalanine ammonia-lyase in heat pretreatment-induced chilling tolerance in banana fruit. Physiol. Plant. 2008, 132, 318–328. [Google Scholar] [CrossRef]
- Chen, B.; Feder, M.E.; Kang, L. Evolution of heat-shock protein expression underlying adaptive responses to environmental stress. Mol. Ecol. 2018, 27, 3040–3054. [Google Scholar] [CrossRef]
- Lin, Q.; Xie, Y.; Guan, W.; Duan, Y.; Wang, Z.; Sun, C. Combined transcriptomic and proteomic analysis of cold stress induced sugar accumulation and heat shock proteins expression during postharvest potato tuber storage. Food Chem. 2019, 297, 124991. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, Y.; Wang, L.; Hou, Y.; Bao, Y.; Jia, Z.; Zheng, Y.; Jin, P. Hot water treatment improves peach fruit cold resistance through PpHSFA4c-mediated HSF-HSP and ROS pathways. Postharvest Biol. Technol. 2023, 199, 112272. [Google Scholar] [CrossRef]
- Enoki, Y.; Sakurai, H. Diversity in DNA recognition by heat shock transcription factors (HSFs) from model organisms. FEBS Lett. 2011, 585, 1293–1298. [Google Scholar] [CrossRef]
- Majee, A.; Kumari, D.; Sane, V.A.; Singh, R.K. Novel roles of HSFs and HSPs, other than relating to heat stress, in temperature-mediated flowering. Ann. Bot. 2023, 132, 1103–1106. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Xu, F.; Song, C.; Yang, X.; Zhang, Z.; Yi, M.; Ma, N.; Zhou, X.; He, J. Small HSPs play an important role in crosstalk between HSF-HSP and ROS pathways in heat stress response through transcriptomic analysis in lilies (Lilium longiflorum). BMC Plant Biol. 2022, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Yuanyuan, M.; Yali, Z.; Jiang, L.; Hongbo, S. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8. [Google Scholar] [CrossRef]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Y.; Chen, Y.; Jiang, S.; Xu, F.; Wang, H.; Shao, X. NMR revealed that trehalose enhances sucrose accumulation and alleviates chilling injury in peach fruit. Sci. Hortic. 2022, 303, 111190. [Google Scholar] [CrossRef]
- Liu, T.; Shi, J.; Li, M.; Ye, X.; Qi, H. Trehalose triggers hydrogen peroxide and nitric oxide to participate in melon seedlings oxidative stress tolerance under cold stress. Environ. Exp. Bot. 2021, 184, 104379. [Google Scholar] [CrossRef]
- Brummell, D.A.; Dal Cin, V.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef]
- Sobkowiak, A.; Jończyk, M.; Adamczyk, J.; Szczepanik, J.; Solecka, D.; Kuciara, I.; Hetmańczyk, K.; Trzcinska-Danielewicz, J.; Grzybowski, M.; Skoneczny, M. Molecular foundations of chilling-tolerance of modern maize. BMC Genom. 2016, 17, 125. [Google Scholar] [CrossRef]
- Yamada, T.; Kuroda, K.; Jitsuyama, Y.; Takezawa, D.; Arakawa, K.; Fujikawa, S. Roles of the plasma membrane and the cell wall in the responses of plant cells to freezing. Planta 2002, 215, 770–778. [Google Scholar] [CrossRef]
- Lampugnani, E.R.; Khan, G.A.; Somssich, M.; Persson, S. Building a plant cell wall at a glance. J. Cell Sci. 2018, 131, jcs207373. [Google Scholar] [CrossRef]
- Mizutani, M.; Ohta, D. Diversification of P450 genes during land plant evolution. Annu. Rev. Plant Biol. 2010, 61, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.; Jugulam, M. Role of cytochrome P450 enzymes in plant stress response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Bilodeau, P.; Udvardi, M.; Peacock, W.; Dennis, E. A prolonged cold treatment-induced cytochrome P450 gene from Arabidopsis thaliana. Plant Cell Environ. 1999, 22, 791–800. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, Y.; Gou, M.; Liu, C.-J. Tissue-Preferential recruitment of electron transfer chains for cytochrome P450-catalyzed phenolic biosynthesis. Sci. Adv. 2023, 9, eade4389. [Google Scholar] [CrossRef]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biol. 2005, 6, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ban, Q.; Li, H.; Hou, Y.; Jin, M.; Han, S.; Rao, J. DkXTH8, a novel xyloglucan endotransglucosylase/hydrolase in persimmon, alters cell wall structure and promotes leaf senescence and fruit postharvest softening. Sci. Rep. 2016, 6, 39155. [Google Scholar] [CrossRef]
- Carvajal, F.; Palma, F.; Jamilena, M.; Garrido, D. Cell wall metabolism and chilling injury during postharvest cold storage in zucchini fruit. Postharvest Biol. Technol. 2015, 108, 68–77. [Google Scholar] [CrossRef]
- Wan, D.; Wan, Y.; Hou, X.; Ren, W.; Ding, Y.; Sa, R. De novo assembly and transcriptomic profiling of the grazing response in Stipa grandis. PLoS ONE 2015, 10, e0122641. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, L.; Wong, D.C.J.; Wang, Y.; Zhu, Z.; Xu, G.; Wang, Q.; Li, S.; Liang, Z.; Xin, H. The ethylene response factor VaERF092 from Amur grape regulates the transcription factor VaWRKY33, improving cold tolerance. Plant J. 2019, 99, 988–1002. [Google Scholar] [CrossRef]
- Hu, C.; Wang, M.; Zhu, C.; Wu, S.; Li, J.; Yu, J.; Hu, Z. A transcriptional regulation of ERF15 contributes to ABA-mediated cold tolerance in tomato. Plant Cell Environ. 2024, 47, 1334–1347. [Google Scholar] [CrossRef]
- Qu, J.; Xiao, P.; Zhao, Z.-Q.; Wang, Y.-L.; Zeng, Y.-K.; Zeng, X.; Liu, J.-H. Genome-wide identification, expression analysis of WRKY transcription factors in Citrus ichangensis and functional validation of CiWRKY31 in response to cold stress. BMC Plant Biol. 2024, 24, 617. [Google Scholar] [CrossRef]
- Jin, C.; Li, K.-Q.; Xu, X.-Y.; Zhang, H.-P.; Chen, H.-X.; Chen, Y.-H.; Hao, J.; Wang, Y.; Huang, X.-S.; Zhang, S.-L. A novel NAC transcription factor, PbeNAC1, of Pyrus betulifolia confers cold and drought tolerance via interacting with PbeDREBs and activating the expression of stress-responsive genes. Front. Plant Sci. 2017, 8, 1049. [Google Scholar] [CrossRef]
- Yin, Q.; Qin, W.; Zhou, Z.; Wu, A.M.; Deng, W.; Li, Z.; Shan, W.; Chen, J.Y.; Kuang, J.F.; Lu, W.J. Banana MaNAC1 activates secondary cell wall cellulose biosynthesis to enhance chilling resistance in fruit. Plant Biotechnol. J. 2024, 22, 413–426. [Google Scholar] [CrossRef]
- Ning, M.; Tang, F.; Zhang, Q.; Zhao, X.; Yang, L.; Cai, W.; Shan, C. The quality of Gold Queen Hami melons stored under different temperatures. Sci. Hortic. 2019, 243, 140–147. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: II. Role of electron transfer. Arch. Biochem. Biophys. 1968, 125, 850–857. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Audic, S.; Claverie, J.-M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef]
- Kim, K.I.; van de Wiel, M.A. Effects of dependence in high-dimensional multiple testing problems. BMC Bioinform. 2008, 9, 114. [Google Scholar] [CrossRef]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T. KEGG for linking genomes to life and the environment. Nucleic acids Res. 2007, 36, D480–D484. [Google Scholar] [CrossRef]




| Gene ID | Gene Name | Fold Change | |||
|---|---|---|---|---|---|
| GE 0.5 °C | JS 0.5 °C | ||||
| 0 vs. 12 d | 0 vs. 24 d | 0 vs. 12 d | 0 vs. 24 d | ||
| Oxidoreductase activity | |||||
| XM_008442029.1 | peroxidase | 9.000 | 13.454 | 16.336 | 17.877 |
| XM_008460995.1 | peroxidase | 0.067 | 0.0112 | 0.025 | 0.006 |
| XM_008467715.2 | peroxidase | 10.196 | 21.407 | 11.551 | 15.562 |
| XM_008441186.2 | GAPD | 9.254 | 10.556 | 5.657 | 8.225 |
| XM_008454735.2 | CATisozyme | 46.527 | 243.8753 | 97.681 | 1136.199 |
| XM_008452678.2 | PAL | 0.139 | 0.102 | 0.178 | 0.129 |
| XM_008442056.2 | HsF | 7.726 | 12.188 | 7.829 | 33.612 |
| XM_008441060.2 | HsP | 11.820 | 22.290 | 44.575 | 104.598 |
| XM_008444288.2 | P5CS | 10.778 | 20.252 | 48.168 | 71.506 |
| Carbohydrate metabolic process | |||||
| XM_008441186.2 | GAPDH | 9.254 | 10.556 | 5.657 | 8.225 |
| XM_008439170.2 | trehalase | 8.456 | 5.063 | 5.242 | 10.339 |
| XM_008448052.2 | GAUT | 5.540 | 14.621 | 4.595 | 13.929 |
| XM_008463183.2 | PE | 53.817 | 333.144 | 36.504 | 142.025 |
| Cell Wall metabolism | |||||
| XM_008439950.2 | XTHs | 29.041 | 51.268 | 11.472 | 6.821 |
| XM_008443187.2 | XTHs | 32.223 | 106.153 | 22.814 | 39.947 |
| XM_008466543.2 | CYP450 | 21.259 | 157.586 | 93.054 | 1217.748 |
| XM_017044655.1 | CYP450 | 0.187 | 0.058 | 0.199 | 0.164 |
| XM_017044466.1 | CYP450 | 0.189 | 0.092 | 0.243 | 0.139 |
| XM_008439072.2 | CYP450 | 86.823 | 23.918 | 165.421 | 398.932 |
| XM_008441679.2 | NAD(P)H- ubiquinone oxidoreductase | 9.849 | 18.001 | 24.933 | 48.5023 |
| Regulation of transcription | |||||
| XM_008442055.2 | ERF109 | 187.403 | 286.026 | 276.282 | 1595.729 |
| NM_001319315.1 | ERF071 | 18.000 | 20.112 | 5.134 | 8.574 |
| XM_008457109.2 | ERF054 | 10.853 | 23.752 | 33.128 | 24.933 |
| XM_008457900.2 | ERF109 | 36.002 | 46.851 | 37.271 | 155.417 |
| XM_008466930.2 | WRKY 40 | 24.084 | 105.420 | 24.420 | 117.148 |
| XM_008468514.2 | NAC 2 | 7.516 | 10.928 | 23.264 | 84.449 |
| XM_008456444.2 | NAC 72 | 3.706 | 4.627 | 5.502 | 6.543 |
| XM_008450150.2 | MYB 48 | 4.028 | 13.737 | 12.553 | 21.706 |
| XM_017046406.1 | MYB 44 | 88.035 | 192.672 | 3061.451 | 11,910.943 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, W.; Xiao, L.; Hao, X.; Shan, C.; Zhou, Z.; Ning, M.; Tang, F. Physiological and Transcriptomic Analysis of Two Types of Hami Melons in Low-Temperature Storage. Plants 2025, 14, 1153. https://doi.org/10.3390/plants14081153
Liao W, Xiao L, Hao X, Shan C, Zhou Z, Ning M, Tang F. Physiological and Transcriptomic Analysis of Two Types of Hami Melons in Low-Temperature Storage. Plants. 2025; 14(8):1153. https://doi.org/10.3390/plants14081153
Chicago/Turabian StyleLiao, Wanqin, Linlu Xiao, Xiangshuai Hao, Chunhui Shan, Zhongkai Zhou, Ming Ning, and Fengxian Tang. 2025. "Physiological and Transcriptomic Analysis of Two Types of Hami Melons in Low-Temperature Storage" Plants 14, no. 8: 1153. https://doi.org/10.3390/plants14081153
APA StyleLiao, W., Xiao, L., Hao, X., Shan, C., Zhou, Z., Ning, M., & Tang, F. (2025). Physiological and Transcriptomic Analysis of Two Types of Hami Melons in Low-Temperature Storage. Plants, 14(8), 1153. https://doi.org/10.3390/plants14081153






