Silicon Supply Improves the Rhizodeposition and Transfer of Nitrogen from Trifolium incarnatum L. to Brassica napus L.
Abstract
1. Introduction
2. Results
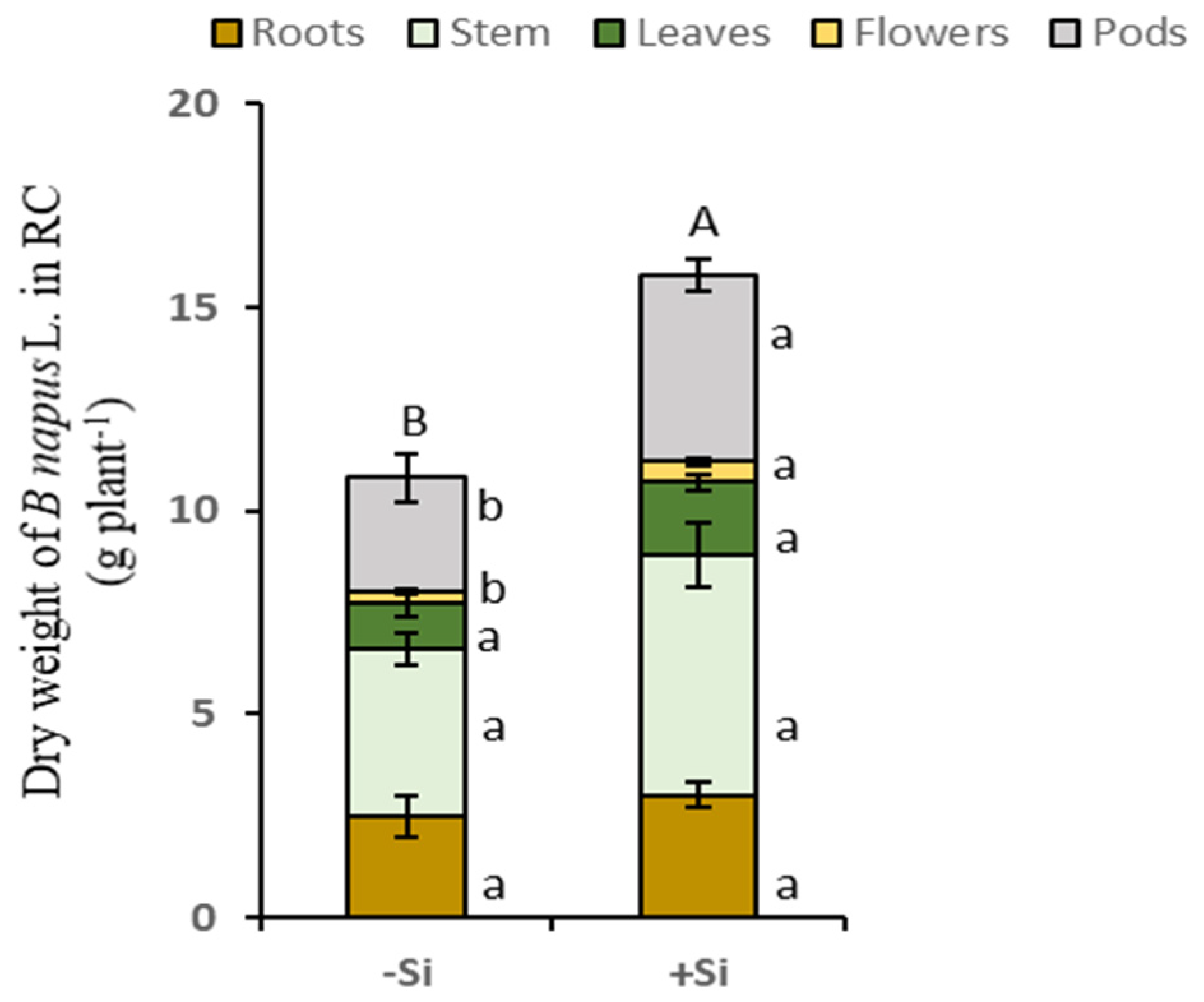
2.1. Effect of Si Supply on Biomass and Total N Amount in B. napus L. and T. incarnatum L.
2.2. Effect of Si Supply on N Rhizodeposition from T. incarnatum L. and N Transfer to B. napus L.
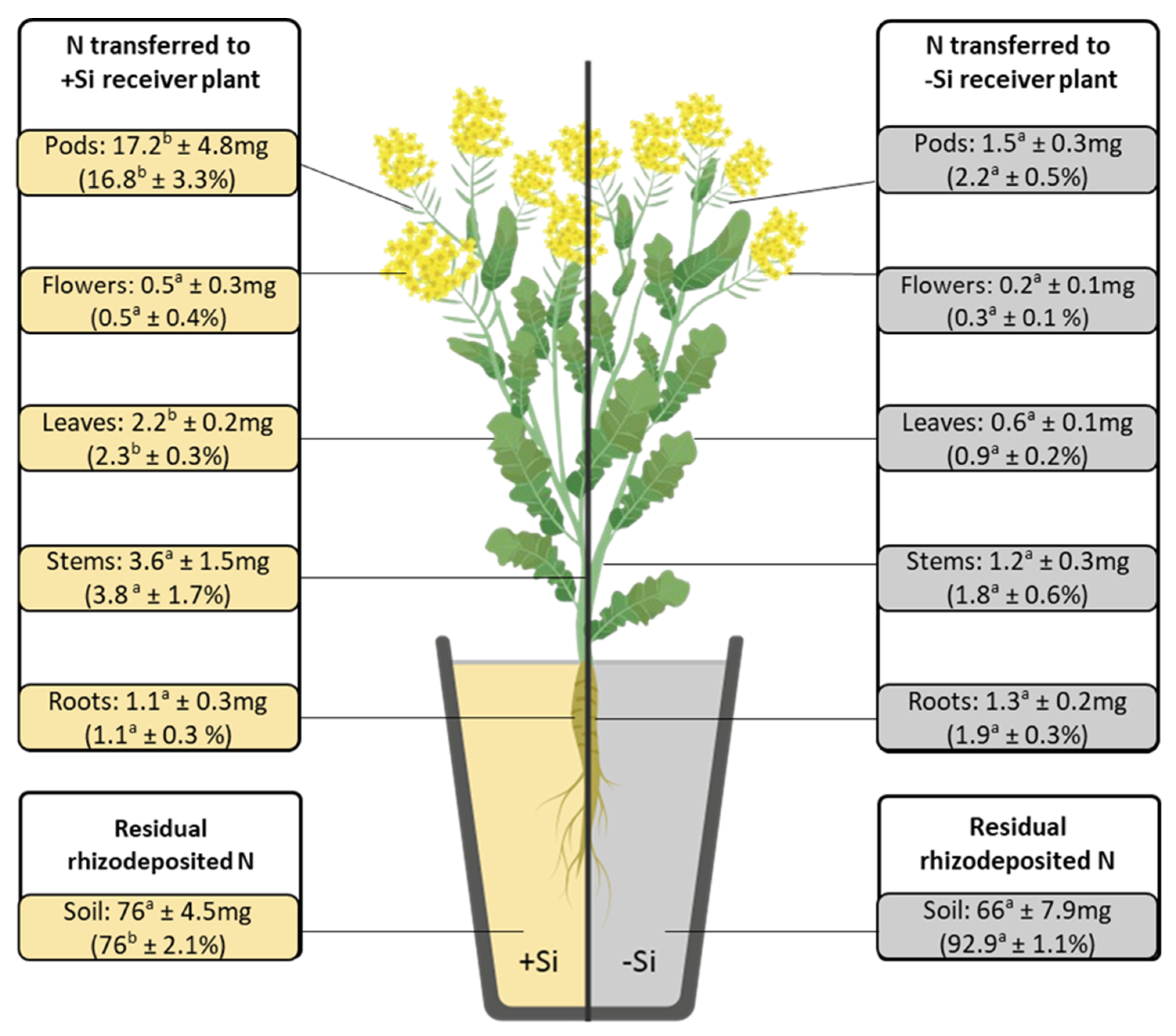
2.3. Si Effect on the Distribution of N Derived from T. incarnatum L. in the Receiver Compartment
| Dry Weight (g Plant−1) | ||||
|---|---|---|---|---|
| Roots | Shoots | Whole Plant | ||
| in RC | in DC | |||
| −Si | 2.6a ± 0.6 | 4.2a ± 0.4 | 43.4a ± 4.2 | 50.2a ± 5.8 |
| +Si | 3.1a ± 0.7 | 4.6a ± 0.5 | 38.7a ± 0.5 | 46.4a ± 0.7 |
| Brassica napus L. | Trifolium incarnatum L. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | Flowers | Pods | Whole Plant | Roots | Shoots | Whole Plant | |||
| in RC | in DC | ||||||||||
| N amount (mg plant−1) | −Si | 18a ± 3 | 27a ± 1 | 20a ± 6 | 3a ± 6 | 53a ± 7 | 122a ± 7 | 64a ± 12 | 116a ± 13 | 1227a ± 74 | 1408a ± 96 |
| +Si | 20a ± 3 | 42.6a ± 6 | 40b ± 4 | 6b ± 0 | 78b ± 1 | 185b ± 12 | 96a ± 17 | 133a ± 5 | 1215a ± 200 | 1445a ± 207 | |
| 15N excess (atom %) | −Si | 3.2a ± 1.1 | 1.5a ± 0.9 | 2.2a ± 1.3 | 1.8a ± 0.4 | 1.0a ± 0.2 | 1.4a ± 0.2 | 19.9a ± 6.3 | 53.9a ± 2.0 | 32.2a ± 4.7 | 33.4a ± 4.5 |
| +Si | 2a ± 0.8 | 3.2a ± 1.6 | 2.3a ± 0.4 | 2.7a ± 1.9 | 5.9b ± 0.6 | 4.1b ± 0.7 | 13.9a ± 1.3 | 59.9a ± 3.7 | 31.4a ± 2.8 | 32.6a ± 2.7 | |
| N Rhizodeposited in the RC (mg) | N Transferred to B. napus (mg) | N Transfer (%) | Ndft (%) | |
|---|---|---|---|---|
| −Si | 70.9a ± 7.6 | 5.7a ± 0.3 | 0.42a ± 0.03 | 4.2a ± 0.8 |
| +Si | 99.8b ± 7.3 | 23.3b ± 3.8 | 1.60b ± 0.1 | 12.2b ± 1.3 |

3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Experimental Design
4.2. Determination of N and 15N Amount
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, Y.; Wang, F.; Shi, X.; Shi, F.; Li, N.; Li, J.; Chenu, K.; Luo, H.; Yang, G. Late Nitrogen Fertilization Improves Cotton Yield through Optimizing Dry Matter Accumulation and Partitioning. Ann. Agric. Sci. 2023, 68, 75–86. [Google Scholar] [CrossRef]
- Yahbi, M.; Nabloussi, A.; Maataoui, A.; El Alami, N.; Boutagayout, A.; Daoui, K. Effects of Nitrogen Rates on Yield, Yield Components, and Other Related Attributes of Different Rapeseed (Brassica napus L.) Varieties. OCL 2022, 29, 8. [Google Scholar] [CrossRef]
- Sainju, U.M.; Ghimire, R.; Pradhan, G.P. Nitrogen Fertilization I: Impact on Crop, Soil, and Environment. In Nitrogen Fixation; Cid Rigobelo, E., Pereira Serra, A., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-648-5. [Google Scholar]
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse Gas Emissions from Global Production and Use of Nitrogen Synthetic Fertilisers in Agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef]
- Tian, D.; Niu, S. A Global Analysis of Soil Acidification Caused by Nitrogen Addition. Environ. Res. Lett. 2015, 10, 024019. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, G.; Luo, C.; Zhou, P. Groundwater Nitrogen Pollution and Assessment of Its Health Risks: A Case Study of a Typical Village in Rural-Urban Continuum, China. PLoS ONE 2012, 7, e33982. [Google Scholar] [CrossRef]
- De Vries, W. Impacts of Nitrogen Emissions on Ecosystems and Human Health: A Mini Review. Curr. Opin. Environ. Sci. Health 2021, 21, 100249. [Google Scholar] [CrossRef]
- Fustec, J.; Lesuffleur, F.; Mahieu, S.; Cliquet, J.-B. Nitrogen Rhizodeposition of Legumes. A Review. Agron. Sustain. Dev. 2010, 30, 57–66. [Google Scholar] [CrossRef]
- Raza, A.; Zahra, N.; Hafeez, M.B.; Ahmad, M.; Iqbal, S.; Shaukat, K.; Ahmad, G. Nitrogen Fixation of Legumes: Biology and Physiology. In The Plant Family Fabaceae; Hasanuzzaman, M., Araújo, S., Gill, S.S., Eds.; Springer: Singapore, 2020; pp. 43–74. ISBN 9789811547515. [Google Scholar]
- Kebede, E. Contribution, Utilization, and Improvement of Legumes-Driven Biological Nitrogen Fixation in Agricultural Systems. Front. Sustain. Food Syst. 2021, 5, 767998. [Google Scholar] [CrossRef]
- Landschoot, S.; Zustovi, R.; Dewitte, K.; Randall, N.P.; Maenhout, S.; Haesaert, G. Cereal-Legume Intercropping: A Smart Review Using Topic Modelling. Front. Plant Sci. 2024, 14, 1228850. [Google Scholar] [CrossRef]
- Cadoux, S.; Sauzet, G.; Valantin-Morison, M.; Pontet, C.; Champolivier, L.; Robert, C.; Lieven, J.; Flénet, F.; Mangenot, O.; Fauvin, P.; et al. Intercropping Frost-Sensitive Legume Crops with Winter Oilseed Rape Reduces Weed Competition, Insect Damage, and Improves Nitrogen Use Efficiency. OCL 2015, 22, D302. [Google Scholar] [CrossRef]
- Gou, X.; Reich, P.B.; Qiu, L.; Shao, M.; Wei, G.; Wang, J.; Wei, X. Leguminous Plants Significantly Increase Soil Nitrogen Cycling across Global Climates and Ecosystem Types. Glob. Change Biol. 2023, 29, 4028–4043. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.C.; Faris, M.A. Species Variation in the Fixation and Transfer of Nitrogen from Legumes to Associated Grasses. Plant Soil 1987, 98, 265–274. [Google Scholar] [CrossRef]
- Génard, T.; Etienne, P.; Diquélou, S.; Yvin, J.-C.; Revellin, C.; Laîné, P. Rapeseed-Legume Intercrops: Plant Growth and Nitrogen Balance in Early Stages of Growth and Development. Heliyon 2017, 3, e00261. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Ma, C.; Dong, S.; Xu, Y.; Gong, Z. Effects of Nitrogen Concentrations on Nodulation and Nitrogenase Activity in Dual Root Systems of Soybean Plants. Soil Sci. Plant Nutr. 2017, 63, 470–482. [Google Scholar] [CrossRef]
- Génard, T.; Etienne, P.; Laîné, P.; Yvin, J.-C.; Diquélou, S. Nitrogen Transfer from Lupinus albus L., Trifolium incarnatum L. and Vicia sativa L. Contribute Differently to Rapeseed (Brassica napus L.) Nitrogen Nutrition. Heliyon 2016, 2, e00150. [Google Scholar] [CrossRef]
- Génard, T.; Laîné, P.; Diquélou, S.; Nési, N.; Yvin, J.-C.; Etienne, P. Impact of Sulfur Applications on the Agronomic Performance of Rapeseed–Clover Mixtures. J. Plant Nutr. Soil Sci. 2017, 180, 676–682. [Google Scholar] [CrossRef]
- Breitenmoser, S.; Steinger, T.; Baux, A.; Hiltpold, I. Intercropping Winter Oilseed Rape (Brassica napus L.) Has the Potential to Lessen the Impact of the Insect Pest Complex. Agronomy 2022, 12, 723. [Google Scholar] [CrossRef]
- Etesami, H.; Adl, S.M. Can Interaction between Silicon and Non–Rhizobial Bacteria Help in Improving Nodulation and Nitrogen Fixation in Salinity–Stressed Legumes? A Review. Rhizosphere 2020, 15, 100229. [Google Scholar] [CrossRef]
- Pavlovic, J.; Samardzic, J.; Kostic, L.; Laursen, K.H.; Natic, M.; Timotijevic, G.; Schjoerring, J.K.; Nikolic, M. Silicon Enhances Leaf Remobilization of Iron in Cucumber under Limited Iron Conditions. Ann. Bot. 2016, 118, 271–280. [Google Scholar] [CrossRef]
- Detmann, K.C.; Araújo, W.L.; Martins, S.C.V.; Sanglard, L.M.V.P.; Reis, J.V.; Detmann, E.; Rodrigues, F.Á.; Nunes-Nesi, A.; Fernie, A.R.; DaMatta, F.M. Silicon Nutrition Increases Grain Yield, Which, in Turn, Exerts a Feed-forward Stimulation of Photosynthetic Rates via Enhanced Mesophyll Conductance and Alters Primary Metabolism in Rice. New Phytol. 2012, 196, 752–762. [Google Scholar] [CrossRef]
- Haddad, C.; Arkoun, M.; Jamois, F.; Schwarzenberg, A.; Yvin, J.-C.; Etienne, P.; Laîné, P. Silicon Promotes Growth of Brassica napus L. and Delays Leaf Senescence Induced by Nitrogen Starvation. Front. Plant Sci. 2018, 9, 516. [Google Scholar] [CrossRef] [PubMed]
- Mabagala, F.S.; Geng, Y.H.; Cao, G.J.; Wang, L.C.; Wang, M.; Zhang, M.L. Effect of silicon on crop yield, and nitrogen use efficiency applied under straw return treatements. Appl. Ecol. Environ. Res. 2020, 18, 5577–5590. [Google Scholar] [CrossRef]
- Wu, X.; Yu, Y.; Baerson, S.R.; Song, Y.; Liang, G.; Ding, C.; Niu, J.; Pan, Z.; Zeng, R. Interactions between Nitrogen and Silicon in Rice and Their Effects on Resistance toward the Brown Planthopper Nilaparvata Lugens. Front. Plant Sci. 2017, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Laîné, P.; Haddad, C.; Arkoun, M.; Yvin, J.-C.; Etienne, P. Silicon Promotes Agronomic Performance in Brassica napus Cultivated under Field Conditions with Two Nitrogen Fertilizer Inputs. Plants 2019, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Putra, R.; Powell, J.R.; Hartley, S.E.; Johnson, S.N. Is It Time to Include Legumes in Plant Silicon Research? Funct. Ecol. 2020, 34, 1142–1157. [Google Scholar] [CrossRef]
- Coquerel, R.; Arkoun, M.; Dupas, Q.; Leroy, F.; Laîné, P.; Etienne, P. Silicon Supply Improves Nodulation and Dinitrogen Fixation and Promotes Growth in Trifolium incarnatum Subjected to a Long-Term Sulfur Deprivation. Plants 2023, 12, 2248. [Google Scholar] [CrossRef]
- Coquerel, R.; Arkoun, M.; Trouverie, J.; Bernay, B.; Laîné, P.; Etienne, P. Ionomic and Proteomic Changes Highlight the Effect of Silicon Supply on the Nodules Functioning of Trifolium incarnatum L. Front. Plant Sci. 2024, 15, 1462149. [Google Scholar] [CrossRef]
- Rubiales, D.; Annicchiarico, P.; Vaz Patto, M.C.; Julier, B. Legume Breeding for the Agroecological Transition of Global Agri-Food Systems: A European Perspective. Front. Plant Sci. 2021, 12, 782574. [Google Scholar] [CrossRef]
- Yan, E.; Munier-Jolain, N.; Martin, P.; Carozzi, M. Intercropping on French Farms: Reducing Pesticide and N Fertiliser Use While Maintaining Gross Margins. Eur. J. Agron. 2024, 152, 127036. [Google Scholar] [CrossRef]
- Jamont, M.; Piva, G.; Fustec, J. Sharing N Resources in the Early Growth of Rapeseed Intercropped with Faba Bean: Does N Transfer Matter? Plant Soil 2013, 371, 641–653. [Google Scholar] [CrossRef]
- Putra, R.; Waterman, J.M.; Mathesius, U.; Wojtalewicz, D.; Powell, J.R.; Hartley, S.E.; Johnson, S.N. Benefits of Silicon-Enhanced Root Nodulation in a Model Legume Are Contingent upon Rhizobial Efficacy. Plant Soil 2022, 477, 201–217. [Google Scholar] [CrossRef]
- Paynel, F.; Cliquet, J.B. N Transfer from White Clover to Perennial Ryegrass, via Exudation of Nitrogenous Compounds. Agronomie 2003, 23, 503–510. [Google Scholar] [CrossRef]
- Liao, M.; Fan, Z.P.; Huang, X.-H.; Yang, X.-M.; Chen, S.S.; Xie, X.-M.; Xu, C.-X.; Gua, J.-W. Effets of Supplying Silicon Nutrient on Utilization Rate of Nitrogen and Phosphorus Nutrients by Rice and its Ecological Mechanisms in a Hybrid Rice Double-cropping System. J. Zhejiang Univ. Sci. B. 2020, 6, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sha, Y.; Huang, Y.; Hao, Z.; Guo, W.; Ke, L.; Chen, F.; Yuan, L.; Mi, G. Efficient Nitrogen Allocation and Reallocation into the Ear in Relation to the Superior Vascular System in Low-Nitrogen Tolerant Maize Hybrid. Field Crops Res. 2022, 284, 108580. [Google Scholar] [CrossRef]
- Mazurier, S. Diversité de Populations Naturelles Nodulantes de Rhizobium Leguminosarum. Ph.D. Thesis, Université Claude Bernard Lyon 1, Lyon, France, 1989. [Google Scholar]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A Uniform Decimal Code for Growth Stages of Crops and Weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- Ledgard, S.F.; Freney, J.R.; Simpson, J.R. Assessing Nitrogen Transfer from Legumes to Associated Grasses. Soil Biol. Biochem. 1985, 17, 575–577. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coquerel, R.; Arkoun, M.; Laîné, P.; Etienne, P. Silicon Supply Improves the Rhizodeposition and Transfer of Nitrogen from Trifolium incarnatum L. to Brassica napus L. Plants 2025, 14, 1246. https://doi.org/10.3390/plants14081246
Coquerel R, Arkoun M, Laîné P, Etienne P. Silicon Supply Improves the Rhizodeposition and Transfer of Nitrogen from Trifolium incarnatum L. to Brassica napus L. Plants. 2025; 14(8):1246. https://doi.org/10.3390/plants14081246
Chicago/Turabian StyleCoquerel, Raphaël, Mustapha Arkoun, Philippe Laîné, and Philippe Etienne. 2025. "Silicon Supply Improves the Rhizodeposition and Transfer of Nitrogen from Trifolium incarnatum L. to Brassica napus L." Plants 14, no. 8: 1246. https://doi.org/10.3390/plants14081246
APA StyleCoquerel, R., Arkoun, M., Laîné, P., & Etienne, P. (2025). Silicon Supply Improves the Rhizodeposition and Transfer of Nitrogen from Trifolium incarnatum L. to Brassica napus L. Plants, 14(8), 1246. https://doi.org/10.3390/plants14081246







