Different Leaf Strategies Between Lithophytic and Terrestrial Orchids in a Subtropical Karst Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Materials
2.2. Photosynthetic Parameters
2.3. Leaf Structural, Water-Related Traits, and Nitrogen Content
2.4. Leaf Anatomical Traits
2.5. Leaf Punch Force
2.6. Calculation of Leaf Nitrogen Allocated to Photosynthetic Apparatus
2.7. Statistical Analyses
3. Results
3.1. Differences in Leaf Functional Traits Between Lithophytic and Terrestrial Orchids
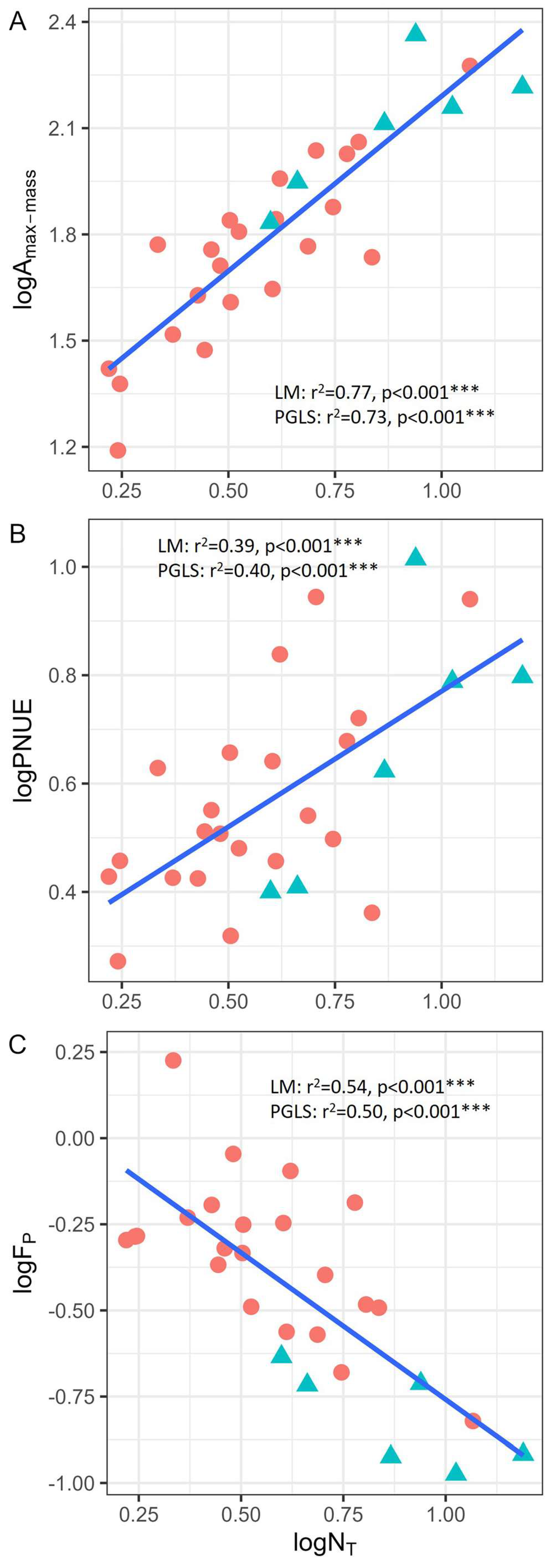
3.2. Correlations Between Leaf Nitrogen Allocation, Photosynthetic Characteristics, and Biomechanical Traits
3.3. Correlations Between Leaf Saturated Water Content and Photosynthesis, Biomechanical Strength, and Leaf Structure
4. Discussion
4.1. Differences in Leaf Traits Between Lithophytic and Terrestrial Orchids
4.2. Correlations Among Photosynthesis, Nitrogen Allocation, and Biomechanical Traits
4.3. Correlation Between SWC and Leaf Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Amax-mass | Mass-standardized maximum photosynthetic rate |
| Jmax | Maximum electron transport rate |
| Vcmax | Maximum carboxylation rate |
| Chl | Leaf chlorophyll content |
| Chla/b | The ratio of Chla to Chlb |
| CT | Upper cuticle thickness |
| FP | Leaf punch force |
| gs | Stomatal conductance |
| LMA | Leaf mass per area |
| LDMC | Leaf dry mass content |
| LD | Leaf density |
| LT | Leaf thickness |
| Nm | Leaf nitrogen content |
| NT | Total nitrogen content in photosynthetic apparatus |
| PNUE | Photosynthetic nitrogen-use efficiency |
| SD | Stomatal density |
| SWC | Leaf saturated water content |
| WUE | Water-use efficiency |
References
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Roth-Nebelsick, A.; Krause, M. The Plant Leaf: A biomimetic resource for multifunctional and economic design. Biomimetics 2023, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- Haynes, A.F. What do we know about parasitic plants and the leaf economic spectrum? J. Plant Ecol. 2022, 15, 691–699. [Google Scholar] [CrossRef]
- Onoda, Y.; Wright, I.; Evans, J.; Hikosaka, K.; Kitajima, K.; Niinemets, Ü.; Poorter, H.; Tosens, T.; Westoby, M. Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol. 2017, 214, 1447–1463. [Google Scholar] [CrossRef]
- He, P.; Wright, I.J.; Zhu, S.; Onoda, Y.; Liu, H.; Li, R.; Liu, X.; Hua, L.; Oyanoghafo, O.O.; Ye, Q. Leaf mechanical strength and photosynthetic capacity vary independently across 57 subtropical forest species with contrasting light requirements. New Phytol. 2019, 223, 607–618. [Google Scholar] [CrossRef]
- Reich, P.B.; Flores-Moreno, H. Peeking beneath the hood of the leaf economics spectrum. New Phytol. 2017, 214, 1395–1397. [Google Scholar] [CrossRef]
- Nadal, M.; Clemente-Moreno, M.J.; Perera-Castro, A.V.; Roig-Oliver, M.; Onoda, Y.; Gulías, J.; Flexas, J. Incorporating pressure-volume traits into the leaf economics spectrum. Ecol. Lett. 2023, 26, 549–562. [Google Scholar] [CrossRef]
- Martinez-Vilalta, J.; Anderegg, W.R.L.; Sapes, G.; Sala, A. Greater focus on water pools may improve our ability to understand and anticipate drought-induced mortality in plants. New Phytol. 2019, 223, 22–32. [Google Scholar] [CrossRef]
- Schwinning, S. The water relations of two evergreen tree species in a karst savanna. Oecologia 2008, 158, 373–383. [Google Scholar] [CrossRef]
- Guo, B.; Zang, W.Q.; Luo, W. Spatial-temporal shifts of ecological vulnerability of karst mountain ecosystem: Impacts of global change and anthropogenic interference. Sci. Total Environ. 2020, 741, 140256. [Google Scholar] [CrossRef]
- Li, Q.; Cao, J.; Yu, L. Biogeochemical effect of karst soil on the element contents in the leaves of two species of Flos Lonicerae. Plant Soil Environ. 2008, 54, 486–492. [Google Scholar] [CrossRef]
- Liu, H.; Feng, C.-L.; Chen, B.-S.; Wang, Z.-S.; Xie, X.-Q.; Deng, Z.-H.; Wei, X.-L.; Liu, S.-Y.; Zhang, Z.-B.; Luo, Y.-B. Overcoming extreme weather challenges: Successful but variable assisted colonization of wild orchids in southwestern China. Biol. Conserv. 2012, 150, 68–75. [Google Scholar] [CrossRef]
- Cao, K.F.; Fu, P.L.; Chen, Y.J.; Jiang, Y.J.; Zhu, S.D. Implications of the ecophysiological adaptation of plants on tropical karst habitats for the ecological restoration of desertified rocky lands in southern China. Sci. Sin. Vitae 2014, 44, 238–247. [Google Scholar] [CrossRef]
- Cribb, P.J.; Kell, S.P.; Dixon, K.W.; Barrett, R.L. Orchid conservation: A global perspective. In Orchid Conservation; Dixon, K.W., Kell, S.P., Barrett, R.L., Cribb, P.J., Eds.; Natural History Publications: Kota Kinabalu, Malaysia, 2003; pp. 1–24. [Google Scholar]
- Cheng, X.; Li, S.; Hu, H.; Wu, T. Leaf traits of orchids in relation to life form in Karst area of southwestern China. J. Plant Classif. Resour. 2011, 33, 595–604. [Google Scholar]
- Zhang, S.; Yang, Y.; Li, J.; Qin, J.; Zhang, W.; Huang, W.; Hu, H. Physiological diversity of orchids. Plant Divers. 2018, 40, 196–208. [Google Scholar] [CrossRef]
- Hietz, P.; Wagner, K.; Ramos, F.N.; Cabral, J.S.; Agudelo, C.; Benavides, A.M.; Cach-Pérez, M.J.; Cardelús, C.L.; Galván, N.C.; da Costa, L.E.N.; et al. Putting vascular epiphytes on the traits map. J. Ecol. 2022, 110, 340–358. [Google Scholar] [CrossRef]
- Wang, K. Study on Leaf Functional Traits of Orchidaceae in Beipan River Karst Area. Master Thesis, Guizhou University, Guiyang, China, 2023. [Google Scholar]
- Wang, M.; Jiang, C.; Sun, O.J. Spatially differentiated changes in regional climate and underlying drivers in southwestern China. J. For. Res. 2022, 33, 755–765. [Google Scholar] [CrossRef]
- Wang, C.X.; Chen, C.; Zhang, S.Q.; Ma, Z.F.; Peng, Y.M. Variation characteristics of extreme climate events in Southwest China from 1961 to 2017. Heliyon 2023, 9, e19648. [Google Scholar] [CrossRef]
- Geekiyanage, N.; Goodale, U.M.; Cao, K.F.; Kitajima, K. Plant ecology of tropical and subtropical karst ecosystems. Biotropica 2019, 51, 626–640. [Google Scholar] [CrossRef]
- Deng, H. The study of Crassulacean Acid Metabolism (CAM) Photosynthesis in Orchids. Ph. D. Thesis, Chinese Academy of Forestry Sciences, Beijing, China, 2015. [Google Scholar]
- Thornley, J.H.M. Mathematical Models in Plant Physiology; Academic Press: London, UK, 1976. [Google Scholar]
- Farquhar, G.D.; von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef]
- Yang, Z.H.; Huang, W.; Yang, Q.Y.; Chang, W.; Zhang, S.B. Anatomical and diffusional determinants inside leaves explain the difference in photosynthetic capacity between Cypripedium and Paphiopedilum, Orchidaceae. Photosynth. Res. 2018, 136, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Onoda, Y.; Westoby, M.; Adler, P.B.; Choong, A.M.F.; Clissold, F.J.; Cornelissen, J.H.C.; Díaz, S.; Dominy, N.J.; Elgart, A.; Enrico, L.; et al. Global patterns of leaf mechanical properties. Ecol. Lett. 2011, 14, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Niinemets, Ü.; Tenhunen, J.D. A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ. 1997, 20, 845–866. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Yang, X.; Wu, X.; Wang, Z.; Zhao, Y.; Lin, C.; Yu, S.; Wang, G.; Zhou, H. Effect of the odour compound from Periploca sepium Bunge on the physiological and biochemical indices, photosynthesis and ultrastructure of the leaves of Humulus scandens (Lour.) Merr. Ecotox. Environ. Safe. 2022, 238, 113556. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Díaz-Espejo, A.; Flexas, J.; Galmés, J.; Warren, C.R. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J. Exp. Bot. 2009, 8, 2249–2270. [Google Scholar] [CrossRef]
- Boughalleb, F.; Hajlaoui, H. Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiol. Plant. 2011, 33, 53–65. [Google Scholar] [CrossRef]
- Galmés, J.; Ochogavía, J.M.; Gago, J.; Roldán, E.J.; Cifre, J.; Conesa, M.À. Leaf responses to drought stress in Mediterranean accessions of Solanum lycopersicum: Anatomical adaptations in relation to gas exchange parameters. Plant Cell Environ. 2013, 36, 920–935. [Google Scholar] [CrossRef]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef]
- Nogués, S.; Baker, N.R. Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J. Exp. Bot. 2000, 51, 1309–1317. [Google Scholar] [CrossRef]
- Saito, T.; Soga, K.; Hoson, T.; Terashima, I. The bulk elastic modulus and the reversible properties of cell walls in developing Quercus leaves. Plant Cell Physiol. 2006, 47, 715–725. [Google Scholar] [CrossRef]
- Sun, S.W.; Zhang, Y.J.; Cao, K.F. Correlations among leaf structure, drought tolerance and photosynthetic capacity in saplings of Euphorbiaceae from different micro-habitats in a seasonal tropical rainforest. Chin. J. Plant Ecol. 2014, 38, 311–324. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology (Fifth Edition); Sinauer Associates: Sunderland, UK, 2015. [Google Scholar]
- Niinemets, Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Yang, S.J.; Sun, M.; Yang, Q.Y.; Ma, R.Y.; Zhang, J.L.; Zhang, S.B. Two strategies by epiphytic orchids for maintaining water balance: Thick cuticles in leaves and water storage in pseudobulbs. AoB Plants 2016, 8, plw046. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.-L.; Zhu, S.-D.; Zhang, J.-L.; Finnegan, P.M.; Jiang, Y.-J.; Lin, H.; Fan, Z.-X.; Cao, K.-F. The contrasting leaf functional traits between a karst forest and a nearby non-karst forest in south-west China. Funct. Plant Biol. 2019, 46, 907–915. [Google Scholar] [CrossRef]
- Chang, W.; Zhang, S.B.; Li, S.Y.; Hu, H. Ecophysiological significance of leaf traits in Cypripedium and Paphiopedilum. Physiol. Plant. 2011, 141, 30–39. [Google Scholar] [CrossRef]
- Kume, A.; Akitsu, T.; Nasahara, K.N. Why is chlorophyll b only used in light-harvesting systems? J. Plant Res. 2018, 131, 961–972. [Google Scholar] [CrossRef]
- Wan, C.Y.; Yu, J.R.; Zhu, S.D. Differences in leaf traits and trait correlation networks between karst and non-karst forest tree species. Chin. J. Plant Ecol. 2023, 47, 1386–1397. [Google Scholar] [CrossRef]
- Takashima, T.; Hikosaka, K.; Hirose, T. Photosynthesis or persistence: Nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ. 2004, 27, 1047–1054. [Google Scholar] [CrossRef]
- Feng, Y.L. Nitrogen allocation and partitioning in invasive and native Eupatorium species. Physiol. Plant. 2008, 132, 350–358. [Google Scholar] [CrossRef]
- Shi, Z.M.; Tang, J.C.; Cheng, R.M.; Luo, D.; Liu, S.R. A review of nitrogen allocation in leaves and factors in its effects. Acta Ecol. Sin. 2015, 35, 5909–5919. [Google Scholar] [CrossRef]
- Lei, Z.-Y.; Wang, H.; Wright, I.J.; Zhu, X.-G.; Niinemets, Ü.; Li, Z.-L.; Sun, D.-S.; Dong, N.; Zhang, W.-F.; Zhou, Z.-L.; et al. Enhanced photosynthetic nitrogen use efficiency and increased nitrogen allocation to photosynthetic machinery under cotton domestication. Photosynth. Res. 2021, 150, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Evens, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Evens, J.R.; Seemann, J.R. The allocation of protein nitrogen in the photosynthetic apparatus: Costs, consequences, and control. Plant Biology 1989, 8, 183–205. [Google Scholar]
- Onoda, Y.; Hikosaka, K.; Hirose, T. Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct. Ecol. 2004, 18, 419–425. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, K.K.; Bi, J.G.; Yu, X.Q.; Luo, L.J.; Hu, L.Y. Mesophyll conductance and N allocation co-explained the variation in photosynthesis in two canola genotypes under contrasting nitrogen supply. Front. Plant Sci. 2023, 14, 1171331. [Google Scholar] [CrossRef]
- Hikosaka, K. Interspecific difference in the photosynthesis-nitrogen relationship: Patterns, physiological causes, and ecological importance. J. Plant Res. 2004, 117, 481–494. [Google Scholar] [CrossRef]
- Henley, W.J.; Levavasseur, G.; Franklin, L.A.; Osmond, C.B.; Ramus, J. Photoacclimation and photoinhibition in Ulva rotundata as influenced by nitrogen availability. Planta 1991, 184, 235–243. [Google Scholar] [CrossRef]
- He, N.; Li, Y.; Liu, C.; Xu, L.; Li, M.; Zhang, J.; He, J.; Tang, Z.; Han, X.; Ye, Q.; et al. Plant trait networks: Improved resolution of the dimensionality of adaptation. Trends Ecol. Evol. 2020, 35, 908–918. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Huang, H.; Wang, H.; Peñuelas, J.; Sardans, J.; Niinemets, Ü. Leaf water content contributes to global leaf trait relationships. Nat. Commun. 2022, 13, 5525. [Google Scholar]
- Blumenthal, D.M.; Mueller, K.E.; Kray, J.A.; Ocheltree, T.W.; Augustine, D.J.; Wilcox, K.R. Traits link drought resistance with herbivore defence and plant economics in semi-arid grasslands: The central roles of phenology and leaf dry matter content. J. Ecol. 2020, 108, 2336–2351. [Google Scholar] [CrossRef]
- Eggli, U.; Nyffeler, R. Living under temporarily arid conditions: Succulence as an adaptive strategy. Bradleya 2009, 27, 13–36. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]

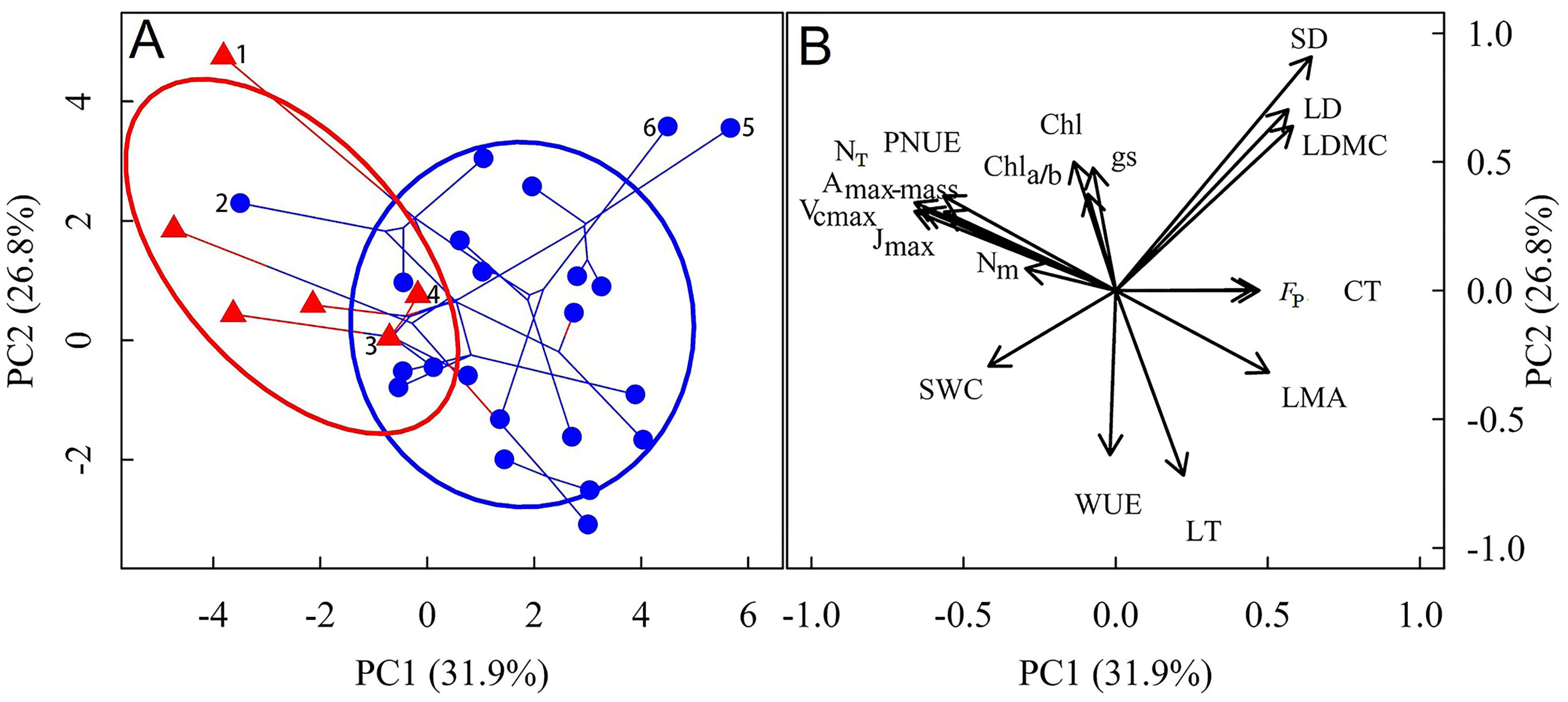

| Variables | Coefficient of Variation | Lithophyte | Terrestrial | p Value |
|---|---|---|---|---|
| Amax-mass (nmol g−1 s−1) | 65.26% | 64.68 ± 8.37 | 137.67 ± 23.61 | 0.0034 |
| SD (No. mm−2) | 50.58% | 59.55 ± 6.43 | 44.84 ± 7.84 | 0.3954 |
| gs (μmol m−2 s−1) | 49.70% | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.8665 |
| PNUE (μmol g−1 s−1) | 51.70% | 3.97 ± 0.41 | 5.34 ± 1.20 | 0.4599 |
| WUE (μmol mol−1) | 20.79% | 96.94 ± 3.72 | 81.56 ± 9.57 | 0.1937 |
| Nm (mg g−1) | 37.68% | 16.16 ± 1.16 | 27.40 ± 1.88 | 0.0002 |
| NT (mg g−1) | 65.53% | 4.07 ± 0.49 | 8.45 ± 1.73 | 0.0080 |
| Chl (mg g−1) | 36.23% | 1.04 ± 0.07 | 1.61 ± 0.17 | 0.0053 |
| Chla/b | 10.50% | 2.69 ± 0.06 | 2.96 ± 0.08 | 0.0386 |
| SWC (g g−1) | 57.56% | 4.53 ± 0.39 | 9.85 ± 1.67 | 0.0034 |
| LMA (g m−2) | 57.47% | 94.47 ± 9.13 | 30.90 ± 4.96 | 0.0002 |
| LDMC (g g−1) | 42.13% | 0.20 ± 0.02 | 0.11 ± 0.02 | 0.0034 |
| CT (μm) | 69.95% | 2.03 ± 0.27 | 0.73 ± 0.05 | 0.0042 |
| LT (μm) | 54.20% | 489.44 ± 53.08 | 286.15 ± 50.44 | 0.0331 |
| LD (g cm−3) | 41.11% | 0.20 ± 0.02 | 0.12 ± 0.02 | 0.0171 |
| FP (kN m−1) | 71.26% | 0.53 ± 0.07 | 0.16 ± 0.02 | 0.0000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Sun, D.; Wang, X.; Zhu, S.; Goodale, U.M. Different Leaf Strategies Between Lithophytic and Terrestrial Orchids in a Subtropical Karst Forest. Plants 2025, 14, 1161. https://doi.org/10.3390/plants14081161
Yang M, Sun D, Wang X, Zhu S, Goodale UM. Different Leaf Strategies Between Lithophytic and Terrestrial Orchids in a Subtropical Karst Forest. Plants. 2025; 14(8):1161. https://doi.org/10.3390/plants14081161
Chicago/Turabian StyleYang, Mei, Dan Sun, Xiaoyin Wang, Shidan Zhu, and Uromi Manage Goodale. 2025. "Different Leaf Strategies Between Lithophytic and Terrestrial Orchids in a Subtropical Karst Forest" Plants 14, no. 8: 1161. https://doi.org/10.3390/plants14081161
APA StyleYang, M., Sun, D., Wang, X., Zhu, S., & Goodale, U. M. (2025). Different Leaf Strategies Between Lithophytic and Terrestrial Orchids in a Subtropical Karst Forest. Plants, 14(8), 1161. https://doi.org/10.3390/plants14081161





