Wind Pollination of Apple Flowers Under Insect Exclusion Nets Questions the Insect-Dependent Pollination Model of Modern Apple Plantations
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Broothaerts, W.; Van Nerum, I.; Keulemans, J. Update on and review of the incompatibility (S-)genotypes of apple cultivars. HortScience 2004, 39, 943–947. [Google Scholar] [CrossRef]
- Yao, J.L.; Dong, Y.H.; Morris, B.A. Parthenocarpic apple fruit production conferred by transposon insertion mutations in a MADS-box transcription factor. Proc. Natl. Acad. Sci. USA 2001, 98, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Janick, J.; Cummins, J.N.; Brown, S.K.; Hemmat, M. Apples. In Fruit Breeding, Volume 1, Tree and Tropical Fruits; John Wiley & Sons: New York, NY, USA, 1996; pp. 1–77. 632p. [Google Scholar]
- Delaplane, K.S.; Mayer, D.F. Crop Pollination by Bees; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- De Witte, K.; Vercammen, J.; Van Daele, G.; Keulemans, J. Fruit set, seed set and fruit weight in apple as influenced by emasculation, self-pollination and cross-pollination. In II Workshop on Pollination 42; ACTA: Leuven, Belgium, 1995; pp. 177–184. Available online: https://www.actahort.org/books/423/423_23.htm (accessed on 8 April 2025).
- Free, J.B.; Spencer-Booth, Y. The effect of distance from pollinizer varieties on the fruit set of apple, pear and sweet-cherry trees. J. Hortic. Sci. 1964, 39, 54–60. [Google Scholar] [CrossRef]
- Free, J.B. Comparison of the importance of insect and wind pollination of apple trees. Nature 1964, 201, 726–727. [Google Scholar] [CrossRef]
- Smith, B.D.; Williams, R.R. Methods of Pollen Transfer at Long Ashton; Annual Report; Long Ashton Research Station: Bristol, UK, 1966; p. 120. [Google Scholar]
- Elsysy, M.; Serra, S.; Schwallier, P.; Musacchi, S.; Einhorn, T. Net enclosure of ‘Honeycrisp’ and ‘Gala’ apple trees at different bloom stages affects fruit set and alters seed production. Agronomy 2019, 9, 478. [Google Scholar] [CrossRef]
- Blitzer, E.J.; Gibbs, J.; Park, M.G.; Danforth, B.N. Pollination services for apple are dependent on diverse wild bee communities. Agric. Ecosyst. Environ. 2016, 221, 1–7. [Google Scholar] [CrossRef]
- Osterman, J.; Theodorou, P.; Radzevičiūtė, R.; Schnitker, P.; Paxton, R.J. Apple pollination is ensured by wild bees when honey bees are drawn away from orchards by a mass co-flowering crop, oilseed rape. Agric. Ecosyst. Environ. 2021, 315, 107383. [Google Scholar] [CrossRef]
- Paudel, Y.P.; Mackereth, R.; Hanley, R.; Qin, W. Honey bees (Apis mellifera L.) and pollination issues: Current status, impacts, and potential drivers of decline. J. Agric. Sci. 2015, 7, 93. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Insect Pollinators Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- IPBES. The Assessment Report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services on Pollinators, Pollination and Food Production; Potts, S.G., Imperatriz-Fonseca, V., Ngo, H.T., Eds.; Secretariat IPBES: Bonn, Germany, 2016; pp. 1–552. [Google Scholar] [CrossRef]
- Ramírez, F.; Davenport, T.L. Apple pollination: A review. Sci. Hortic. 2013, 162, 188–203. [Google Scholar] [CrossRef]
- Burchill, R.T. Airborne Pollen in Apple Orchards; Annual Report 1962; East Malling Research Station, Niab East Malling: West Malling, UK, 1962; p. 109. [Google Scholar]
- Langridge, D.F. Effects of temperature, humidity, and caging on the concentration of fruit pollen in the air. Aust. J. Exp. Agric. Anim. Husb. 1969, 9, 549. [Google Scholar] [CrossRef]
- Fulford, R., XI. Regular and Irregular Bearing in Fruit Plants; Annual Report 1964; East Malling Research Station, Niab East Malling: West Malling, UK, 1964; p. 71. [Google Scholar]
- Janssen, I.; Geissler, S.; Müller, W. Analyse ökologischerAuswirkungen von Land-Und forstwirtschaftlichenNutzpflanzen und Eingeführten Standortfremden Pflanzen; Bericht des Österreichischen Ökologischen Instituts: Wien, Austria, 1995. [Google Scholar]
- Reim, S.; Flachowsky, H.; Michael, M.; Hanke, M.V. Assessing gene flow in apple using a descendant of Malus sieversii var. sieversii f. niedzwetzkyana as an identifier for pollen dispersal. Environ. Biosaf. Res. 2006, 5, 89–104. [Google Scholar]
- Stephen, W.P. Pear Pollination Studies in Oregon. 1958. Available online: https://scholar.google.com/scholar?hl=en&as_sdt=0%2C23&q=21.%09Stephen%2C+W.P.+Pear+Pollination+Studies+in+Oregon%3B+1958+.&btnG= (accessed on 8 April 2025).
- Westwood, M.N.; Grim, J. Effect of pollinizer placement on long term yield of Anjou, Bartlett and Bosc pears. Proc. Am. Soc. Hort. Sci. 1962, 81, 103–107. [Google Scholar]
- Westwood, M.N.; Stephen, W.P.; Cordy, C.B. The possibility of wind pollination in pear. Hortic. Sci. 1966, 1, 28–29. [Google Scholar] [CrossRef]
- Soejima, J. Estimation of gene flow via pollen spread for the orchard layout prior to the field release of apple transformants. In International Symposium on Biotechnology of Temperate Fruit Crops and Tropical Species 738; ACTA: London, UK, 2005; pp. 341–345. Available online: https://www.actahort.org/books/738/738_40.htm (accessed on 8 April 2025).
- Tyson, R.C.; Wilson, J.B.; Lane, W.D. Beyond diffusion: Modelling local and long-distance dispersal for organisms exhibiting intensive and extensive search modes. Theor. Popul. Biol. 2011, 79, 70–81. [Google Scholar] [CrossRef]
- Kron, P.; Brian, C.; Peter, G.; Kevan, G. Across-and along-row pollen dispersal in high-density apple orchards: Insights from allozyme markers. J. Hortic. Sci. Biotechnol. 2001, 76, 286–294. [Google Scholar] [CrossRef]
- Larsen, A.S.; Kjær, E.D. Pollen mediated gene flow in a native population of Malus sylvestris and its implications for contemporary gene conservation management. Conserv. Genet. 2009, 10, 1637–1646. [Google Scholar] [CrossRef]
- Kremen, C.; Williams, N.M.; Aizen, M.A.; Gemmill-Herren, B.; LeBuhn, G.; Minckley, R.; Packer, L.; Potts, S.G.; Roulston, T.; Steffan-Dewenter, I.; et al. Pollination and other ecosystem services produced by mobile organisms: A conceptual framework for the effects of land-use change. Ecol. Lett. 2007, 10, 299–314. [Google Scholar] [CrossRef]
- Carisio, L.; Diaz, S.S.; Ponso, S.; Manino, A.; Porporato, M. Effects of pollinizer density and apple tree position on pollination efficiency in cv. Gala. Sci. Hortic. 2020, 273, 109629. [Google Scholar] [CrossRef]
- Manja, K.; Aoun, M. The use of nets for tree fruit crops and their impact on the production: A review. Sci. Hortic. 2019, 246, 110–122. [Google Scholar] [CrossRef]
- Elsysy, M.; Einhorn, T.C. Enclosure of apple canopies with insect exclusion, anti-hail nets during early bloom stages reduces fruit set and seed content of ‘Honeycrisp’, ‘SweeTango’ and ‘Fuji’ but does not negatively affect fruit size. Acta Hortic. 2022, 1346, 175–182. [Google Scholar] [CrossRef]
- Langridge, D.F.; Jenkins, P.T. The role of honeybees in pollination of apples. Aust. J. Exp. Agric. 1970, 10, 366–368. [Google Scholar] [CrossRef]
- Schlathölter, I.; Dalbosco, A.; Meissle, M.; Knauf, A.; Dallemulle, A.; Keller, B.; Romeis, J.; Broggini, G.A.; Patocchi, A. Low outcrossing from an apple field trial protected with nets. Agronomy 2021, 11, 1754. [Google Scholar] [CrossRef]
- Meissle, M.; Waldburger, M.; Jeanneret, P.; Broggini, G.A.; Patocchi, A.; Romeis, J. Insect pollinator monitoring in and around a netted plot of apple trees—Biosafety implications for genetically engineered fruit trees. Agronomy 2022, 13, 84. [Google Scholar] [CrossRef]
- Sharma, H.K.; Gupta, J.K.; Thakur, J.R. Effect of bee pollination and polliniser proportion on apple productivity. In VII International Symposium on Temperate Zone Fruits in the Tropics and Subtropics 662; ACTA: London, UK, 2003; pp. 451–454. Available online: https://www.actahort.org/books/662/662_68.htm (accessed on 8 April 2025).
- Whiting, M.; Taylor, K.; Das, P. Supplemental artificial pollination can improve fruit set in tree fruit. In XII International Symposium on Integrating Canopy, Rootstock and Environmental Physiology in Orchard Systems 1346; ACTA: London, UK, 2021; pp. 121–128. Available online: https://www.actahort.org/books/1346/1346_16.htm (accessed on 8 April 2025).
- Boyle, R.M.D.; Philogène, B.J.R. The native pollinators of an apple orchard: Variations and significance. J. Hortic. Sci. 1983, 58, 355–363. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gratton, C. Species richness of wild bees, but not the use of managed honeybees, increases fruit set of a pollinator-dependent crop. J. Appl. Ecol. 2015, 52, 323–330. [Google Scholar] [CrossRef]
- Culley, T.M.; Weller, S.G.; Sakai, A.K. The evolution of wind pollination in angiosperms. Trends Ecol. Evol. 2002, 17, 361–369. [Google Scholar] [CrossRef]
- Vizzotto, G.; Driussi, E.; Pontoni, M.; Testolin, R. Effect of flower pollination on fruit set and cropping in apple. Am. J. Agric. For. 2018, 6, 156–161. [Google Scholar] [CrossRef]
- Kelderer, M.; Casera, C.; Lardscheider, E.; Rainer, A. Controlling codling moth with different netting structures and their influence on crop yield and quality. In Proceedings of the 14th International Conference in Organic Fruit-Growing–Eco-Fruit, Weinsberg, Germany, 22–24 February 2010; pp. 183–190. [Google Scholar]
- Yoder, K.; Yuan, R.; Combs, L.; Byers, R.; McFerson, J.; Schmidt, T. Effects of temperature and the combination of liquid lime sulfur and fish oil on pollen germination, pollen tube growth, and fruit set in apples. HortScience 2009, 44, 1277–1283. [Google Scholar] [CrossRef]
- Jahed, K.R.; Hirst, P.M. Pollen tube growth and fruit set in apple. HortScience 2017, 52, 1054–1059. [Google Scholar] [CrossRef]
- Estornell, L.H.; Agustí, J.; Merelo, P.; Talón, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, A.; Begheldo, M.; Zermiani, M.; Ramina, A. Signaling pathways mediating the induction of apple fruitlet abscission. Plant Physiol. 2011, 155, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Bangerth, F. Dominance among fruits/sinks and the search for a correlative signal. Physiol. Plant. 1989, 76, 608–614. [Google Scholar] [CrossRef]
- Greene, D.W.; Lakso, A.N.; Robinson, T.L.; Schwallier, P. Development of a fruitlet growth model to predict thinner response on apples. HortScience 2013, 48, 584–587. [Google Scholar] [CrossRef]
- Heinicke, A.J. Factors influencing the abscission of flowers and partially developed fruits of the apple (Pyrus malus L.). N. Y. Agric. Exp. Stn. Bull. 1917, 393, 114. [Google Scholar]
- Elsysy, M.A.; Hirst, P.M. Flowering in ‘Honeycrisp’ Apple Shows That Spurs Are Semiautonomous Organs. J. Am. Soc. Hortic. Sci. 2023, 148, 108–116. [Google Scholar] [CrossRef]
- Winfield, M.; Burridge, A.; Ordidge, M.; Harper, H.; Wilkinson, P.; Thorogood, D.; Copas, L.; Edwards, K.; Barker, G. Development of a minimal KASP marker panel for distinguishing genotypes in apple collections. PLoS ONE 2020, 15, e0242940. [Google Scholar] [CrossRef]
- Schouten, H.J.; Van De Weg, W.E.; Carling, J.; Khan, S.A.; McKay, S.J.; van Kaauwen, M.P.; Wittenberg, A.H.; Koehorst-van Putten, H.J.; Noordijk, Y.; Gao, Z.; et al. Diversity arrays technology (DArT) markers in apple for genetic linkage maps. Mol. Breed. 2012, 29, 645–660. [Google Scholar] [CrossRef]
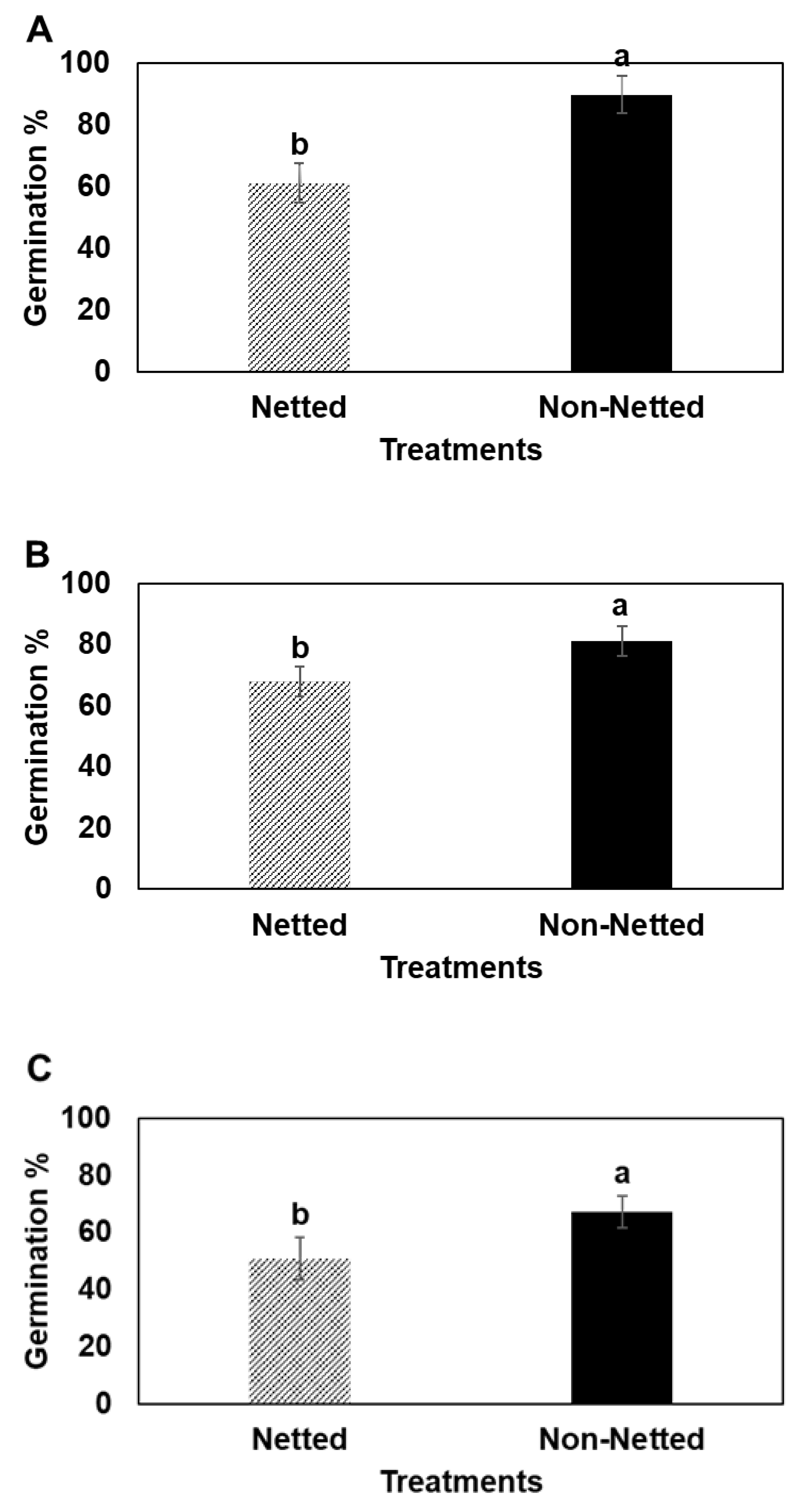
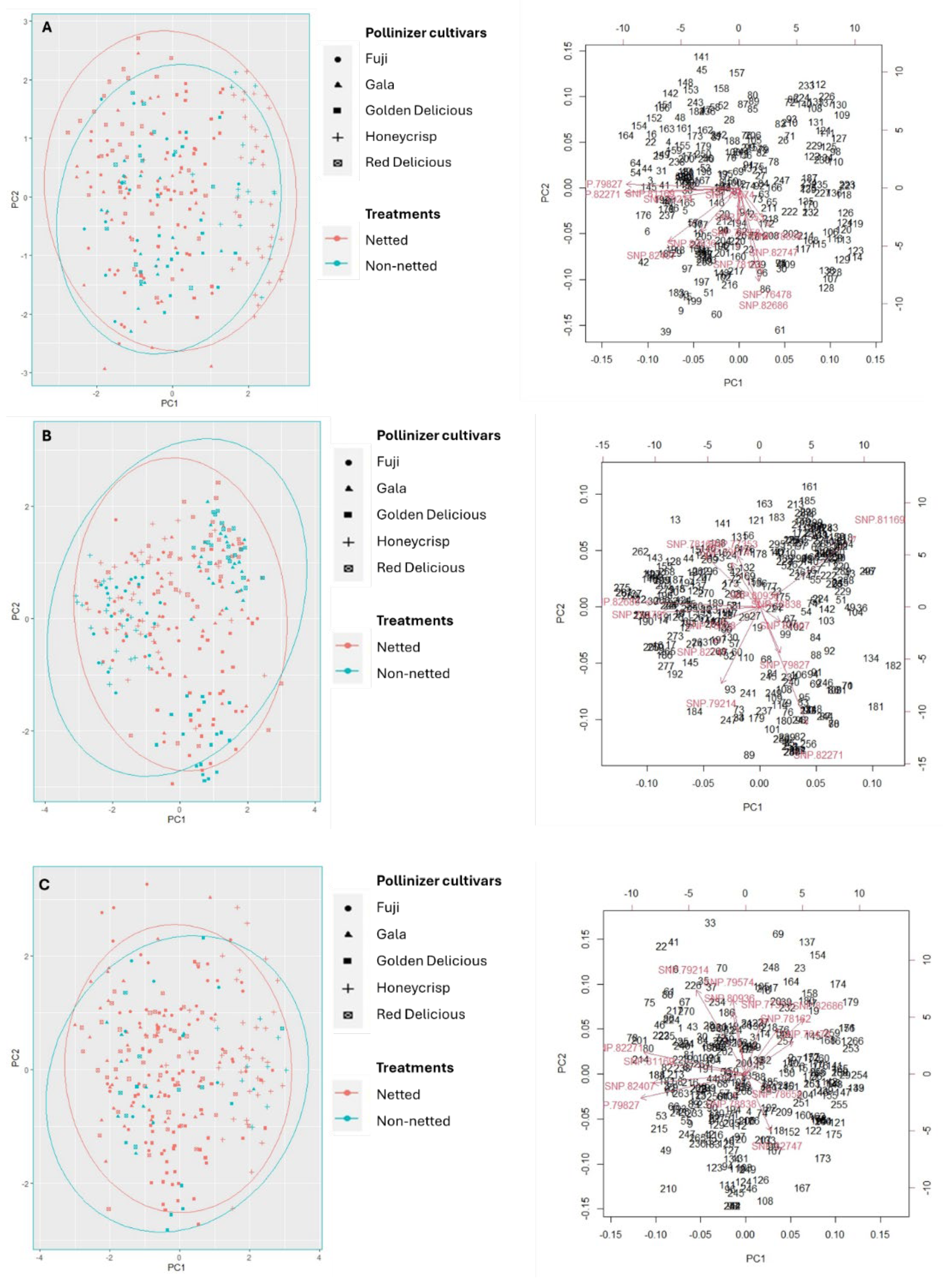


| Fruit Set | Fruit Number | Fruit Size | ||||
|---|---|---|---|---|---|---|
| (Fruit Number/Cluster) | (Fruit/TCSA) | (g) | ||||
| 2021 | 2022 | 2021 | 2022 | 2021 | 2022 | |
| ‘Gala’ | ||||||
| Non-netted | 1.1 ± 0.1 | 1.8 ± 0.1 | 17.7 ± 1.5 | 13.9 ± 1.6 | 131.5 ± 2.6 | 171.1 ± 6.4 |
| Netted | 0.9 ± 0.1 | 1.1 ± 0.1 | 5.2 ± 0.6 | 8.5 ± 0.8 | 151.5 ± 5.5 | 173.4 ± 4.3 |
| p | ** | *** | * | |||
| ‘Honeycrisp’ | ||||||
| Non-netted | 1.5 ± 0.2 | 1.8 ± 0.2 | 10.1 ± 1.8 | 10.8 ± 0.9 | 245.5 ± 9.6 | 295.9 ± 7.1 |
| Netted | 0.5 ± 0.04 | 0.9 ± 0.06 | 1.4 ± 0.1 | 8.5 ± 0.6 | 311.1 ± 8.1 | 286.6 ± 13.9 |
| p | *** | *** | * | |||
| ‘Fuji’ | ||||||
| Non-netted | 0.3 ± 0.01 | 1.4 ± 0.1 | 2.1 ± 0.6 | 12.1 ± 0.9 | 250.9 ± 7.1 | 187 ± 4.6 |
| Netted | 0.2 ± 0.07 | 0.8 ± 0.06 | 1 ± 0.2 | 6.8 ± 0.6 | 248.9 ± 5.7 | 198.8 ± 7.4 |
| p | *** | *** | 0.39 | |||
| Treatment | Fruit Set Status | Seed Number Per Fruit | p Abscission | |
|---|---|---|---|---|
| 2021 | 2022 | |||
| ‘Gala’ | ||||
| Netted | Retained | 4.43 ± 0.36 a | 8.2 ± 0.5 a y | *** z |
| Abscised | 2.25 ± 0.26 b | 7.5 ± 0.57 a | ||
| Non netted | Retained | 1.75 ± 0.37 b | 5.2 ± 0.24 b | * |
| Abscised | 0.34 ± 0.07 c | 4.1 ± 0.18 b | ||
| ‘Honeycrisp’ | ||||
| Netted | Retained | 6.02 ± 0.55 a | 6.93 ± 0.68 a | *** |
| Abscised | 3.06 ± 0.27 b | 4.37 ± 0.77 ab | ||
| Non netted | Retained | 1.17 ± 0.48 c | 5.09 ± 0.57 a | *** |
| Abscised | 0.12 ± 0.08 c | 2.6 ± 0.62 b | ||
| ‘Fuji’ | ||||
| Netted | Retained | 6.35 ± 0.59 a | 8.45 ± 0.62 a | *** |
| Abscised | 1.67 ± 1.12 b | 3.6 ± 0.81 b | ||
| Non netted | Retained | 4 ± 2 ab | 5.41 ± 0.72 b | *** |
| Abscised | 2.2 ± 1.46 b | 2.6 ± 0.7 b | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsysy, M.; Ebrahimi, A.; Einhorn, T. Wind Pollination of Apple Flowers Under Insect Exclusion Nets Questions the Insect-Dependent Pollination Model of Modern Apple Plantations. Plants 2025, 14, 1196. https://doi.org/10.3390/plants14081196
Elsysy M, Ebrahimi A, Einhorn T. Wind Pollination of Apple Flowers Under Insect Exclusion Nets Questions the Insect-Dependent Pollination Model of Modern Apple Plantations. Plants. 2025; 14(8):1196. https://doi.org/10.3390/plants14081196
Chicago/Turabian StyleElsysy, Mokhles, Aziz Ebrahimi, and Todd Einhorn. 2025. "Wind Pollination of Apple Flowers Under Insect Exclusion Nets Questions the Insect-Dependent Pollination Model of Modern Apple Plantations" Plants 14, no. 8: 1196. https://doi.org/10.3390/plants14081196
APA StyleElsysy, M., Ebrahimi, A., & Einhorn, T. (2025). Wind Pollination of Apple Flowers Under Insect Exclusion Nets Questions the Insect-Dependent Pollination Model of Modern Apple Plantations. Plants, 14(8), 1196. https://doi.org/10.3390/plants14081196






