Identification of Causal Agents of Rust of Saccharum spp. and Assessment of Resistance to Brown Rust in Erianthus arundinaceus Clones and Their Offspring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Rust Samples and Fungal Microscopy in China
2.2. Polymerase Chain Reaction (PCR) Detection of Rust
2.3. Cloning and Sequencing of PCR Fragments
2.4. Multiple Sequence Alignment and Phylogenetic Analysis
2.5. Molecular Detection of Bru1 Gene
2.6. Collection of Spores of P. melanocephala
2.7. Inoculation Method for Brown Rust
2.8. Experimental Designs to Identify Resistance to Brown Rust
2.9. Data Analysis
3. Results
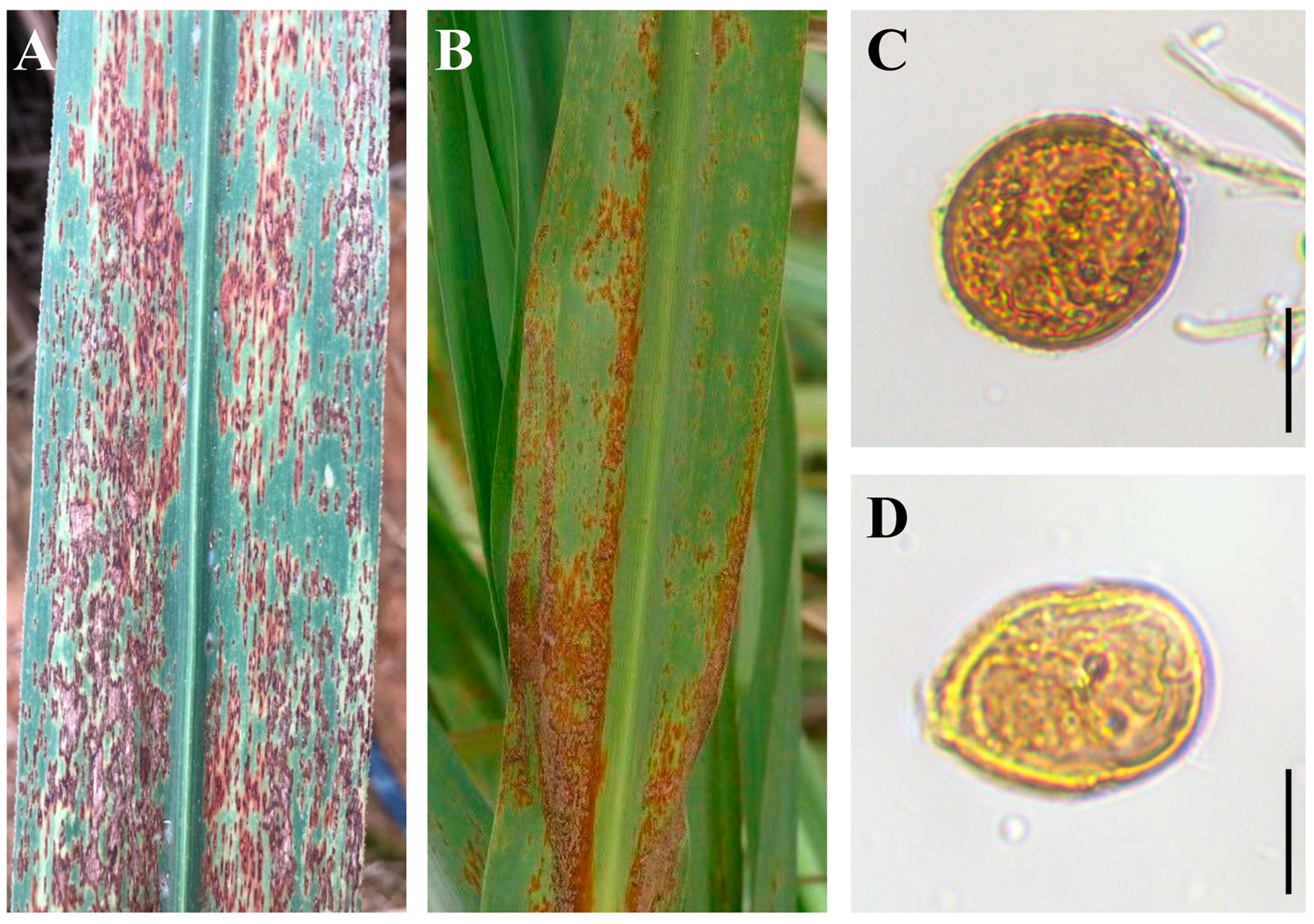
3.1. Morphology of Rust Fungi in Sugarcane
3.2. Sugarcane Rust Detection Using PCR Method
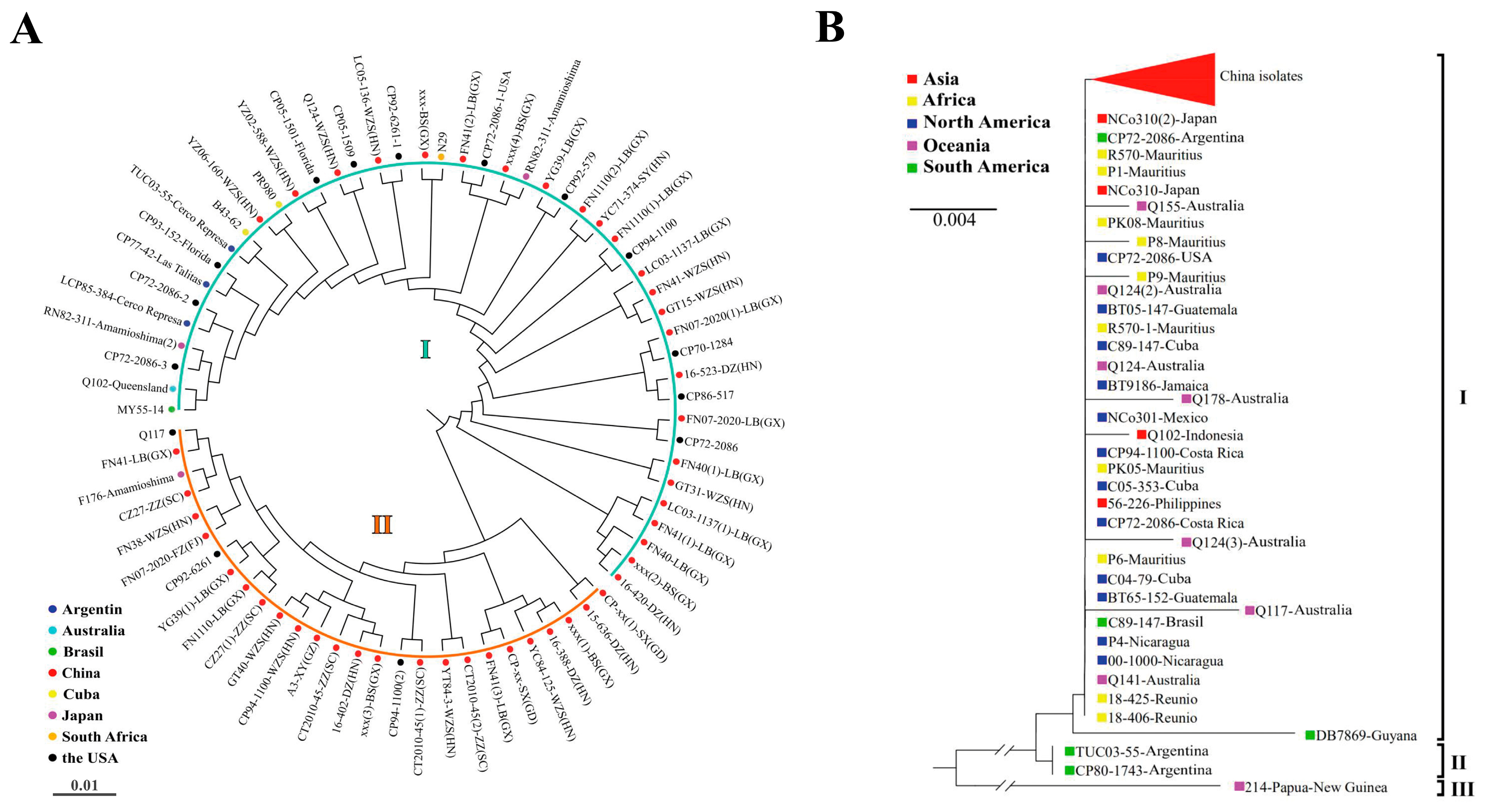
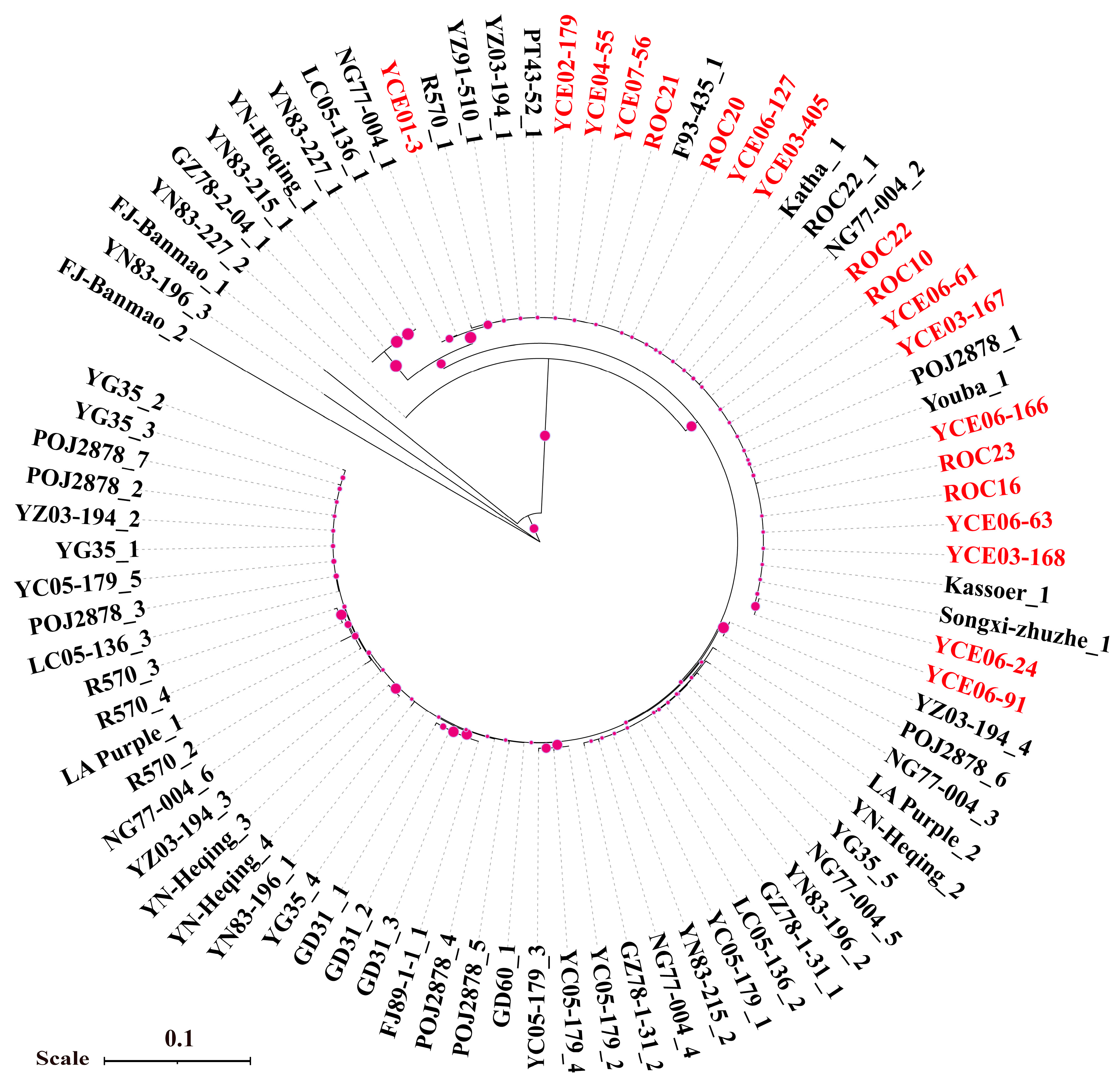
3.3. Phylogenetic Analysis of P. melanocephala and P. kuehnii
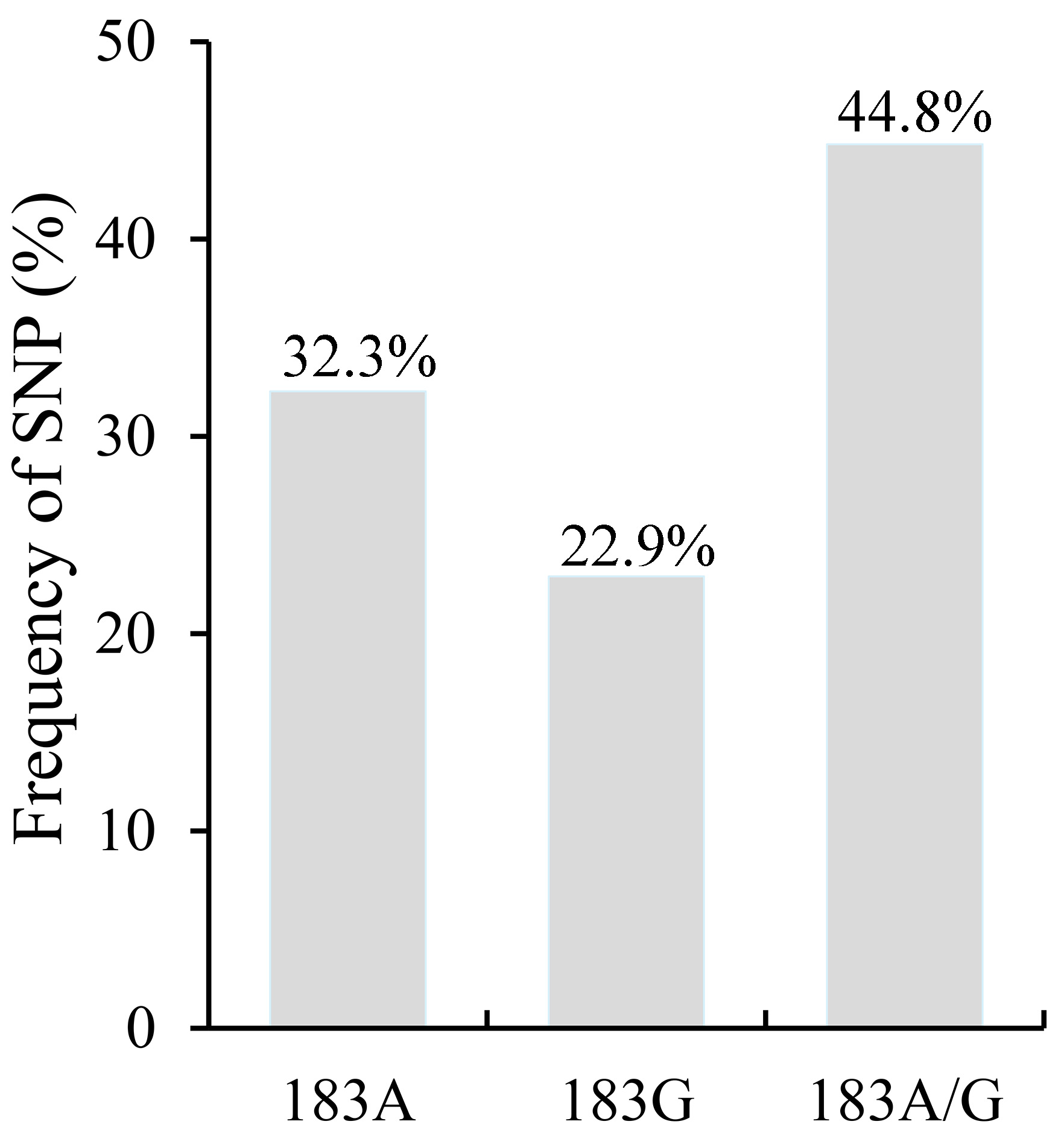
3.4. Single Nucleotide Polymorphism (SNP) Analysis of P. kuehnii
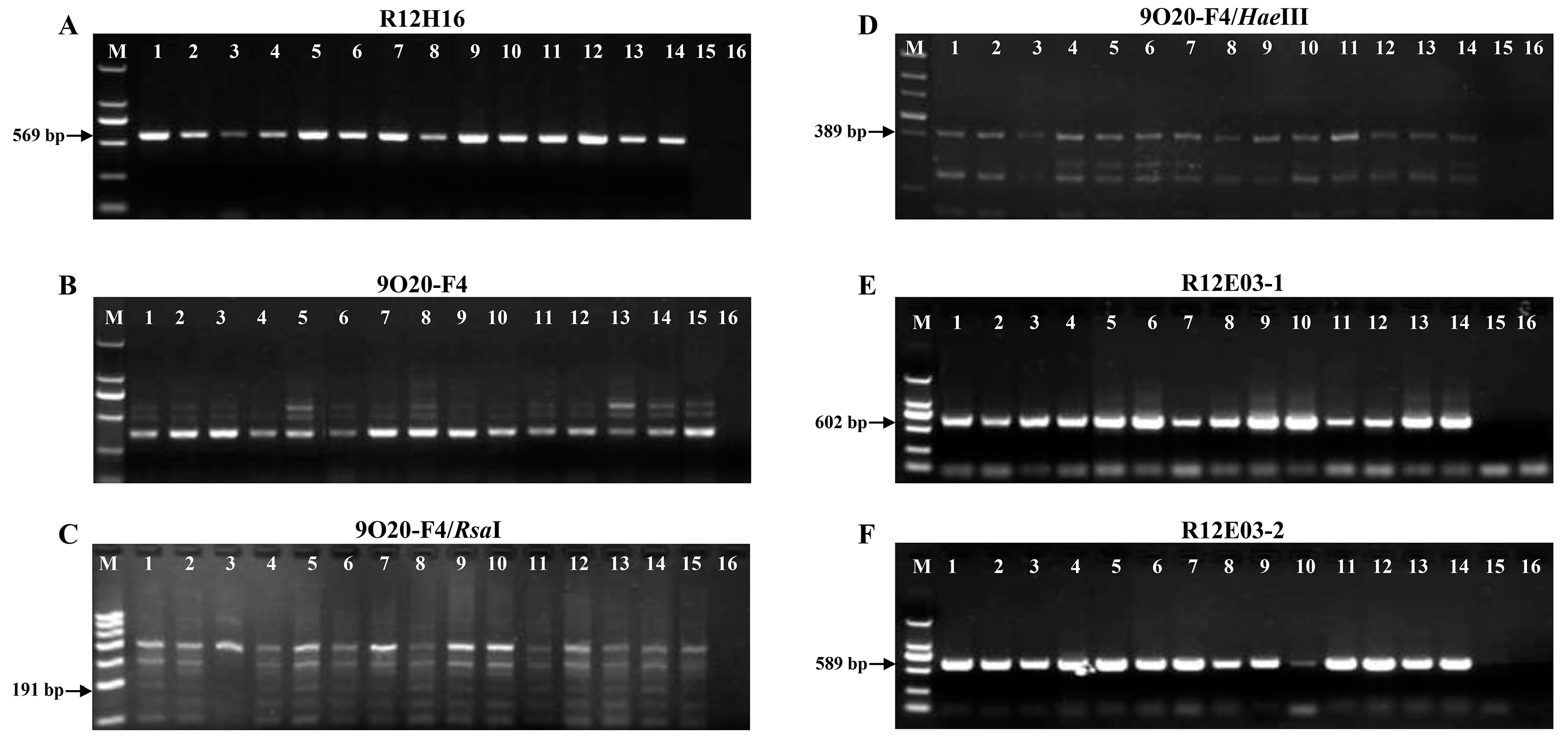
3.5. Detection of Bru1 Gene in E. arundinaceus Hybrids and Their Parents
3.6. SNP Analysis of Bru1 Gene
3.7. Phylogenetic Analysis of Bru1 Gene
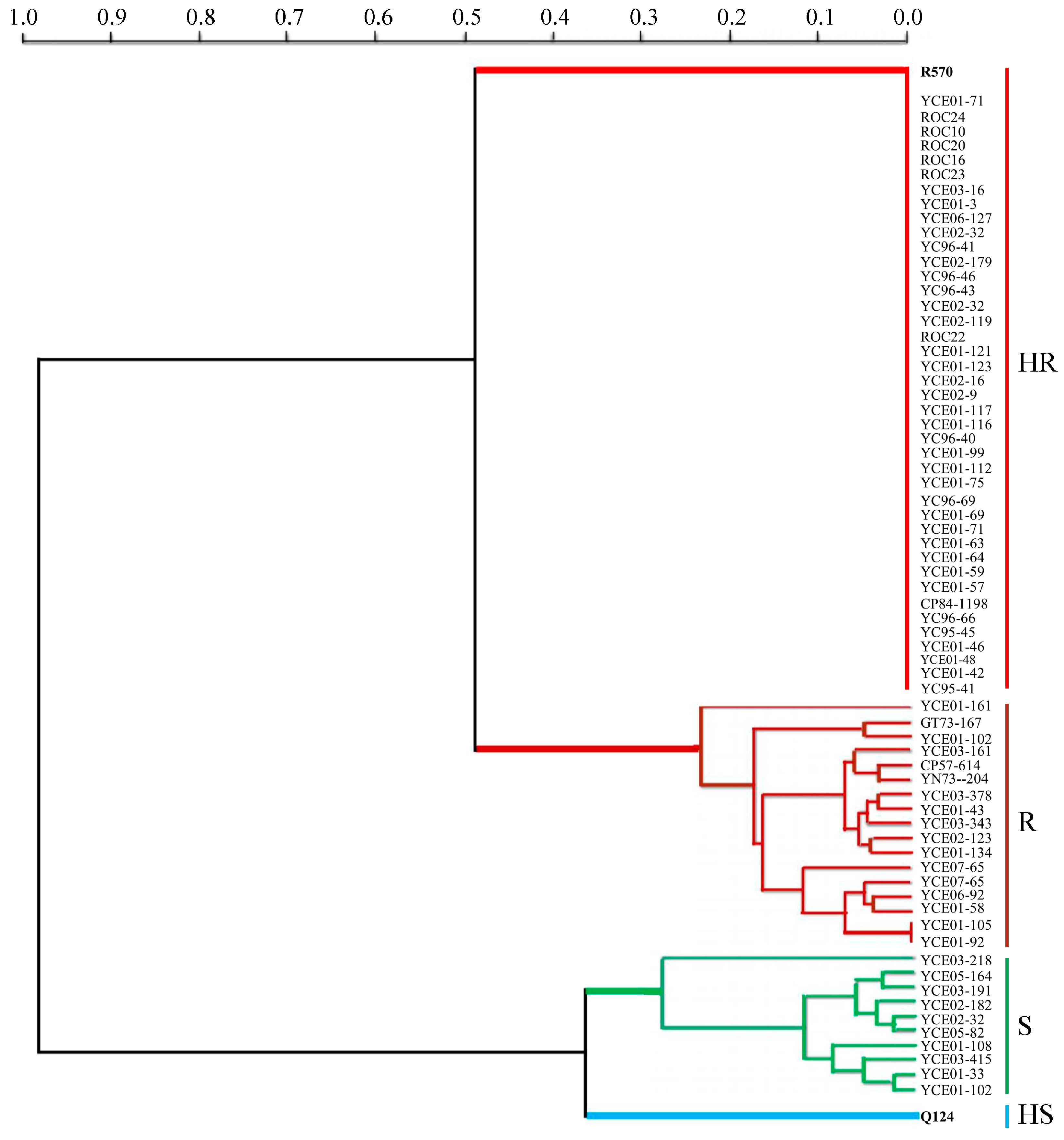
3.8. Identification of Resistance to Brown Rust in E. arundinaceus Hybrids and Their Parents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cárdenas, D.E.; Carrillo-Tarazona, Y.; Sood, S.G.; Hincapie, M.A.; Wang, J.; Rott, P.C.; Cano, L.M. A diagnostic guide for orange rust disease in sugarcane. Plant Health Prog. 2024, 25, 514–526. [Google Scholar] [CrossRef]
- Viswanathan, R. Degeneration in sugarcane varieties: Does the Sugar Industry realize it? Sugar Tech. 2024, 26, 1501–1504. [Google Scholar] [CrossRef]
- Comstock, J.; Shine Jr, J.; Raid, R. Effect of rust on sugarcane growth and biomass. Plant Dis. 1992, 76, 175–177. [Google Scholar] [CrossRef]
- Sanjel, S.; Chaulagain, B.; Small, I.M.; Comstock, J.C.; Hincapie, M.; Raid, R.N.; Rott, P. Comparison of progress of brown rust and orange rust and conditions conducive for severe epidemic development during the sugarcane crop season in Florida. Plant Dis. 2019, 103, 825–831. [Google Scholar] [CrossRef]
- Sanjel, S.; Hincapie, M.; Wang, Y.; Todd, J.; Chaulagain, B.; Sood, S.; Comstock, J.C.; Raid, R.N.; Rott, P. Occurrence of two races of Puccinia kuehnii causing orange rust of sugarcane in Florida. Plant Pathol. 2021, 70, 1616–1625. [Google Scholar] [CrossRef]
- Rott, P.; Bailey, R.A.; Comstock, J.C.; Croft, B.J.; Saumtally, A.S. A Guide to Sugarcane Diseases; CIRAD/ISSCT: Montpellier, France, 2000; pp. 85–89, 121–125. [Google Scholar]
- Fu, H.Y.; Xiao, S.H.; Liu, Y.H.; Sun, S.R.; Wu, X.B.; Chen, R.K.; Gao, S.J. Molecular detection of causal pathogens causing rust disease and Bru1 resistance-gene in elite Sugarcane Clones. Chin. J. Trop. Crops 2016, 37, 958–963. [Google Scholar]
- Li, W.F.; Wang, X.Y.; Shan, H.L.; Zhang, R.Y.; Li, Y.H.; Lu, W.J.; Huang, Y.K. Occurrence and damage of epidemic fungal diseases in middle and late stages of sugarcane growth in Yunnan Province of China. Eur. J. Plant Pathol. 2022, 164, 353–364. [Google Scholar] [CrossRef]
- Koch, G.; Ruaro, L.; Calegario, R.F.; Filho, J.C.B.; Daros, E.; Oliveira, R.A.; Duarte, H.S.S. Control of orange rust and brown rust of sugarcane with systemic fungicides. Sugar Tech. 2021, 23, 606–614. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Shan, H.L.; Yang, K.; Wang, X.Y.; Cang, X.Y.; Wang, C.M.; Luo, Z.M.; Li, W.F.; Huang, Y.K. Identification of brown rust resistance in the field and detection of the Bru1 gene in sugarcane varieties. Crop Breed. Appl. Biotechnol. 2021, 21, e32542121. [Google Scholar] [CrossRef]
- Li, W.F.; Wang, X.Y.; Huang, Y.K.; Zhang, R.Y.; Luo, Z.M. Identification of resistance to brown rust and molecular detection of Bru1 gene in 31 wild core sugarcane germplasms. Acta Agron. Sin. 2015, 41, 806–812. [Google Scholar] [CrossRef]
- Asnaghi, C.; Roques, D.; Ruffel, S.; Kaye, C.; Hoarau, J.Y.; Telismart, H.; Girard, J.C.; Raboin, L.M.; Risterucci, A.M.; Grivet, L. Targeted mapping of a sugarcane rust resistance gene (Bru1) using bulked segregant analysis and AFLP markers. Theor. Appl. Genet. 2004, 108, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Costet, L.; Le Cunff, L.; Royaert, S.; Raboin, L.M.; Hervouet, C.; Toubi, L.; Telismart, H.; Garsmeur, O.; Rousselle, Y.; Pauquet, J. Haplotype structure around Bru1 reveals a narrow genetic basis for brown rust resistance in modern sugarcane cultivars. Theor. Appl. Genet. 2012, 125, 825–836. [Google Scholar] [CrossRef]
- Wang, H.B.; Chen, P.H.; Yang, Y.Q.; D’hont, A.; Lu, Y.H. Molecular insights into the origin of the brown rust resistance gene Bru1 among Saccharum species. Theor. Appl. Genet. 2017, 130, 2431–2443. [Google Scholar] [CrossRef] [PubMed]
- Racedo, J.; Noguera, A.S.; Castagnaro, A.P.; Perera, M.F. Biotechnological strategies adopted for sugarcane disease management in Tucumán, Argentina. Plants 2023, 12, 3994. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Islam, M.S.; Sood, S.; Maya, S.; Hanson, E.A.; Comstock, J.; Wang, J. Identifying quantitative trait loci (QTLs) and developing diagnostic markers linked to orange rust resistance in sugarcane (Saccharum spp.). Front. Plant Sci. 2018, 9, 350. [Google Scholar] [CrossRef]
- Dijoux, J.; Rio, S.; Hervouet, C.; Garsmeur, O.; Barau, L.; Dumont, T.; Rott, P.; D’Hont, A.; Hoarau, J.Y. Unveiling the predominance of Saccharum spontaneum alleles for resistance to orange rust in sugarcane using genome-wide association. Theor. Appl. Genet. 2024, 137, 81. [Google Scholar] [CrossRef]
- Amalraj, V.A.; Balasundaram, N. On the taxonomy of the members of ‘Saccharum complex’. Genet. Resour. Crop Evol. 2006, 53, 35–41. [Google Scholar] [CrossRef]
- Parco, A.S.; Hale, A.L.; Avellaneda, M.C.; Hoy, J.W.; Kimbeng, C.A.; Pontif, M.J.; McCord, P.H.; Ayala, S.T.; Todd, J.R.; Baisakh, N. Distribution and frequency of Bru1, a major brown rust resistance gene, in the sugarcane world collection. Plant Breed. 2017, 136, 637–651. [Google Scholar] [CrossRef]
- Glynn, N.; Dixon, L.; Castlebury, L.; Szabo, L.; Comstock, J. PCR assays for the sugarcane rust pathogens Puccinia kuehnii and P. melanocephala and detection of a SNP associated with geographical distribution in P. kuehnii. Plant Pathol. 2010, 59, 703–711. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/nt. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Moreira, A.S.; Nogueira, J.A.F.; Gonçalves, C.R.N.B.; Souza, N.A.; Bergamin, F.A. Pathogenic and molecular comparison of Puccinia kuehnii isolates and reactions of sugarcane varieties to orange rust. Plant Pathol. 2018, 67, 1687–1696. [Google Scholar] [CrossRef]
- Urashima, A.S.; Mistura, T.F.; Porto, L.N.R.; Austin, P.D.; Arias, R.S. Genetic diversity of Puccinia kuehnii, the causal agent of orange rust of sugarcane, from Brazil. J. Phytopathol. 2020, 168, 581–590. [Google Scholar] [CrossRef]
- Shine Jr, J.; Comstock, J.; Dean, J. Comparison of five isolates of sugarcane brown rust and differential reaction on six sugarcane clones. Sugar Cane Int. 2005, 23, 638–647. [Google Scholar]
- Peixoto, R.F.J.; Creste, S.; Landell, M.G.A.; Nunes, D.S.; Sanguino, A.; Campos, M.F.; Vencovsky, R.; Tambarussi, E.V.; Figueira, A. Genetic diversity among Puccinia melanocephala isolates from Brazil assessed using simple sequence repeat markers. Genet. Mol. Res. 2014, 13, 7852–7863. [Google Scholar] [CrossRef] [PubMed]
- Hoy, J.; Avellaneda, M.; Bombecini, J. Variability in Puccinia melanocephala pathogenicity and resistance in sugarcane cultivars. Plant Dis. 2014, 98, 1728–1732. [Google Scholar] [CrossRef]
- Ye, X.; Li, J.; Cheng, Y.; Yao, F.; Long, L.; Yu, C.; Wang, Y.; Wu, Y.; Li, J.; Wang, J.; et al. Genome-wide association study of resistance to stripe rust (Puccinia striiformis f. Sp. Tritici) in Sichuan wheat. BMC Plant Biol. 2019, 19, 147. [Google Scholar]
- Le, C.L.; Garsmeur, O.; Raboin, L.M.; Pauquet, J.; Telismart, H.; Selvi, A.; Grivet, L.; Philippe, R.; Begum, D.; Deu, M. Diploid/polyploid syntenic shuttle mapping and haplotype-specific chromosome walking toward a rust resistance gene (Bru1) in highly polyploid sugarcane (2n∼12x∼115). Genetics 2008, 180, 649–660. [Google Scholar]
- Oliveira, G.K.; Barreto, F.Z.; Balsalobre, T.W.A.; Chapola, R.G.; Hoffmann, H.P.; Carneiro, M.S. Molecular evaluation and phenotypic screening of brown and orange rust in Saccharum germplasm. PLoS ONE 2024, 19, 20. [Google Scholar] [CrossRef]
| Province | No. of Tested Samples | Rates of Pm-Positive Samples | Rates of Pk-Positive Samples | Rates of Mixed Infection Samples | No. of Negative Samples |
|---|---|---|---|---|---|
| Guangxi | 78 | 7.7% (6/78) | 42.3% (33/78) | 25.6% (20/78) | 19 |
| Yunnan | 30 | 43.3% (13/30) | 6.7% (2/30) | - | 15 |
| Guangdong | 22 | - | 72.7% (16/22) | 9.1% (2/22) | 4 |
| Hainan | 45 | 33.3% (15/45) | 22.2% (10/45) | 6.7% (3/45) | 17 |
| Guizhou | 12 | 8.3% (1/12) | 58.3% (7/12) | - | 4 |
| Sichuan | 9 | - | 22.2% (2/9) | 11.1% (1/9) | 6 |
| Fujian | 5 | 20% (1/5) | - | - | 4 |
| Total | 201 | 17.9% (36/201) | 34.8% (70/201) | 12.9% (26/201) | 69 |
| Sample No. | Clones | R12H16 | 9O20-F4 | 9O20-F4 | R12E03-1 | R12E03-2 | |
|---|---|---|---|---|---|---|---|
| RsaI | HaeIII | ||||||
| 36 | YCE02-179 | + | + | + | + | + | + |
| 42 | YCE01-3 | + | + | + | + | + | + |
| 43 | YCE06-127 | + | + | − | + | + | + |
| 44 | YCE06-91 | + | + | + | + | + | + |
| 46 | YCE06-63 | + | + | + | + | + | + |
| 48 | YCE03-168 | + | + | + | + | + | + |
| 50 | YCE03-167 | + | + | + | + | + | + |
| 54 | YCE03-405 | + | + | + | + | + | + |
| 60 | YCE06-24 | + | + | + | + | + | + |
| 61 | YCE04-55 | + | + | + | + | + | + |
| 67 | YCE06-166 | + | + | + | + | + | + |
| 68 | YCE06-61 | + | + | + | + | + | + |
| 70 | YCE07-56 | + | + | + | + | + | + |
| 78 | ROC21 | + | + | + | + | + | + |
| 81 | ROC16 | + | + | + | + | + | + |
| 82 | ROC20 | + | + | + | + | + | + |
| 83 | ROC10 | + | + | + | + | + | + |
| 84 | ROC22 | + | + | + | + | + | + |
| 85 | ROC23 | + | + | + | + | + | + |
| RC | R570 | + | + | + | + | + | + |
| SC | Q124 | − | + | − | − | − | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-L.; Sun, S.-R.; Wang, Z.-Q.; Fu, H.-Y.; Xu, H.-Y.; Chang, H.-L.; Gao, S.-J.; Wang, Q.-N. Identification of Causal Agents of Rust of Saccharum spp. and Assessment of Resistance to Brown Rust in Erianthus arundinaceus Clones and Their Offspring. Plants 2025, 14, 1221. https://doi.org/10.3390/plants14081221
Chen J-L, Sun S-R, Wang Z-Q, Fu H-Y, Xu H-Y, Chang H-L, Gao S-J, Wang Q-N. Identification of Causal Agents of Rust of Saccharum spp. and Assessment of Resistance to Brown Rust in Erianthus arundinaceus Clones and Their Offspring. Plants. 2025; 14(8):1221. https://doi.org/10.3390/plants14081221
Chicago/Turabian StyleChen, Jun-Lv, Sheng-Ren Sun, Zhu-Qing Wang, Hua-Ying Fu, Huan-Yin Xu, Hai-Long Chang, San-Ji Gao, and Qin-Nan Wang. 2025. "Identification of Causal Agents of Rust of Saccharum spp. and Assessment of Resistance to Brown Rust in Erianthus arundinaceus Clones and Their Offspring" Plants 14, no. 8: 1221. https://doi.org/10.3390/plants14081221
APA StyleChen, J.-L., Sun, S.-R., Wang, Z.-Q., Fu, H.-Y., Xu, H.-Y., Chang, H.-L., Gao, S.-J., & Wang, Q.-N. (2025). Identification of Causal Agents of Rust of Saccharum spp. and Assessment of Resistance to Brown Rust in Erianthus arundinaceus Clones and Their Offspring. Plants, 14(8), 1221. https://doi.org/10.3390/plants14081221






