Leaves and Tree Rings as Biomonitoring Archives of Atmospheric Mercury Deposition: An Ecophysiological Perspective
Abstract
1. Introduction: The Role of Trees in Atmospheric-Terrestrial Mercury Exchange Dynamics
2. Vegetation-Atmosphere Mercury Exchange: Biogeochemical and Ecological Significance
2.1. Atmospheric Mercury–Vegetation Exchange: Processes, Dynamics, and Cycling
- Litterfall transfer (60–90% of total terrestrial Hg deposition): retained foliar Hg, including β-HgS nanoparticles and Hg(SR)2 complexes, ultimately enters forest soils upon senescence at rates of 10–34 μg m−2 yr−1 across diverse biomes [16]. The estimated global litterfall deposition ranges from 1180 to 1410 Mg yr−1 [6].
- Photoreduction and re-emission (10–25%): Under high light and temperature, foliar Hg can undergo photoreduction to GEM and re-enter the atmosphere, exhibiting strong diurnal cycles with peak emissions at midday [27,28]. This process has an exponential temperature dependence and is significantly enhanced by UVB radiation.
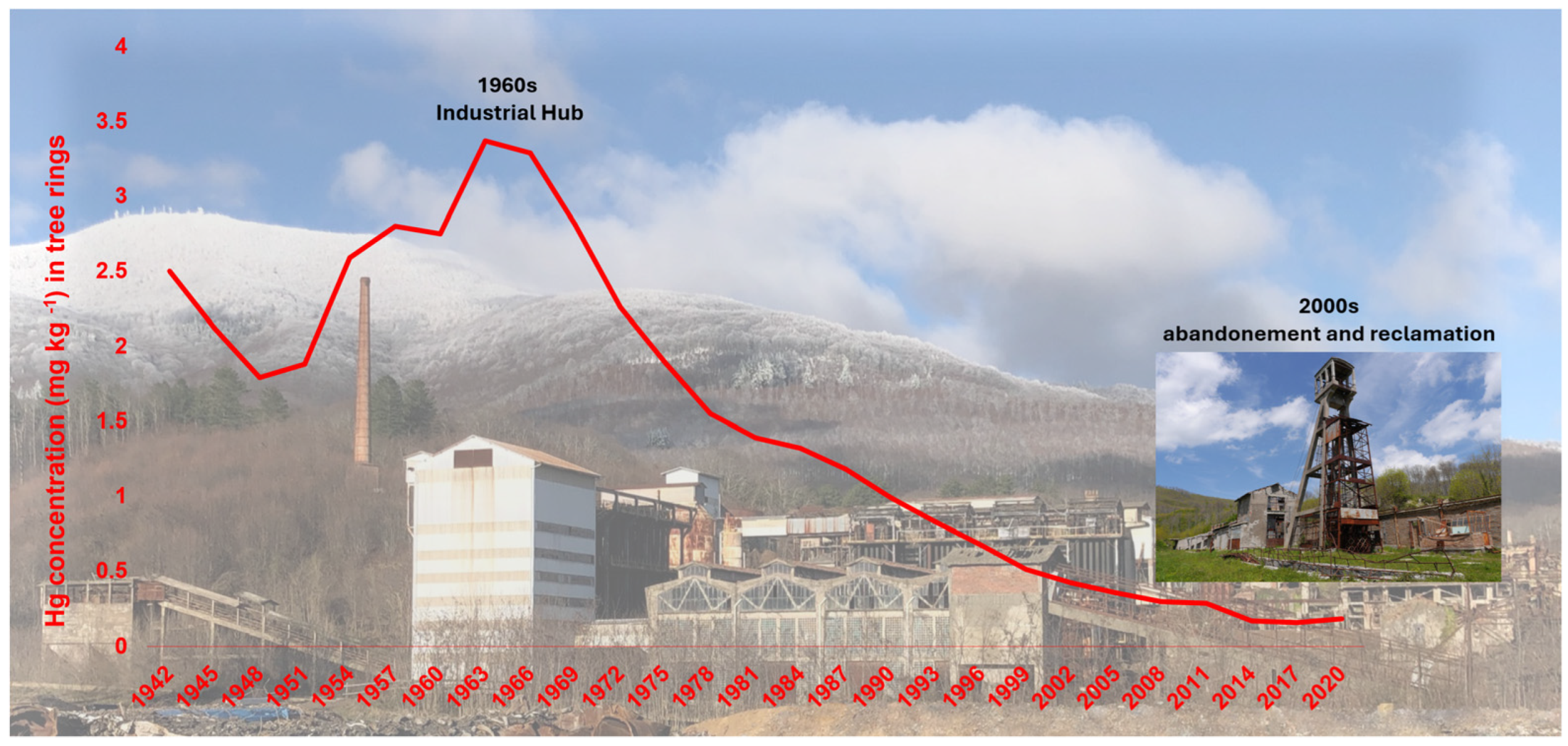
- Phloem translocation (5–15%): A smaller fraction is transported via the phloem to woody tissues, forming dendrochemical records of atmospheric Hg [23,25]. Multi-decadal tree-ring analyses documented a 2–3-fold increase in Hg during peak industrial emissions (1950–1980), followed by a gradual decline corresponding to regulatory efforts [29].
2.2. Environmental Modulators of Mercury Assimilation in Vegetation
2.3. Landscape- and Ecosystem-Scale Mercury Dynamics
3. Mercury Ecophysiology in Trees: Uptake Mechanisms and Transport Pathways
3.1. Stomatal and Non-Stomatal Uptake Pathways
3.2. Leaf Morphological and Anatomical Determinants of Mercury Assimilation
3.3. Physiological and Functional Determinants of Species-Specific Mercury Accumulation
- 1.
- Rapid initial uptake during leaf expansion (0.28–0.45 ng g−1 day−1)
- 2.
- Moderate accumulation under peak photosynthetic activity (0.12–0.26 ng g−1 day−1)
- 3.
3.4. Mercury Transport and Retention in Woody Tissues
3.5. Climatic and Hydrological Influences on Mercury Partitioning in Trees
3.6. Integrated Effects of Physiological, Morphological, and Environmental Drivers
4. Tree Leaves as Biomonitors of Atmospheric Mercury
4.1. Historical Development and Conceptual Framework
4.2. Spatial-Temporal Assessment Using Leaf Sampling
4.3. Applications in Environmental Monitoring
4.4. Methodological Considerations for Mercury Biomonitoring with Tree Leaves
5. Tree Rings as Historical Archives of Atmospheric Mercury
5.1. Theoretical Framework for Dendrochronological Mercury Archives
5.2. Choosing Suitable Species and Minimizing Radial Translocation of Mercury
5.3. Applications in Historical Reconstruction
5.4. Analytical Methodologies for Tree-Ring Mercury Quantification
- Rigorous cross-dating procedures to confirm the exact calendar year of each ring.
- High-resolution sampling, preferably at annual or biennial scales, to capture fine temporal trends in atmospheric Hg uptake.
- Elimination of potential contamination through careful sample preparation and handling.
- Annual-resolution ring separation through rigorous cross-dating,
- Careful method selection (TDA-AAS, CVAFS, LA-ICP-MS, or stable isotope approaches) matched to the study’s resolution and sensitivity needs, and
- Physiological data integration to assess species-specific mobility effects.
6. Future Directions and Conclusions
6.1. Integrative Perspectives on Leaves and Tree Rings
6.2. Ecophysiological Integration
6.3. Climate Change Implications
6.4. Policy Relevance Under the Minamata Convention
6.5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| GEM | Gaseous Elemental Mercury |
| GOM | Gaseous Oxidased Mercury |
| PBM | Particulate-Bound Mercury |
References
- Augusto, L.; Boča, A. Tree Functional Traits, Forest Biomass, and Tree Species Diversity Interact with Site Properties to Drive Forest Soil Carbon. Nat. Commun. 2022, 13, 1097. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Crowther, T.W.; Mo, L.; Maynard, D.S.; Renner, S.S.; Van Den Hoogen, J.; Zou, Y.; Liang, J.; de-Miguel, S.; Nabuurs, G.-J.; et al. The Global Biogeography of Tree Leaf Form and Habit. Nat. Plants 2023, 9, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Taylor, A.R.; Reich, P.B.; Hisano, M.; Chen, H.Y.H.; Chang, S.X. Tree Diversity Increases Decadal Forest Soil Carbon and Nitrogen Accrual. Nature 2023, 618, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Dubbert, M.; Werner, C. Water Fluxes Mediated by Vegetation: Emerging Isotopic Insights at the Soil and Atmosphere Interfaces. New Phytol. 2019, 221, 1754–1763. [Google Scholar] [CrossRef]
- Jiskra, M.; Sonke, J.E.; Obrist, D.; Bieser, J.; Ebinghaus, R.; Myhre, C.L.; Pfaffhuber, K.A.; Wängberg, I.; Kyllönen, K.; Worthy, D.; et al. A Vegetation Control on Seasonal Variations in Global Atmospheric Mercury Concentrations. Nat. Geosci. 2018, 11, 244. [Google Scholar] [CrossRef]
- Zhou, J.; Obrist, D.; Dastoor, A.; Jiskra, M.; Ryjkov, A. Vegetation Uptake of Mercury and Impacts on Global Cycling. Nat. Rev. Earth Environ. 2021, 2, 269–284. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, W.; Lin, C.-J.; Feng, X. Mercury Cycling and Isotopic Fractionation in Global Forests. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3763–3786. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Y.; Li, P.; Fu, X.; Zhang, Q.; Wang, X.; Chen, L.; Wang, S.; Wang, F.; Feng, X. Ecosystem Mercury Recovery and Health Benefit Under the Minamata Convention in a Changing Climate. Rev. Environ. Contam. 2022, 260, 15. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Z.; Huang, S.; Zhang, P.; Peng, Y.; Wu, P.; Gu, J.; Dutkiewicz, S.; Zhang, H.; Wu, S.; et al. Global Health Effects of Future Atmospheric Mercury Emissions. Nat. Commun. 2021, 12, 3035. [Google Scholar] [CrossRef]
- Lyman, S.N.; Cheng, I.; Gratz, L.E.; Weiss-Penzias, P.; Zhang, L. An Updated Review of Atmospheric Mercury. Sci. Total Environ. 2020, 707, 135575. [Google Scholar] [CrossRef]
- Sonke, J.E.; Angot, H.; Zhang, Y.; Poulain, A.; Björn, E.; Schartup, A. Global Change Effects on Biogeochemical Mercury Cycling. Ambio 2023, 52, 853–876. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, H.M.; Jacob, D.J.; Zhang, Y.; Dibble, T.S.; Slemr, F.; Amos, H.M.; Schmidt, J.A.; Corbitt, E.S.; Marais, E.A.; Sunderland, E.M. A New Mechanism for Atmospheric Mercury Redox Chemistry: Implications for the Global Mercury Budget. Atmos. Chem. Phys. 2017, 17, 6353–6371. [Google Scholar] [CrossRef]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A Review of Global Environmental Mercury Processes in Response to Human and Natural Perturbations: Changes of Emissions, Climate, and Land Use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Laacouri, A.; Nater, E.A.; Kolka, R.K. Distribution and Uptake Dynamics of Mercury in Leaves of Common Deciduous Tree Species in Minnesota, U.S.A. Environ. Sci. Technol. 2013, 47, 10462–10470. [Google Scholar] [CrossRef]
- Manceau, A.; Wang, J.; Rovezzi, M.; Glatzel, P.; Feng, X. Biogenesis of Mercury–Sulfur Nanoparticles in Plant Leaves from Atmospheric Gaseous Mercury. Environ. Sci. Technol. 2018, 52, 3935–3948. [Google Scholar] [CrossRef]
- Wang, X.; Bao, Z.; Lin, C.-J.; Yuan, W.; Feng, X. Assessment of Global Mercury Deposition through Litterfall. Environ. Sci. Technol. 2016, 50, 8548. [Google Scholar] [CrossRef]
- Zhou, J.; Bollen, S.W.; Roy, E.M.; Hollinger, D.Y.; Wang, T.; Lee, J.T.; Obrist, D. Comparing Ecosystem Gaseous Elemental Mercury Fluxes over a Deciduous and Coniferous Forest. Nat. Commun. 2023, 14, 2722. [Google Scholar] [CrossRef]
- Feinberg, A.; Dlamini, T.; Jiskra, M.; Shah, V.; Selin, N.E. Evaluating Atmospheric Mercury (Hg) Uptake by Vegetation in a Chemistry-Transport Model. Environ. Sci. Process. Impacts 2022, 24, 1303–1318. [Google Scholar] [CrossRef]
- Obrist, D.; Roy, E.M.; Harrison, J.L.; Kwong, C.F.; Munger, J.W.; Moosmueller, H.; Romero, C.D.; Sun, S.; Zhou, J.; Commane, R. Previously Unaccounted Atmospheric Mercury Deposition in a Midlatitude Deciduous Forest. Proc. Natl. Acad. Sci. USA 2021, 118, e2105477118. [Google Scholar] [CrossRef]
- Wohlgemuth, L.; Rautio, P.; Ahrends, B.; Russ, A.; Vesterdal, L.; Waldner, P.; Timmermann, V.; Eickenscheidt, N.; Fürst, A.; Greve, M.; et al. Physiological and Climate Controls on Foliar Mercury Uptake by European Tree Species. Biogeosciences 2022, 19, 1335–1353. [Google Scholar] [CrossRef]
- Zhang, X.; Kang, H.; Liu, X.; Zhou, J.; Liu, M.; Wang, L.; Xing, X.; Lu, Q.; Zeng, X.; Wei, N.; et al. Comparative Foliar Atmospheric Mercury Accumulation across Functional Types in Temperate Trees. Environ. Sci. Technol. 2025, 59, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Siegwolf, R.T.W.; Lehmann, M.M.; Goldsmith, G.R.; Churakova (Sidorova), O.V.; Mirande-Ney, C.; Timoveeva, G.; Weigt, R.B.; Saurer, M. Updating the Dual C and O Isotope—Gas-exchange Model: A Concept to Understand Plant Responses to the Environment and Its Implications for Tree Rings. Plant Cell Environ. 2023, 46, 2606–2627. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, X.; Bishop, K.; Marshall, J.; Nilsson, M.B.; Li, C.; Björn, E.; Zhu, W. Tree Rings Mercury Controlled by Atmospheric Gaseous Elemental Mercury and Tree Physiology. Environ. Sci. Technol. 2024, 58, 16833–16842. [Google Scholar] [CrossRef]
- Sa, R.; Wang, Z.; Xu, Z.; Zhao, Q.; Zhang, Q.; Zhang, X. Distribution Characteristics of Mercury Concentration and Estimation of Mercury Pools in Different Age Groups of Larix gmelinii Forests of Daxing’an Mountain. Environ. Pollut. 2023, 338, 122653. [Google Scholar] [CrossRef]
- McLagan, D.S.; Biester, H.; Navrátil, T.; Kraemer, S.M.; Schwab, L. Internal Tree Cycling and Atmospheric Archiving of Mercury: Examination with Concentration and Stable Isotope Analyses. Biogeosciences 2022, 19, 4415–4429. [Google Scholar] [CrossRef]
- Demers, J.D.; Blum, J.D.; Zak, D.R. Mercury Isotopes in a Forested Ecosystem: Implications for Air-surface Exchange Dynamics and the Global Mercury Cycle. Glob. Biogeochem. Cycles 2013, 27, 222–238. [Google Scholar] [CrossRef]
- Yuan, W.; Sommar, J.; Lin, C.-J.; Wang, X.; Li, K.; Liu, Y.; Zhang, H.; Lu, Z.; Wu, C.; Feng, X. Stable Isotope Evidence Shows Re-Emission of Elemental Mercury Vapor Occurring after Reductive Loss from Foliage. Environ. Sci. Technol. 2019, 53, 651. [Google Scholar] [CrossRef]
- Wohlgemuth, L.; Osterwalder, S.; Joseph, C.; Kahmen, A.; Hoch, G.; Alewell, C.; Jiskra, M. A Bottom-up Quantification of Foliar Mercury Uptake Fluxes across Europe. Biogeosciences 2020, 17, 6441–6456. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Yuan, W.; Wang, D.; Feng, X. Tree Rings Recording Historical Atmospheric Mercury: A Review of Progresses and Challenges. Crit. Rev. Environ. Sci. Technol. 2023, 54, 445–462. [Google Scholar] [CrossRef]
- Yuan, T.; Huang, S.; Zhang, P.; Song, Z.; Ge, J.; Miao, X.; Wang, Y.; Pang, Q.; Peng, D.; Wu, P.; et al. Potential Decoupling of CO2 and Hg Uptake Process by Global Vegetation in the 21st Century. Nat. Commun. 2024, 15, 4490. [Google Scholar] [CrossRef]
- Wohlgemuth, L.; Feinberg, A.; Buras, A.; Jiskra, M. A Spatial Assessment of Current and Future Foliar Hg Uptake Fluxes Across European Forests. Glob. Biogeochem. Cycles 2023, 37, e2023GB007833. [Google Scholar] [CrossRef]
- Teixeira, D.C.; Lacerda, L.D.; Silva-Filho, E.V. Foliar Mercury Content from Tropical Trees and Its Correlation with Physiological Parameters in Situ. Environ. Pollut. 2018, 242, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Wang, X.; Lin, C.-J.; Zhang, G.; Wu, F.; Liu, N.; Jia, L.; Zhang, H.; Lu, H.; Dong, J.; et al. Fate and Transport of Mercury through Waterflows in a Tropical Rainforest. Environ. Sci. Technol. 2024, 58, 4968. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yin, S.; Zhang, X.; Lyu, J.; Zhang, Y.; Zhu, Y.; Yan, J. A High-Resolution Study of PM2.5 Accumulation inside Leaves in Leaf Stomata Compared with Non-Stomatal Areas Using Three-Dimensional X-Ray Microscopy. Sci. Total Environ. 2022, 852, 158543. [Google Scholar] [CrossRef]
- Steinparzer, M.; Schaubmayr, J.; Godbold, D.L.; Rewald, B. Particulate Matter Accumulation by Tree Foliage Is Driven by Leaf Habit Types, Urbanization- and Pollution Levels. Environ. Pollut. 2023, 335, 122289. [Google Scholar] [CrossRef]
- Xu, S.; Li, B.; Li, P.; He, X.; Chen, W.; Yan, K.; Li, Y.; Wang, Y. Soil High Cd Exacerbates the Adverse Impact of Elevated O3 on Populus alba “Berolinensis” L. Ecotoxicol. Environ. Saf. 2019, 174, 35. [Google Scholar] [CrossRef]
- Goyal, D.; Yadav, A.; Vats, T. Air Pollution and Its Role in Stress Physiology. In Air Pollution and Environmental Health; Saxena, P., Srivastava, A., Eds.; Springer: Singapore, 2020; pp. 115–140. ISBN 978-981-15-3481-2. [Google Scholar]
- Peckham, M.A.; Gustin, M.S.; Weisberg, P.J. Assessment of the Suitability of Tree Rings as Archives of Global and Regional Atmospheric Mercury Pollution. Environ. Sci. Technol. 2019, 53, 3663–3671. [Google Scholar] [CrossRef]
- Jiskra, M.; Wiederhold, J.G.; Skyllberg, U.; Kronberg, R.-M.; Hajdas, I.; Kretzschmar, R. Mercury Deposition and Re-Emission Pathways in Boreal Forest Soils Investigated with Hg Isotope Signatures. Environ. Sci. Technol. 2015, 49, 7188–7196. [Google Scholar] [CrossRef]
- Landis, J.D.; Obrist, D.; Zhou, J.; Renshaw, C.E.; McDowell, W.H.; Nytch, C.J.; Palucis, M.C.; Del Vecchio, J.; Montano Lopez, F.; Taylor, V.F. Quantifying Soil Accumulation of Atmospheric Mercury Using Fallout Radionuclide Chronometry. Nat. Commun. 2024, 15, 5430. [Google Scholar] [CrossRef]
- Gerson, J.R.; Szponar, N.; Zambrano, A.A.; Bergquist, B.; Broadbent, E.; Driscoll, C.T.; Erkenswick, G.; Evers, D.C.; Fernandez, L.E.; Hsu-Kim, H.; et al. Amazon Forests Capture High Levels of Atmospheric Mercury Pollution from Artisanal Gold Mining. Nat. Commun. 2022, 13, 559. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, X.; Yuan, W.; Luo, J.; Wang, D. Mercury Accumulation and Dynamics in Montane Forests along an Elevation Gradient in Southwest China. J. Environ. Sci. 2022, 119, 1. [Google Scholar] [CrossRef] [PubMed]
- Gerson, J.R.; Driscoll, C.T.; Demers, J.D.; Sauer, A.K.; Blackwell, B.D.; Montesdeoca, M.R.; Shanley, J.B.; Ross, D.S. Deposition of Mercury in Forests across a Montane Elevation Gradient: Elevational and Seasonal Patterns in Methylmercury Inputs and Production. JGR Biogeosciences 2017, 122, 1922–1939. [Google Scholar] [CrossRef]
- Wang, X.; Luo, J.; Yuan, W.; Lin, C.-J.; Wang, F.; Liu, C.; Wang, G.; Feng, X. Global Warming Accelerates Uptake of Atmospheric Mercury in Regions Experiencing Glacier Retreat. Proc. Natl. Acad. Sci. USA 2020, 117, 2049. [Google Scholar] [CrossRef] [PubMed]
- Woś, B.; Gruba, P.; Socha, J.; Pietrzykowski, M. Biomonitoring of Mercury Contamination in Poland Based on Its Concentration in Scots Pine (Pinus sylvestris L.) Foliage. Int. J. Environ. Res. Public Health 2021, 18, 10366. [Google Scholar] [CrossRef]
- Lodenius, M. Use of Plants for Biomonitoring of Airborne Mercury in Contaminated Areas. Environ. Res. 2013, 125, 113. [Google Scholar] [CrossRef]
- Arnold, J.; Gustin, M.S.; Weisberg, P.J. Evidence for Nonstomatal Uptake of Hg by Aspen and Translocation of Hg from Foliage to Tree Rings in Austrian Pine. Environ. Sci. Technol. 2018, 52, 1174–1182. [Google Scholar] [CrossRef]
- Gačnik, J.; Gustin, M.S. Tree Rings as Historical Archives of Atmospheric Mercury: A Critical Review. Sci. Total Environ. 2023, 898, 165562. [Google Scholar] [CrossRef]
- Stamenkovic, J.; Gustin, M.S. Nonstomatal versus Stomatal Uptake of Atmospheric Mercury. Environ. Sci. Technol. 2009, 43, 1367. [Google Scholar] [CrossRef]
- Bishop, K.H.; Lee, Y.-H.; Munthe, J.; Dambrine, E. Xylem Sap as a Pathway for Total Mercury and Methylmercury Transport from Soils to Tree Canopy in the Boreal Forest. Biogeochmistry 1998, 40, 101–113. [Google Scholar] [CrossRef]
- Yanai, R.D.; Yang, Y.; Wild, A.D.; Smith, K.T.; Driscoll, C.T. New Approaches to Understand Mercury in Trees: Radial and Longitudinal Patterns of Mercury in Tree Rings and Genetic Control of Mercury in Maple Sap. Water Air Soil. Pollut. 2020, 231, 248. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, C.-J.; Yuan, W.; Lu, Z.; Feng, X. Translocation and Distribution of Mercury in Biomasses from Subtropical Forest Ecosystems: Evidence from Stable Mercury Isotopes. Acta Geochim. 2021, 40, 42–50. [Google Scholar] [CrossRef]
- Bardelli, F.; Rimondi, V.; Lattanzi, P.; Rovezzi, M.; Isaure, M.-P.; Giaccherini, A.; Costagliola, P. Pinus nigra Bark from a Mercury Mining District Studied with High Resolution XANES Spectroscopy. Environ. Sci. Process. Impacts 2022, 24, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Rutter, A.P.; Schauer, J.J.; Shafer, M.M.; Creswell, J.; Olson, M.R.; Clary, A.; Robinson, M.; Parman, A.M.; Katzman, T.L. Climate Sensitivity of Gaseous Elemental Mercury Dry Deposition to Plants: Impacts of Temperature, Light Intensity, and Plant Species. Environ. Sci. Technol. 2011, 45, 569. [Google Scholar] [CrossRef] [PubMed]
- Naharro, R.; Maria Esbri, J.; Angel Amoros, J.; Higueras, P.L. Experimental Assessment of the Daily Exchange of Atmospheric Mercury in Epipremnum aureum. Environ. Geochem. Health 2020, 42, 3185. [Google Scholar] [CrossRef]
- Yang, Y.H.; Kim, M.-S.; Park, J.; Kwon, S.Y. Atmospheric Mercury Uptake and Accumulation in Forests Dependent on Climatic Factors. Environ. Sci. Process. Impacts 2024, 26, 519–529. [Google Scholar] [CrossRef]
- Sommar, J.; Zhu, W.; Shang, L.; Lin, C.-J.; Feng, X. Seasonal Variations in Metallic Mercury (Hg0) Vapor Exchange over Biannual Wheat–Corn Rotation Cropland in the North China Plain. Biogeosciences 2016, 13, 2029–2049. [Google Scholar] [CrossRef]
- Converse, A.D.; Riscassi, A.L.; Scanlon, T.M. Seasonal Variability in Gaseous Mercury Fluxes Measured in a High-Elevation Meadow. Atmos. Environ. 2010, 44, 2176–2185. [Google Scholar] [CrossRef]
- Navrátil, T.; Šimeček, M.; Shanley, J.B.; Rohovec, J.; Hojdová, M.; Houška, J. The History of Mercury Pollution near the Spolana Chlor-Alkali Plant (Neratovice, Czech Republic) as Recorded by Scots Pine Tree Rings and Other Bioindicators. Sci. Total Environ. 2017, 586, 1182–1192. [Google Scholar] [CrossRef]
- Sun, M.; Yuan, W.; Liu, N.; Jia, L.; Wu, F.; Huang, J.-H.; Wang, X.; Feng, X. Combined Impacts of Climate and Tree Physiology on Mercury Accumulation in Tropical and Subtropical Foliage and Robust Model Parametrization. Environ. Sci. Technol. 2025, 59, 1661–1672. [Google Scholar] [CrossRef]
- Gustin, M.S.; Ingle, B.; Dunham-Cheatham, S.M. Further Investigations into the Use of Tree Rings as Archives of Atmospheric Mercury Concentrations. Biogeochemistry 2022, 158, 167–180. [Google Scholar] [CrossRef]
- Gustin, M.S.; Dunham-Cheatham, S.M.; Harper, J.F.; Choi, W.-G.; Blum, J.D.; Johnson, M.W. Investigation of the Biochemical Controls on Mercury Uptake and Mobility in Trees. Sci. Total Environ. 2022, 851, 158101. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Luo, L.; Zhang, J.; Christie, P.; Zhang, S. Adsorption of Mercury on Lignin: Combined Surface Complexation Modeling and X-Ray Absorption Spectroscopy Studies. Environ. Pollut. 2012, 162, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Zhang, J.; Shi, W.; Wang, Y.; Tang, Y.; Liu, Z.; Sun, W.; Wang, H.; Guo, J.; Meng, Y.; et al. Mercury Stress Tolerance in Wheat and Maize Is Achieved by Lignin Accumulation Controlled by Nitric Oxide. Environ. Pollut. 2022, 307, 119488. [Google Scholar] [CrossRef] [PubMed]
- Nováková, T.; Navrátil, T.; Demers, J.D.; Roll, M.; Rohovec, J. Contrasting Tree Ring Hg Records in Two Conifer Species: Multi-Site Evidence of Species-Specific Radial Translocation Effects in Scots Pine versus European Larch. Sci. Total Environ. 2021, 762, 144022. [Google Scholar] [CrossRef]
- Chellman, N.; Csank, A.; Gustin, M.S.; Arienzo, M.M.; Vargas Estrada, M.; McConnell, J.R. Comparison of Co-Located Ice-Core and Tree-Ring Mercury Records Indicates Potential Radial Translocation of Mercury in Whitebark Pine. Sci. Total Environ. 2020, 743, 140695. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Wang, D. Assessment of Tree-Ring Mercury Radial Translocation and Age Effect in Masson Pine: Implications for Historical Atmospheric Mercury Reconstruction. J. Environ. Sci. 2024, 138, 266–276. [Google Scholar] [CrossRef]
- Navrátil, T.; Nováková, T.; Shanley, J.B.; Rohovec, J.; Matoušková, Š.; Vaňková, M.; Norton, S.A. Larch Tree Rings as a Tool for Reconstructing 20th Century Central European Atmospheric Mercury Trends. Environ. Sci. Technol. 2018, 52, 11060–11068. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, W.; Lin, C.-J.; Wu, F.; Feng, X. Stable Mercury Isotopes Stored in Masson Pinus Tree Rings as Atmospheric Mercury Archives. J. Hazard. Mater. 2021, 415, 125678. [Google Scholar] [CrossRef]
- Cutter, B.; Guyette, R. Anatomical, Chemical, and Ecological Factors Affecting Tree Species Choice in Dendrochemistry Studies. J. Environ. Qual. 1993, 22, 611–619. [Google Scholar] [CrossRef]
- Lehmann, M.M.; Schuler, P.; Cormier, M.-A.; Allen, S.T.; Leuenberger, M.; Voelker, S. The Stable Hydrogen Isotopic Signature: From Source Water to Tree Rings. In Stable Isotopes in Tree Rings; Siegwolf, R.T.W., Brooks, J.R., Roden, J., Saurer, M., Eds.; Tree Physiology; Springer International Publishing: Cham, Switzerland, 2022; Volume 8, pp. 331–359. ISBN 978-3-030-92697-7. [Google Scholar]
- Dannoura, M.; Epron, D.; Desalme, D.; Massonnet, C.; Tsuji, S.; Plain, C.; Priault, P.; Gérant, D. The Impact of Prolonged Drought on Phloem Anatomy and Phloem Transport in Young Beech Trees. Tree Physiol. 2019, 39, 201–210. [Google Scholar] [CrossRef]
- Quant, M.I.; Feigis, M.; Mistry, S.; Lei, Y.D.; Mitchell, C.P.J.; Staebler, R.; Di Guardo, A.; Terzaghi, E.; Wania, F. Using Passive Air Samplers to Quantify Vertical Gaseous Elemental Mercury Concentration Gradients Within a Forest and Above Soil. JGR Atmos. 2021, 126, e2021JD034981. [Google Scholar] [CrossRef]
- Roy, E.M.; Zhou, J.; Wania, F.; Obrist, D. Use of Atmospheric Concentrations and Passive Samplers to Assess Surface-Atmosphere Exchange of Gaseous Mercury in Forests. Chemosphere 2023, 341, 140113. [Google Scholar] [CrossRef]
- Gaggi, C.; Chemello, G.; Bacci, E. Mercury Vapour Accumulation in Azalea Leaves. Chemosphere 1991, 22, 869–872. [Google Scholar] [CrossRef]
- Loppi, S.; Nelli, L.; Ancora, S.; Bargagli, R. Passive Monitoring of Trace Elements by Means of Tree Leaves, Epiphytic Lichens and Bark Substrate. Environ. Monit. Assess. 1997, 45, 81–88. [Google Scholar] [CrossRef]
- Barquero, J.I.; Rojas, S.; Esbrí, J.M.; García-Noguero, E.M.; Higueras, P. Factors Influencing Mercury Uptake by Leaves of Stone Pine (Pinus pinea L.) in Almadén (Central Spain). Environ. Sci. Pollut. Res. 2019, 26, 3129–3137. [Google Scholar] [CrossRef]
- Fantozzi, L.; Ferrara, R.; Dini, F.; Tamburello, L.; Pirrone, N.; Sprovieri, F. Study on the Reduction of Atmospheric Mercury Emissions from Mine Waste Enriched Soils through Native Grass Cover in the Mt. Amiata Region of Italy. Environ. Res. 2013, 125, 69–74. [Google Scholar] [CrossRef]
- Poissant, L.; Pilote, M.; Yumvihoze, E.; Lean, D. Mercury Concentrations and Foliage/Atmosphere Fluxes in a Maple Forest Ecosystem in Québec, Canada. J. Geophys. Res. 2008, 113, 2007JD009510. [Google Scholar] [CrossRef]
- McClenahen, J.R.; Hutnik, R.J.; Davis, D.D. Spatial and Temporal Patterns of Bioindicator Mercury in Pennsylvania Oak Forest. J. Environ. Qual. 2013, 42, 305–311. [Google Scholar] [CrossRef]
- Monaci, F.; Ancora, S.; Paoli, L.; Loppi, S.; Franzaring, J. Air Quality in Post-Mining Towns: Tracking Potentially Toxic Elements Using Tree Leaves. Environ. Geochem. Health 2023, 45, 843–859. [Google Scholar] [CrossRef]
- Viso, S.; Rivera, S.; Martinez-Coronado, A.; Esbrí, J.M.; Moreno, M.M.; Higueras, P. Biomonitoring of Hg0, Hg2 and Particulate Hg in a Mining Context Using Tree Barks+. Int. J. Environ. Res. Public Health 2021, 18, 5191. [Google Scholar] [CrossRef]
- Franzaring, J.; Haneke, J.; Sannino, A.; Radermacher, G.; Schweiger, A. Effects of Legacy Mining on Mercury Concentrations in Conifer Needles and Mushrooms in Northern Palatinate, Germany. Environ. Pollut. 2024, 357, 124406. [Google Scholar] [CrossRef] [PubMed]
- Nováková, T.; Navrátil, T.; Schütze, M.; Rohovec, J.; Matoušková, Š.; Hošek, M.; Matys Grygar, T. Reconstructing Atmospheric Hg Levels near the Oldest Chemical Factory in Central Europe Using a Tree Ring Archive. Environ. Pollut. 2022, 304, 119215. [Google Scholar] [CrossRef]
- Pleijel, H.; Klingberg, J.; Nerentorp, M.; Broberg, M.C.; Nyirambangutse, B.; Munthe, J.; Wallin, G. Mercury Accumulation in Leaves of Different Plant Types—The Significance of Tissue Age and Specific Leaf Area. Biogeosciences 2021, 18, 6313. [Google Scholar] [CrossRef]
- Pleijel, H.; Klingberg, J.; Sjöman, H.; Wallin, G. Leaf Age Affects Mercury Accumulation in Evergreen Plants. Water Air Soil. Pollut. 2025, 236, 115. [Google Scholar] [CrossRef]
- Frank, D.; Fang, K.; Fonti, P. Dendrochronology: Fundamentals and Innovations. In Stable Isotopes in Tree Rings; Siegwolf, R.T.W., Brooks, J.R., Roden, J., Saurer, M., Eds.; Tree Physiology; Springer International Publishing: Cham, Swizerland, 2022; Volume 8, pp. 21–59. ISBN 978-3-030-92697-7. [Google Scholar]
- Kang, H.; Liu, X.; Zhang, X.; Guo, J.; Huang, J.; Ying, X.; Wang, Y.; Zhang, Q.; Kang, S. Important Accumulated Mercury Pool in a Remote Alpine Forest and Dynamic Accumulation Revealed by Tree Rings in China’s Qilian Mountains. Sci. Total Environ. 2024, 951, 175441. [Google Scholar] [CrossRef]
- Canning, C.M.; Laroque, C.P.; Muir, D. Critical Analysis of the Past, Present, and Future of Dendrochemistry: A Systematic Literature Review. Forests 2023, 14, 1997. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, J.-L.; Planas, D. Mercury Concentration in Tree Rings of Black Spruce (Picea mariana Mill. B.S.P.) in Boreal Quebec, Canada. Water Air Soil. Pollut. 1995, 81, 163–173. [Google Scholar] [CrossRef]
- Cooke, C.A.; Martínez-Cortizas, A.; Bindler, R.; Sexauer Gustin, M. Environmental Archives of Atmospheric Hg Deposition—A Review. Sci. Total Environ. 2020, 709, 134800. [Google Scholar] [CrossRef]
- Binda, G.; Di Iorio, A.; Monticelli, D. The What, How, Why, and When of Dendrochemistry: (Paleo)Environmental Information from the Chemical Analysis of Tree Rings. Sci. Total Environ. 2021, 758, 143672. [Google Scholar] [CrossRef]
- Cocozza, C.; Alterio, E.; Bachmann, O.; Guillong, M.; Sitzia, T.; Cherubini, P. Monitoring Air Pollution Close to a Cement Plant and in a Multi-Source Industrial Area through Tree-Ring Analysis. Environ. Sci. Pollut. Res. 2021, 28, 54030–54040. [Google Scholar] [CrossRef]
- Ballikaya, P.; Marshall, J.; Cherubini, P. Can Tree-Ring Chemistry Be Used to Monitor Atmospheric Nanoparticle Contamination over Time? Atmos. Environ. 2022, 268, 118781. [Google Scholar] [CrossRef]
- Miyahara, A.A.L.; Locosselli, G.M. Challenges and Advances in Intra-Annual Tree-Ring Stable Isotope Research, a Systematic Review. Dendrochronologia 2024, 85, 126218. [Google Scholar] [CrossRef]
- Scanlon, T.M.; Riscassi, A.L.; Demers, J.D.; Camper, T.D.; Lee, T.R.; Druckenbrod, D.L. Mercury Accumulation in Tree Rings: Observed Trends in Quantity and Isotopic Composition in Shenandoah National Park, Virginia. JGR Biogeosci. 2020, 125, e2019JG005445. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Wang, Z.; Guo, Y.; Yin, Y.; Zhang, X.; Cai, Y.; Jiang, G. Understanding Foliar Accumulation of Atmospheric Hg in Terrestrial Vegetation: Progress and Challenges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4331–4352. [Google Scholar] [CrossRef]
- Tatzber, M.; Fürst, A. Mercury in Tree Rings Close to Emission Sources in Austria. Environ. Sci. Pollut. Res. 2023, 30, 86084–86096. [Google Scholar] [CrossRef]
- Ghotra, A.; Lehnherr, I.; Porter, T.J.; Pisaric, M.F.J. Tree-Ring Inferred Atmospheric Mercury Concentrations in the Mackenzie Delta (NWT, Canada) Peaked in the 1970s but Are Increasing Once More. ACS Earth Space Chem. 2020, 4, 457–466. [Google Scholar] [CrossRef]
- Clackett, S.P.; Porter, T.J.; Lehnherr, I. The Tree-Ring Mercury Record of Klondike Gold Mining at Bear Creek, Central Yukon. Environ. Pollut. 2021, 268, 115777. [Google Scholar] [CrossRef]
- Baroni, D.; Ancora, S.; Franzaring, J.; Loppi, S.; Monaci, F. Tree-Rings Analysis to Reconstruct Atmospheric Mercury Contamination at a Historical Mining Site. Front. Plant Sci. 2023, 14, 1260431. [Google Scholar] [CrossRef]
- Fornasaro, S.; Ciani, F.; Nannoni, A.; Morelli, G.; Rimondi, V.; Lattanzi, P.; Cocozza, C.; Fioravanti, M.; Costagliola, P. Tree Rings Record of Long-Term Atmospheric Hg Pollution in the Monte Amiata Mining District (Central Italy): Lessons from the Past for a Better Future. Minerals 2023, 13, 688. [Google Scholar] [CrossRef]
- Maillard, F.; Girardclos, O.; Assad, M.; Zappelini, C.; Pérez Mena, J.M.; Yung, L.; Guyeux, C.; Chrétien, S.; Bigham, G.; Cosio, C.; et al. Dendrochemical Assessment of Mercury Releases from a Pond and Dredged-Sediment Landfill Impacted by a Chlor-Alkali Plant. Environ. Res. 2016, 148, 122–126. [Google Scholar] [CrossRef]
- Rodríguez Martín, J.A.; Nanos, N.; Miranda, J.C.; Carbonell, G.; Gil, L. Volcanic Mercury in Pinus canariensis. Naturwissenschaften 2013, 100, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Martin, J.A.; Gutiérrez, C.; Torrijos, M.; Nanos, N. Wood and Bark of Pinus halepensis as Archives of Heavy Metal Pollution in the Mediterranean Region. Environ. Pollut. 2018, 239, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Allen, K.; Walker, M.; Morgan, C.; Haberle, S. Using Tree Rings to Track Atmospheric Mercury Pollution in Australia: The Legacy of Mining in Tasmania. Environ. Sci. Technol. 2019, 53, 5697–5706. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yanai, R.D.; Montesdeoca, M.; Driscoll, C.T. Measuring Mercury in Wood: Challenging but Important. Int. J. Environ. Anal. Chem. 2017, 97, 456–467. [Google Scholar] [CrossRef]
- Yang, Y.; Yanai, R.D.; Driscoll, C.T.; Montesdeoca, M.; Smith, K.T. Concentrations and Content of Mercury in Bark, Wood, and Leaves in Hardwoods and Conifers in Four Forested Sites in the Northeastern USA. PLoS ONE 2018, 13, e0196293. [Google Scholar] [CrossRef]
- Watmough, S.A.; Hutchinson, T.C.; Evans, R.D. Development of Solid Calibration Standards for Trace Elemental Analyses of Tree Rings by Laser Ablation Inductively Coupled Plasma-Mass Spectrometry. Environ. Sci. Technol. 1998, 32, 2185–2190. [Google Scholar] [CrossRef]
- Perone, A.; Cocozza, C.; Cherubini, P.; Bachmann, O.; Guillong, M.; Lasserre, B.; Marchetti, M.; Tognetti, R. Oak Tree-Rings Record Spatial-Temporal Pollution Trends from Different Sources in Terni (Central Italy). Environ. Pollut. 2018, 233, 278–289. [Google Scholar] [CrossRef]
- Gačnik, J.; Živković, I.; Horvat, M. Mercury Isotopes in the Atmosphere: Synthesis, Perspectives and Analytical Considerations. TrAC Trends Anal. Chem. 2025, 189, 118257. [Google Scholar] [CrossRef]
- Yuan, T.; Zhang, P.; Song, Z.; Huang, S.; Wang, X.; Zhang, Y. Buffering Effect of Global Vegetation on the Air-Land Exchange of Mercury: Insights from a Novel Terrestrial Mercury Model Based on CESM2-CLM5. Environ. Int. 2023, 174, 107904. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monaci, F.; Baroni, D. Leaves and Tree Rings as Biomonitoring Archives of Atmospheric Mercury Deposition: An Ecophysiological Perspective. Plants 2025, 14, 1275. https://doi.org/10.3390/plants14091275
Monaci F, Baroni D. Leaves and Tree Rings as Biomonitoring Archives of Atmospheric Mercury Deposition: An Ecophysiological Perspective. Plants. 2025; 14(9):1275. https://doi.org/10.3390/plants14091275
Chicago/Turabian StyleMonaci, Fabrizio, and Davide Baroni. 2025. "Leaves and Tree Rings as Biomonitoring Archives of Atmospheric Mercury Deposition: An Ecophysiological Perspective" Plants 14, no. 9: 1275. https://doi.org/10.3390/plants14091275
APA StyleMonaci, F., & Baroni, D. (2025). Leaves and Tree Rings as Biomonitoring Archives of Atmospheric Mercury Deposition: An Ecophysiological Perspective. Plants, 14(9), 1275. https://doi.org/10.3390/plants14091275






