Water Stress Effects on Biomass Allocation and Secondary Metabolism in CBD-Dominant Cannabis sativa L.
Abstract
1. Introduction
2. Results
2.1. Regression Analysis on Biomass Allocation and Cannabinoid Profiles for Stress During Vegetative Phase
2.2. Regression Analysis on Biomass Allocation and Cannabinoid Profiles for Stress During Flowering Phase
2.3. Analysis of Metabolite Profiles
3. Discussion
3.1. Plant Growth and Biomass Allocation Under Water Stress
3.2. Cannabinoid Profiles Under Different Stress Conditions
3.3. Terpene Modulation Under Water Stress
4. Materials and Methods
4.1. Experimental Setup
4.2. Gas Chromatography-Mass Spectrometry Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pant, S.P.; Joshi, S.; Bisht, D.; Bisht, M. Exploring the Historical, Botanical, and Taxonomical Foundations of Cannabis. In Cannabis and Derivatives; Elsevier: Amsterdam, The Netherlands, 2024; pp. 3–36. ISBN 978-0-443-15489-8. [Google Scholar]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.; Bressan, R.A.; Song, C.; Zhu, J.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Jogawat, A.; Yadav, B.; Chhaya; Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between Phytohormones and Secondary Metabolites in the Drought Stress Tolerance of Crop Plants: A Review. Physiol. Plant. 2021, 172, 1106–1132. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Fracasso, A.; Struik, P.C.; Yin, X.; Amaducci, S. Water- and Nitrogen-Use Efficiencies of Hemp (Cannabis sativa L.) Based on Whole-Canopy Measurements and Modeling. Front. Plant Sci. 2018, 9, 951. [Google Scholar] [CrossRef] [PubMed]
- Caplan, D.; Dixon, M.; Zheng, Y. Increasing Inflorescence Dry Weight and Cannabinoid Content in Medical Cannabis Using Controlled Drought Stress. HortScience 2019, 54, 964–969. [Google Scholar] [CrossRef]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L.; Matthäus, B. Oil Content, Tocopherol Composition and Fatty Acid Patterns of the Seeds of 51 Cannabis sativa L. Genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Marchini, M.; Charvoz, C.; Dujourdy, L.; Baldovini, N.; Filippi, J.-J. Multidimensional Analysis of Cannabis Volatile Constituents: Identification of 5,5-Dimethyl-1-Vinylbicyclo[2.1.1]Hexane as a Volatile Marker of Hashish, the Resin of Cannabis sativa L. J. Chromatogr. A 2014, 1370, 200–215. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. New Insights Explain That Drought Stress Enhances the Quality of Spice and Medicinal Plants: Potential Applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; ElSohly, M.A. (Eds.) Cannabis sativa L.—Botany and Biotechnology; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-54563-9. [Google Scholar]
- Saloner, A.; Bernstein, N. Response of Medical Cannabis (Cannabis sativa L.) to Nitrogen Supply Under Long Photoperiod. Front. Plant Sci. 2020, 11, 572293. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Shape Matters: Plant Architecture Affects Chemical Uniformity in Large-Size Medical Cannabis Plants. Plants 2021, 10, 1834. [Google Scholar] [CrossRef]
- Small, E. Cannabis; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-6164-2. [Google Scholar]
- Gao, C.; Cheng, C.; Zhao, L.; Yu, Y.; Tang, Q.; Xin, P.; Liu, T.; Yan, Z.; Guo, Y.; Zang, G. Genome-Wide Expression Profiles of Hemp (Cannabis sativa L.) in Response to Drought Stress. Int. J. Genom. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Llusià, J.; Peñuelas, J. Changes in Terpene Content and Emission in Potted Mediterranean Woody Plants under Severe Drought. Can. J. Bot. 1998, 76, 1366–1373. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are They Important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential Cannabis Synergy and Phytocannabinoid-terpenoid Entourage Effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.A. Influence of Water Stress on Growth, Essential Oil, and Chemical Composition of Herbs: Ocimum sp. Int. Agrophysics 2006, 20, 289–296. [Google Scholar]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding Plant Responses to Drought—From Genes to the Whole Plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wahid, A.; Siddique, K.H.M. Drought Stress in Plants: An Overview. In Plant Responses to Drought Stress; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–33. ISBN 978-3-642-32652-3. [Google Scholar]
- Amaducci, S.; Zatta, A.; Pelatti, F.; Venturi, G. Influence of Agronomic Factors on Yield and Quality of Hemp (Cannabis sativa L.) Fibre and Implication for an Innovative Production System. Field Crops Res. 2008, 107, 161–169. [Google Scholar] [CrossRef]
- Gorelick, J.; Bernstein, N. Chemical and Physical Elicitation for Enhanced Cannabinoid Production in Cannabis. In Cannabis sativa L.—Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 439–456. ISBN 978-3-319-54563-9. [Google Scholar]
- Allen, K.D.; McKernan, K.; Pauli, C.; Roe, J.; Torres, A.; Gaudino, R. Genomic Characterization of the Complete Terpene Synthase Gene Family from Cannabis sativa. PLoS ONE 2019, 14, e0222363. [Google Scholar] [CrossRef]
- Sinclair, T.; Ludlow, M. Influence of Soil Water Supply on the Plant Water Balance of Four Tropical Grain Legumes. Funct. Plant Biol. 1986, 13, 329. [Google Scholar] [CrossRef]
- Sinclair, T.R. Theoretical Analysis of Soil and Plant Traits Influencing Daily Plant Water Flux on Drying Soils. Agron. J. 2005, 97, 1148–1152. [Google Scholar] [CrossRef]
- Jones, M.; Turner, N. Osmotic Adjustment in Expanding and Fully Expanded Leaves of Sunflower in Response to Water Deficits. Funct. Plant Biol. 1980, 7, 181. [Google Scholar] [CrossRef]
- Pellegrino, A.; Lebon, E.; Voltz, M.; Wery, J. Relationships between Plant and Soil Water Status in Vine (Vitis vinifera L.). Plant Soil 2005, 266, 129–142. [Google Scholar] [CrossRef]
- Sadras, V.O.; Milroy, S.P. Soil-Water Thresholds for the Responses of Leaf Expansion and Gas Exchange: A Review. Field Crops Res. 1996, 47, 253–266. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Fulvio, F.; Pieracci, Y.; Ascrizzi, R.; Bassolino, L.; Flamini, G.; Paris, R. Insights into Terpenes Profiling and Transcriptional Analyses during Flowering of Different Cannabis sativa L. Chemotypes. Phytochemistry 2025, 229, 114294. [Google Scholar] [CrossRef] [PubMed]
- Blanton, H.; Yin, L.; Duong, J.; Benamar, K. Cannabidiol and Beta-Caryophyllene in Combination: A Therapeutic Functional Interaction. Int. J. Mol. Sci. 2022, 23, 15470. [Google Scholar] [CrossRef]
- Szabó, K.; Zubay, P.; Németh-Zámboriné, É. What Shapes Our Knowledge of the Relationship between Water Deficiency Stress and Plant Volatiles? Acta Physiol. Plant. 2020, 42, 130. [Google Scholar] [CrossRef]
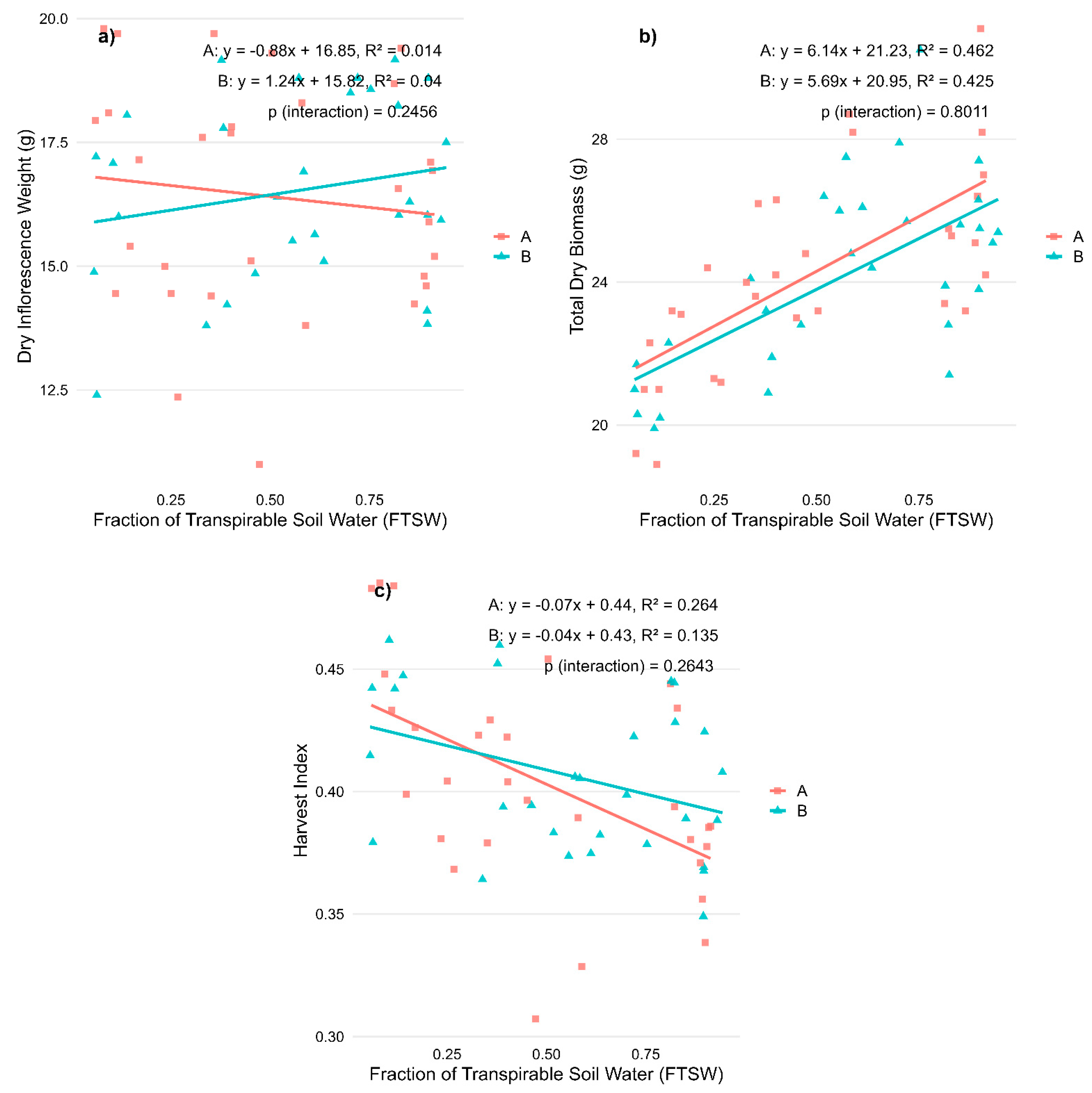
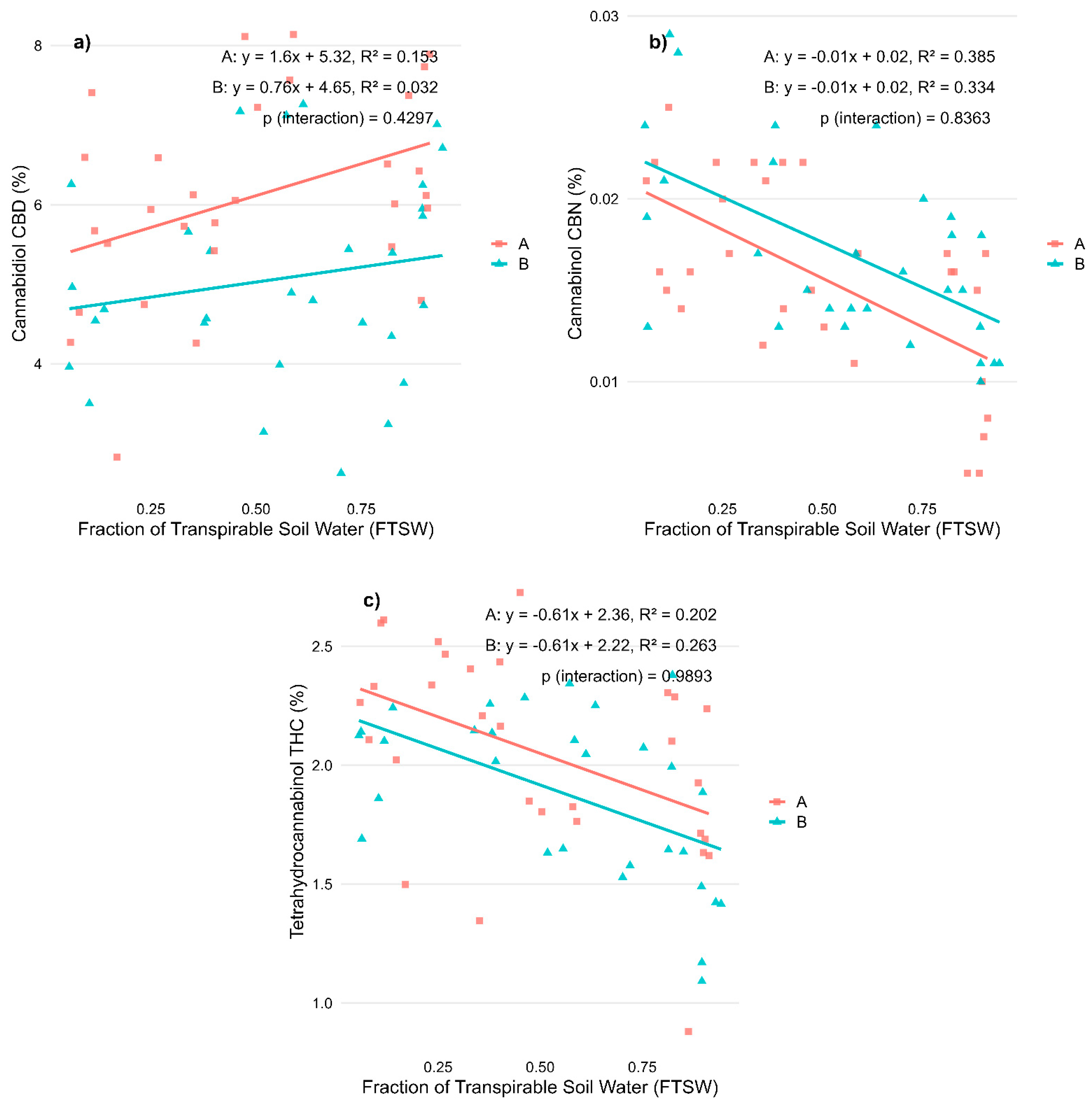
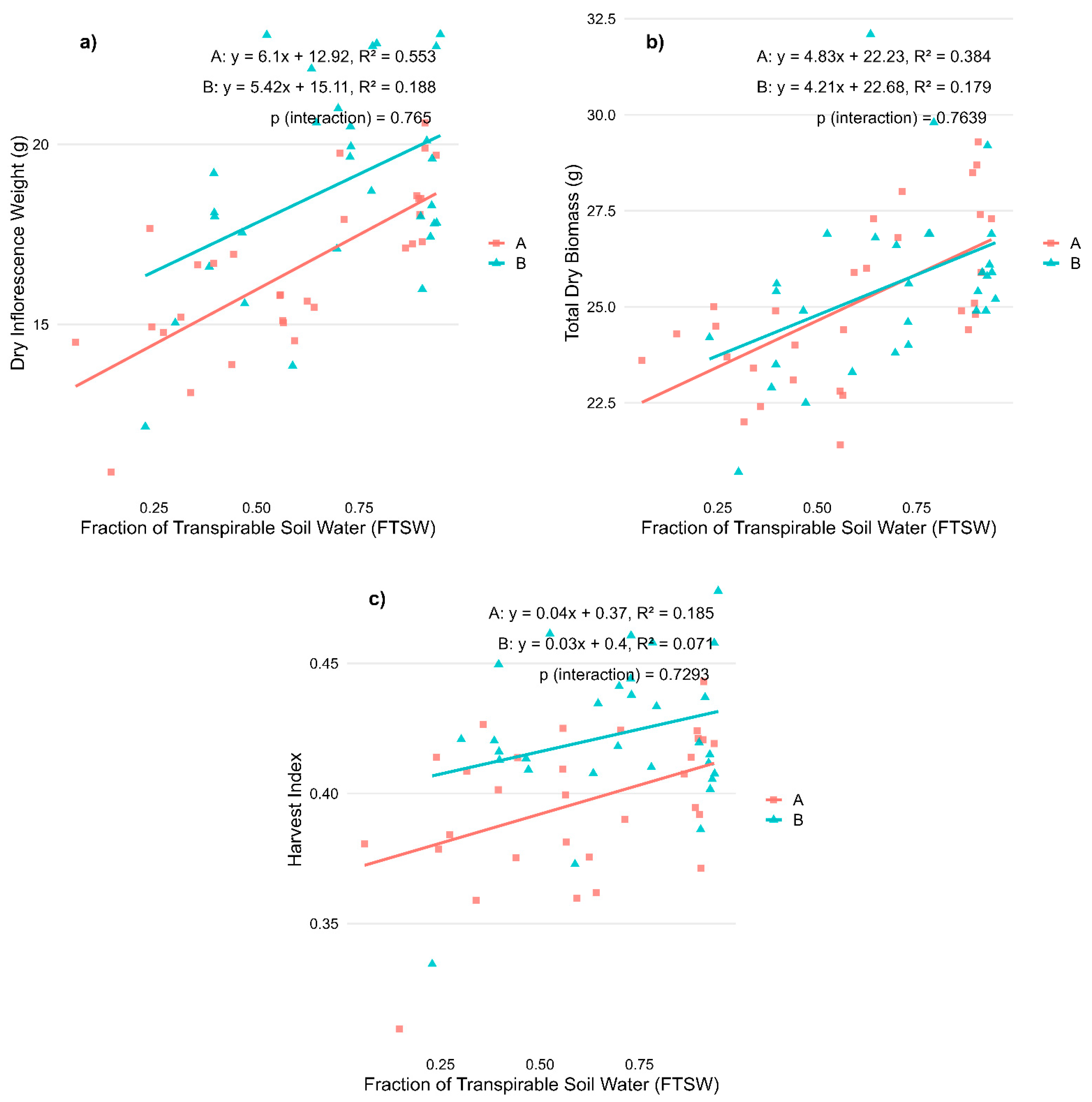

| Variable | R2—A | p-Value—A | R2—B | p-Value—B | p-Value—Interaction | R2—Total | p-Value—Total |
|---|---|---|---|---|---|---|---|
| Dry weight inflorescences (g) | 0.014 | 0.040 | 0.001 | ||||
| Dry weight biomass (g) | 0.462 | *** | 0.425 | *** | 0.435 | *** | |
| Harvest index | 0.264 | ** | 0.135 | * | 0.194 | *** | |
| CBD (%) | 0.153 | * | 0.032 | 0.047 | |||
| CBN (%) | 0.385 | *** | 0.334 | *** | 0.332 | *** | |
| THC (%) | 0.202 | * | 0.263 | ** | 0.239 | *** |
| Variable | R2—A | p-Value—A | R2—B | p-Value—B | p-Value—Interaction | R2—Total | p-Value—Total |
|---|---|---|---|---|---|---|---|
| Dry weight inflorescences (g) | 0.553 | *** | 0.188 | * | 0.348 | *** | |
| Dry weight biomass (g) | 0.384 | *** | 0.179 | * | 0.283 | *** | |
| Harvest index | 0.185 | * | 0.071 | 0.156 | ** | ||
| CBD (%) | 0.147 | * | 0.217 | ** | 0.067 | * | |
| CBN (%) | 0.002 | 0.022 | 0.004 | ||||
| THC (%) | 0.146 | * | 0.127 | 0.052 |
| Compound | Vegetative | Flowering | |||||
|---|---|---|---|---|---|---|---|
| Variety | Stress | V × S | Variety | Stress | V × S | ||
| 1. | Dimethyl sulfide | ** | . | ||||
| 2. | Propanal, 2-methyl- | *** | ** | ||||
| 3. | 2,3-Butanedione | ** | * | ||||
| 4. | Acetic acid, methyl ester | *** | |||||
| 5. | Acetic acid | *** | ** | ||||
| 6. | Furan, 3-methyl- | *** | *** | ||||
| 7. | Butanal, 2-methyl- | ** | * | . | |||
| 8. | Propanoic acid | * | |||||
| 9. | Furan, 2-ethyl- | *** | ** | * | |||
| 10. | 2-Butanone, 3-hydroxy- | *** | ** | ||||
| 11. | 1-Butanol, 2-methyl- | * | |||||
| 12. | Butanoic acid | ** | ** | . | |||
| 13. | 2,3-Butanediol | *** | *** | . | |||
| 14. | Hexanal | * | |||||
| 15. | 3-Hexen-1-ol, (E)- | ** | |||||
| 16. | 2-Hexenal | ** | |||||
| 17. | Ethylbenzene | *** | ** | ** | |||
| 18. | 1-Hexanol | . | * | ||||
| 19. | p-Xylene | *** | ** | * | |||
| 20. | Heptanal | *** | *** | ||||
| 21. | Bicyclo[2.2.1]hept-2-ene, 2,7,7-trimethyl- | *** | ** | *** | |||
| 22. | .alpha.-Pinene | *** | *** | ||||
| 23. | Camphene | *** | ** | ||||
| 24. | Benzaldehyde | *** | . | * | ** | ||
| 25. | .beta.-Pinene Phellandreene | *** | *** | ||||
| 26. | Myrcene | *** | *** | ||||
| 27. | Acetic acid, hexyl ester | *** | *** | ||||
| 28. | Benzene, 1-methyl-3-(1-methylethyl)- | *** | |||||
| 29. | D-Limonene | *** | . | *** | . | * | |
| 30. | 1,3,6-Octatriene, 3,7-dimethyl-, (E)- | *** | . | *** | *** | ||
| 31. | 1,3,6-Octatriene, 3,7-dimethyl-, (Z)- | *** | * | *** | * | ||
| 32. | 1,4-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- | *** | *** | ||||
| 33. | 1,6-Octadien-3-ol, 3,7-dimethyl- | ** | ** | *** | |||
| 34. | Phenylethyl Alcohol | *** | *** | ** | |||
| 35. | trans-2-Pinanol | *** | *** | ||||
| 36. | 2,4,6-Octatriene, 2,6-dimethyl- | *** | . | *** | |||
| 37. | Bicyclo[3.1.1]heptan-3-ol, 6,6-dimethyl-2-methylene- | *** | . | * | * | ||
| 38. | 2,7-Octadien-4-ol, 2-methyl-6-methylene-, (S)- | *** | *** | * | |||
| 39. | Borneol | *** | *** | * | |||
| 40. | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)- | * | |||||
| 41. | 3-Cyclohexene-1-methanol, .alpha., .alpha.4-trimethyl- | * | *** | *** | * | ||
| 42. | 6-Octen-1-ol, 3,7-dimethyl-, (R)- | *** | * | *** | |||
| 43. | Benzeneacetic acid, ethyl ester | * | *** | . | |||
| 44. | Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S-endo)- | *** | *** | ||||
| 45. | 3a,7-Methano-3aH-cyclopentacyclooctene, 1,4,5,6,7,8,9,9a-octahydro-1,1,7-trimethyl-, [3aR-(3a.alpha.,7.alpha.,9a.beta.)]- | *** | ** | *** | |||
| 46. | Ylangene | *** | *** | ||||
| 47. | Copaene | *** | * | *** | |||
| 48. | Hexanoic acid, hexyl ester | *** | * | *** | |||
| 49. | Caryophyllene | *** | * | . | *** | ||
| 50. | Bicyclo[3.1.1]hept-2-ene, 2,6-dimethyl-6-(4-methyl-3-pentenyl)- | *** | . | *** | |||
| 51. | .alpha.-Caryophyllene | *** | . | * | *** | ||
| 52. | 1,6,10-Dodecatriene, 7,11-dimethyl-3-methylene-, (E)- | *** | *** | ||||
| 53. | Naphthalene, 1,2,3,4,4a,5,6,8a-octahydro-7-methyl-4-methylene-1-(1-methylethyl)-, (1.alpha.,4a.alpha.,8a.alpha.)- | *** | * | ||||
| 54. | .gamma.-Elemene | *** | . | * | |||
| 55. | Naphthalene, 1,2,4a,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1.alpha.,4a.alpha.,8a.alpha.)- | ||||||
| 56. | .alpha.-Calacorene | *** | * | ||||
| 57. | 1H-Cycloprop[e]azulene, decahydro-1,1,7-trimethyl-4-methylene-, [1aR-(1a.alpha.,4a.alpha.,7.alpha.,7a.beta.,7b.alpha.)]- | * | |||||
| 58. | Caryophyllene oxide | * | |||||
| 59. | Guaiol | *** | |||||
| 60. | 2-Naphthalenemethanol, 1,2,3,4,4a,5,6,7-octahydro-.alpha.,.alpha.,4a,8-tetramethyl-, (2R-cis)- | *** | |||||
| 61. | 2-Naphthalenemethanol, decahydro-.alpha.,.alpha.,4a-trimethyl-8-methylene-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]- | *** | |||||
| 62. | 2-Naphthalenemethanol, 1,2,3,4,4a,5,6,8a-octahydro-.alpha.,.alpha.,4a,8-tetramethyl-, [2R-(2.alpha.,4a.alpha.,8a.beta.)]- | *** | * | ||||
| 63. | 5-Azulenemethanol, 1,2,3,3a,4,5,6,7-octahydro-.alpha.,.alpha.,3,8-tetramethyl-, [3S-(3.alpha.,3a.beta.,5.alpha.)]- | *** | |||||
| 64. | .alpha.-Bisabolol | *** | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappello Fusaro, M.; Lucchetta, I.; Bona, S. Water Stress Effects on Biomass Allocation and Secondary Metabolism in CBD-Dominant Cannabis sativa L. Plants 2025, 14, 1267. https://doi.org/10.3390/plants14081267
Cappello Fusaro M, Lucchetta I, Bona S. Water Stress Effects on Biomass Allocation and Secondary Metabolism in CBD-Dominant Cannabis sativa L. Plants. 2025; 14(8):1267. https://doi.org/10.3390/plants14081267
Chicago/Turabian StyleCappello Fusaro, Maddalena, Irene Lucchetta, and Stefano Bona. 2025. "Water Stress Effects on Biomass Allocation and Secondary Metabolism in CBD-Dominant Cannabis sativa L." Plants 14, no. 8: 1267. https://doi.org/10.3390/plants14081267
APA StyleCappello Fusaro, M., Lucchetta, I., & Bona, S. (2025). Water Stress Effects on Biomass Allocation and Secondary Metabolism in CBD-Dominant Cannabis sativa L. Plants, 14(8), 1267. https://doi.org/10.3390/plants14081267






