Response of Soil Microbial Diversity to Triple-Cropping System in Paddy Fields in Middle Reaches of Yangtze River
Abstract
1. Introduction
2. Results
2.1. The Effects of Triple-Cropping System on Soil Chemical Properties in Paddy Fields in the Middle Reaches of the Yangtze River
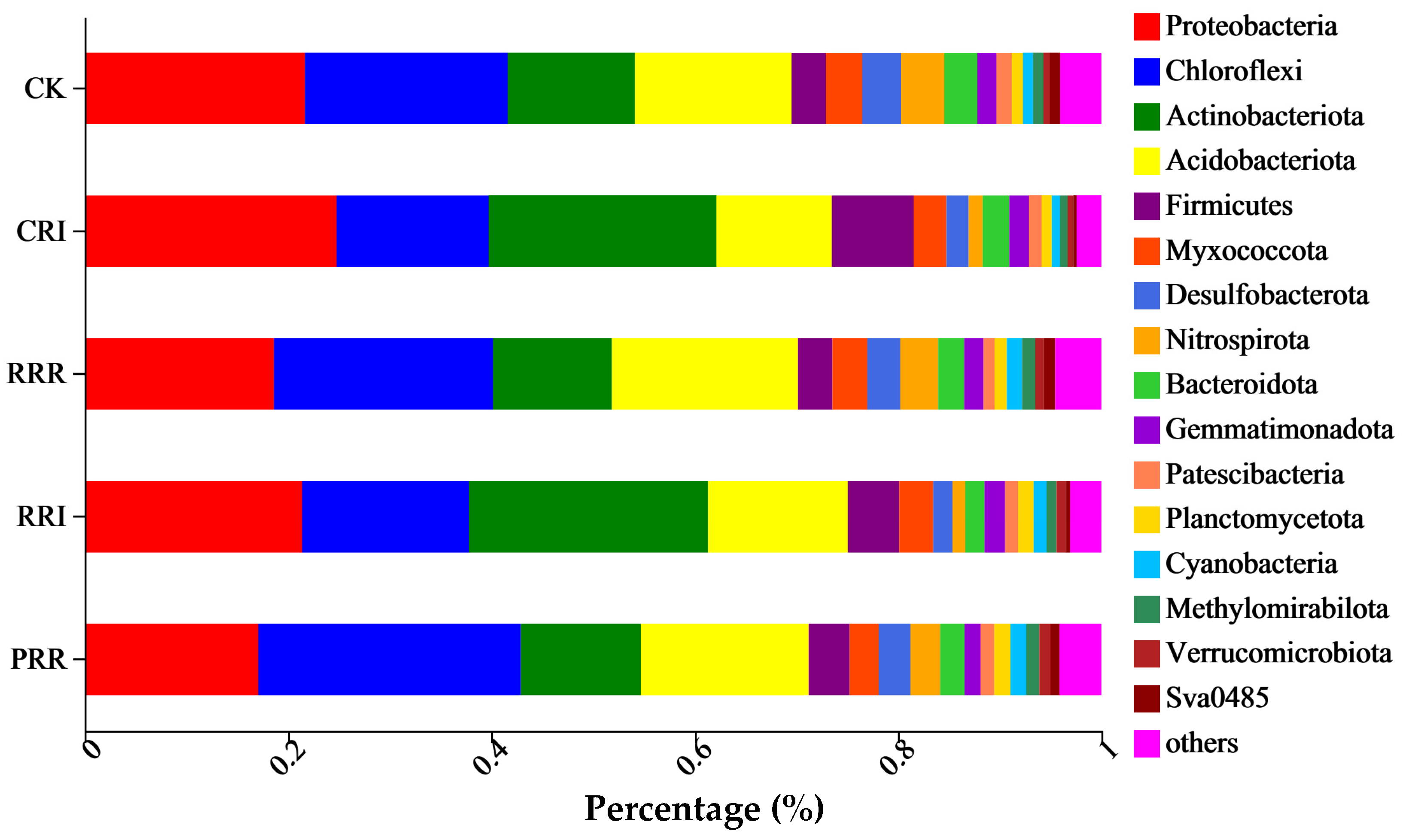
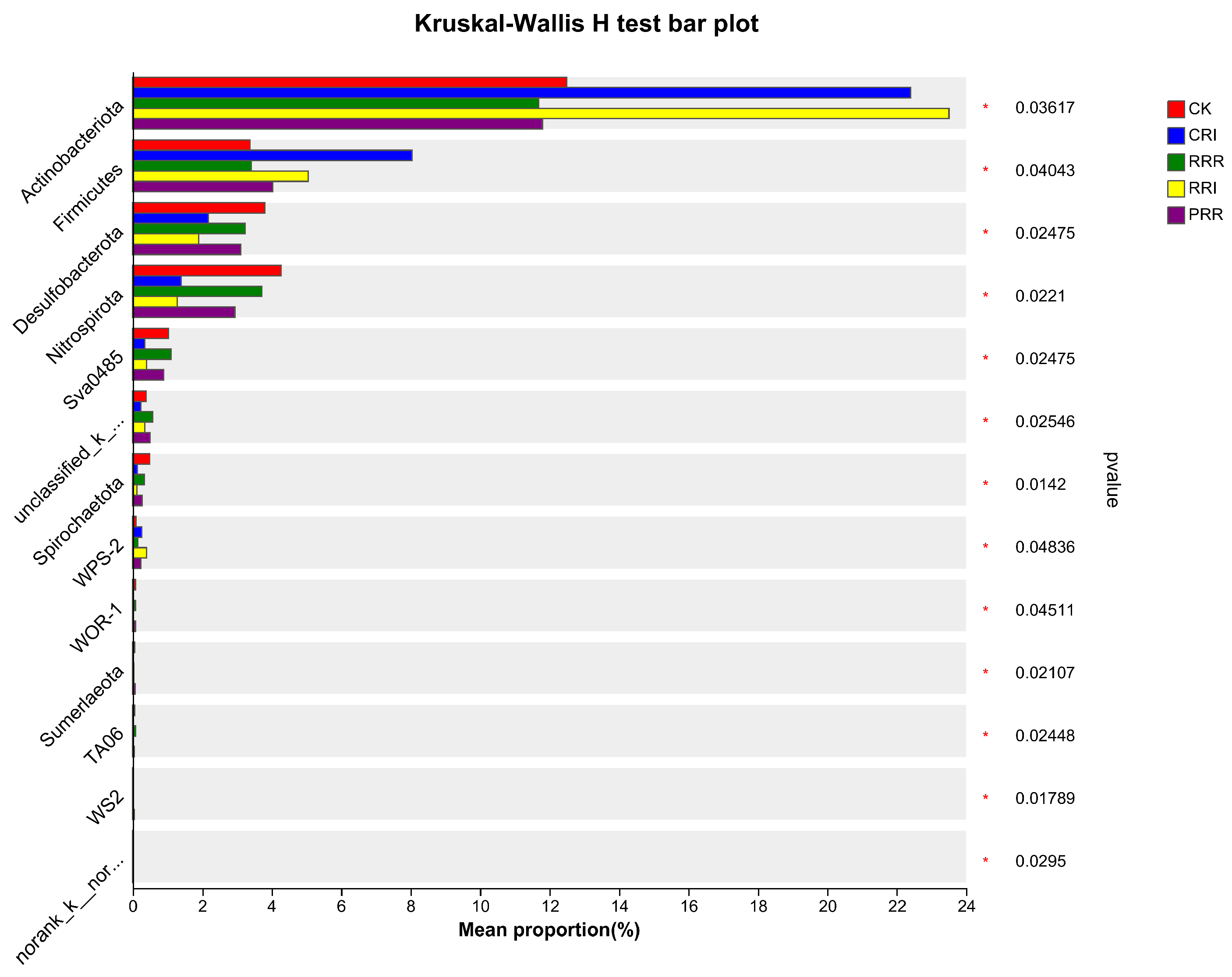
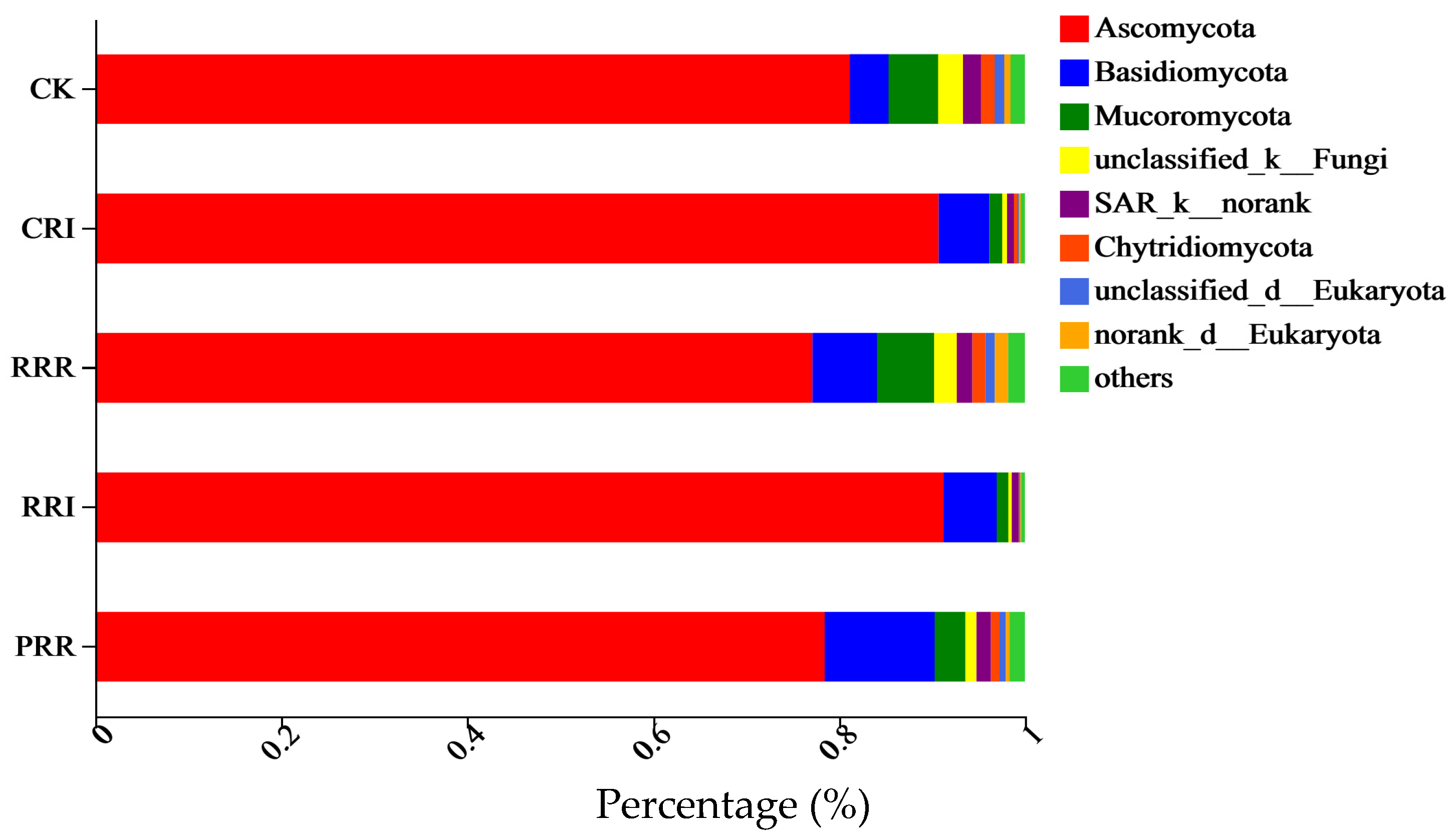
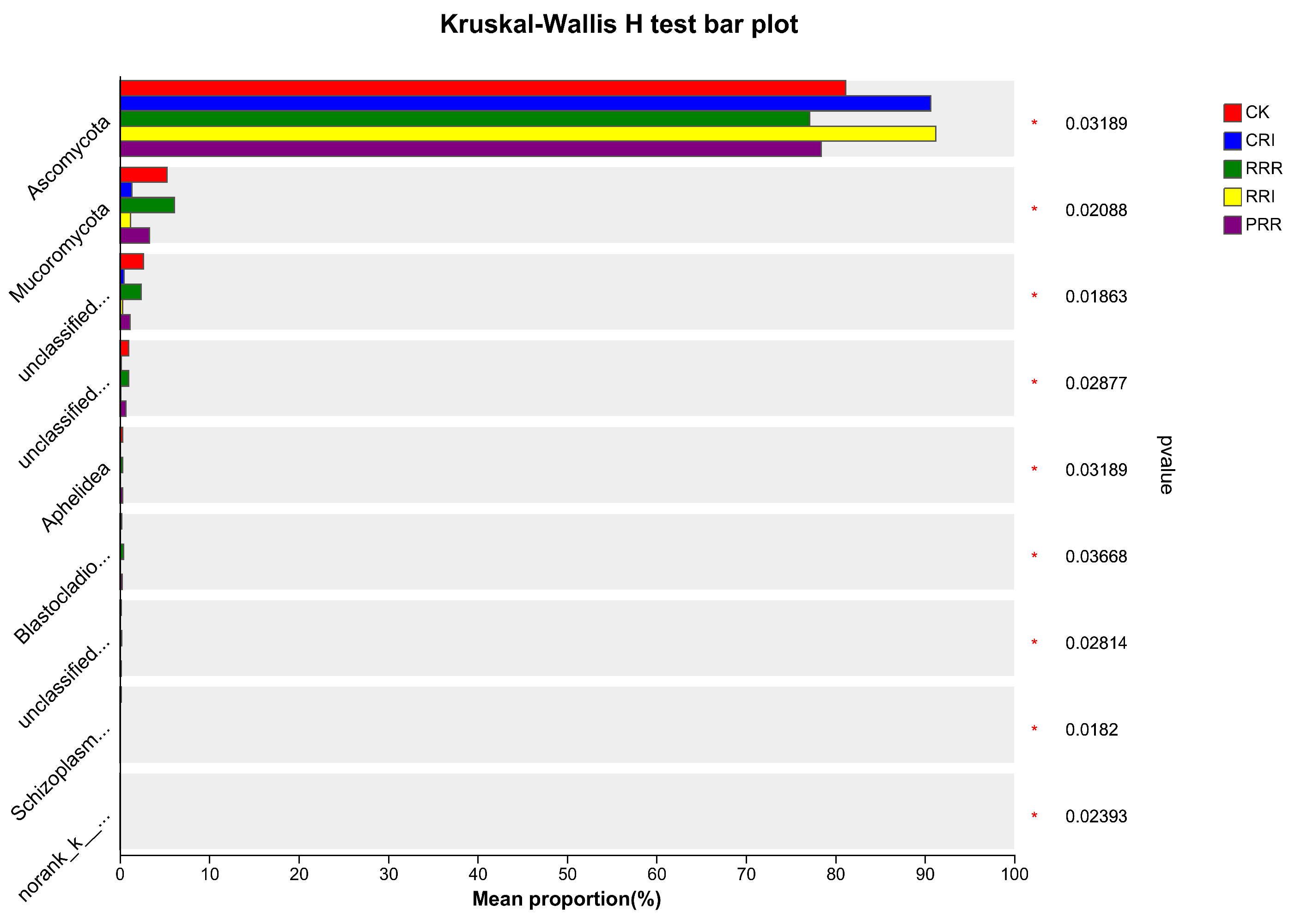
2.2. The Effects of Triple-Cropping System on Soil Microbial Community Diversity in Paddy Fields in the Middle Reaches of the Yangtze River
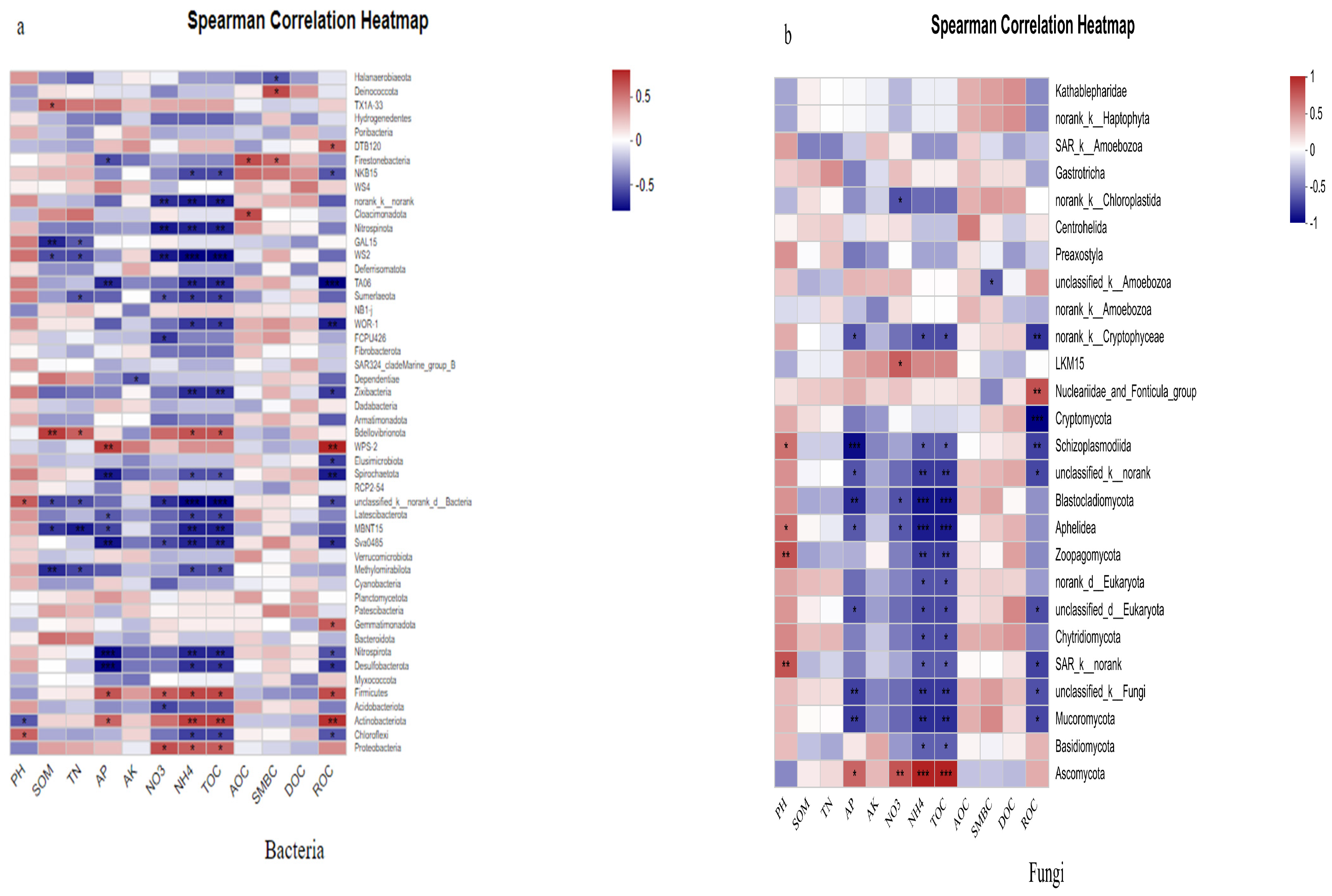
2.3. Correlation Analysis Between Soil Environmental Factors and Soil Microbial Community Structure Diversity
3. Discussions
3.1. Effects of Triple-Cropping System on Soil Chemical Properties in Double-Cropping Rice Area of Middle Reaches of Yangtze River
3.2. Effects of Triple-Cropping System on Soil Microbial Community Structure Diversity in Double-Cropping Rice Paddy Field in Middle Reaches of Yangtze River
3.2.1. Soil Microbial α Diversity
3.2.2. Soil Microbial Species Composition
3.3. Correlation Analysis Between Soil Environmental Factors and Soil Microorganisms
4. Materials and Methods
4.1. Experimental Site
4.2. Test Materials and Field Experiment Design
4.3. Determination of Soil Chemical Properties
4.4. Determination of Soil Microbial Community Structure Diversity
4.5. Data Processing
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Chi, Y.; Song, S. Important soil microbiota’s effects on plants and soils: A comprehensive 30-year systematic literature review. Front. Microbiol. 2024, 15, 1347745. [Google Scholar] [CrossRef] [PubMed]
- Nabi, M. Role of microorganisms in plant nutrition and soil health. In Sustainable Plant Nutrition; Academic Press: Cambridge, MA, USA, 2023; pp. 263–282. [Google Scholar]
- Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K. Enhancing soil health and plant growth through microbial fertilizers: Mechanisms, benefits, and sustainable agricultural practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Vincze, É.B.; Becze, A.; Laslo, É.; Mara, G. Beneficial Soil Microbiomes and Their Potential Role in Plant Growth and Soil Fertility. Agriculture 2024, 14, 152. [Google Scholar] [CrossRef]
- Islam, W.; Noman, A.; Naveed, H.; Huang, Z.; Chen, H.Y.H. Role of environmental factors in shaping the soil microbiome. Environ. Sci. Pollut. Res. 2020, 27, 41225–41247. [Google Scholar] [CrossRef]
- Khaziev, F.K. Soil and biodiversity. Russ. J. Ecol. 2011, 42, 199–204. [Google Scholar] [CrossRef]
- Li, Y.M.; Lin, Q.Y.; Wang, S.P.; Li, X.Z.; Liu, W.T.; Luo, C.Y.; Zhang, Z.H.; Zhu, X.X.; Jiang, L.L.; Li, X.N. Soil bacterial community responses to warming and grazing in a Tibetan alpine meadow. FEMS Microbiol. Ecol. 2016, 92, fiv152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.P.; Huang, Y.T.; Liu, B.Y.; Chen, J.Y.; Lei, Z.Y.; Zhang, Y.H.; Cheng, B.H.; Zhou, T.; Peng, S.L. Effects of daytime and nighttime warming on soil microbial diversity. Geoderma 2024, 447, 116909. [Google Scholar] [CrossRef]
- Mo, Y.; Bier, R.; Li, X.; Daniels, M.; Smith, A.; Yu, L.; Kan, J. Agricultural practices influence soil microbiome assembly and interactions at different depths identified by machine learning. Communi. Biol. 2024, 7, 1349. [Google Scholar] [CrossRef]
- Suman, J.; Rakshit, A.; Ogireddy, S.D.; Singh, S.; Gupta, C.; Chandrakala, J. Microbiome as a key player in sustainable agriculture and human health. Front. Soil Sci. 2022, 2, 821589. [Google Scholar] [CrossRef]
- Khan, M.F.; Hof, C.; Niemcova, P.; Murphy, C.D. Biotransformation of fluorinated drugs and xenobiotics by the model fungus Cunninghamella elegans. Methods Enzymol. 2024, 696, 251–285. [Google Scholar]
- Khan, M.F.; Chowdhary, S.; Koksch, B.; Murphy, C.D. Biodegradation of amphipathic fluorinated peptides reveals a new bacterial defluorinating activity and a new source of natural organofluorine compounds. Environ. Sci. Technol. 2023, 57, 9762–9772. [Google Scholar] [CrossRef]
- Feng, C.; Yi, Z.; Qian, W.; Liu, H.; Jiang, X. Rotations improve the diversity of rhizosphere soil bacterial communities, enzyme activities and tomato yield. PLoS ONE 2023, 18, e0270944. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.; Wang, R.; Guo, S.; Gao, C. Impact of root diversity upon coupling between soil C and N accumulation and bacterial community dynamics and activity: Result of a 30 year rotation experiment. Geoderma 2017, 292, 87–95. [Google Scholar] [CrossRef]
- Tang, J.W.; Zeng, B.J.; Liang, Y.G.; Li, C.; Chen, J.H. Research advances in effects of different patterns on soil microbial communities. Agric. Res. Appl. 2014, 5, 65–69. [Google Scholar]
- Zhang, J.; Cao, W.; Xu, C.; Liu, J. Effects of incorporation of milk vetch (Astragalus sinicus) on microbial populations and enzyme activities of paddy soil in Jiangxi. Soil Fertil. Sci. China 2012, 49, 19–25. [Google Scholar]
- Zhang, X.; Zhang, R.; Gao, J.; Wang, X.; Fan, F.; Ma, X.; Yin, H.; Zhang, C.; Feng, K.; Deng, Y. Thirty-one years of rice-rice-green manure rotations shape the rhizosphere microbial community and enrich beneficial bacteria. Soil Biol. Biochem. 2017, 104, 208–217. [Google Scholar] [CrossRef]
- Bottner, P.; Austrui, F.; Cortez, J.; Billes, G.; Couteaux, M. Decomposition of 14C-and 15N-labelled plant material, under controlled conditions, in coniferous forest soils from a north–south climatic sequence in Western Europe. Soil Biol. Biochem. 1998, 30, 597–610. [Google Scholar] [CrossRef]
- Huang, M.; Tian, A.; Chen, J.; Cao, F.; Chen, Y.; Liu, L. Soil bacterial communities in three rice-based cropping systems differing in productivity. Sci. Rep. 2020, 10, 9867. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Meng, X.; Wang, R.; Chen, L.; Zhou, J.; Chen, C.; Huang, H. Effects of long-term application of rice-turtle co-culture on soil bacterial community structure and diversity. J. South. Agric. 2021, 52, 1860–1868. [Google Scholar]
- Hu, R.; Zheng, L.; Liu, H.; Huang, J. Effects of straw returning on microbial diversity in rice rhizosphere and occurrence of rice sheath blight. J. Plant Prot. 2020, 47, 1261–1269. [Google Scholar]
- Xie, Y.; Chen, X.; Hu, Z.; Chen, S.; Zhang, H.; Ling, H.; Shen, S. Effects of short-time conservation tillage managements on greenhouse gases emissions from soybean-winter wheat rotation system. Huan Jing Ke Xue 2016, 37, 1499–1506. [Google Scholar]
- Wang, S.; Yang, W.; Yang, B.; Wang, L.; Huang, G. Effects of different winter plantings on soil aggregate structure, organic carbon and total nitrogen in double rice field. Acta Agric. Univ. 2018, 40, 1–9. [Google Scholar]
- Yuan, J.; Yang, B.; Hu, Q.; Tang, H.; Li, S.; Huang, G. Effects of paddy field cropping patterns on soil organic carbon and carbon pool management index in the middle reaches of the Yangtze River. Chin. J. Eco-Agric. 2021, 29, 1205–1214. [Google Scholar]
- Wang, Z.; Miu, J.; Liu, Y.; Tang, H.; Zhang, P.; Zhong, C.; Huang, G.; Zhao, Q. Effect of various crops rotations on soil quality in double cropping rice field in the middle reaches of the Yangtze River. Chin. J. Eco-Agric. 2020, 28, 1703–1714. [Google Scholar]
- Yang, B.; Huang, G.; Chen, H.; Yan, L. Optimum combination of winter green manure plowed and nitrogen application levels for high nitrogen uptake and utilization in rice. J. Plant Nutr. Fertil. 2016, 22, 1187–1195. [Google Scholar]
- Pan, F.; Lu, J.; Liu, W.; Geng, M.; Li, X.; Cao, W. Study on characteristics of decomposing and nutrients releasing of three kinds of green manure crops. J. Plant Nutr. Fertil. 2011, 17, 216–223. [Google Scholar]
- Hou, X.; Jia, Z.; Han, Q.; Sun, H.; Wang, W.; Nie, J.; Yang, B. Effects of different rotational tillage patterns on soil structure, infiltration and water storage characteristics in dryland. Trans. Chin. Soc. Agric. Mach. 2012, 28, 85–94. [Google Scholar]
- Yang, B.; Huang, G. Circulating agricultural model of planting Astragalus sinensis in winter in double-cropping paddy field. Jiangsu Agric. Sci. 2018, 46, 51–56. [Google Scholar]
- Lin, Z.H.; Chen, T.B.; Zhou, L.X. Characteristics of the application of chemical fertilizers and their rational. Resour. Sci. 1998, 20, 29–34. [Google Scholar]
- Ji, W.Y.; Si, L.L.; Wang, J.H. Effect of Astragalus sinicus application instead of chemical fertilizer on soil nutrients and rice yield. J. Zhejiang Agr. Sci. 2022, 63, 29–31. [Google Scholar]
- Wan, S.; Zhu, H.; Tang, S.; Xi, G.; Wang, Y. Effects of Astragalus sinicus manure and fertilizer combined application on biological properties of soil in Anhui double cropping rice areas along the Yangtze River. J. Plant Nutr. Fertil. 2015, 21, 387–395. [Google Scholar]
- Wang, L.M.; Huang, D.F.; He, C.M. Impacts of the Chinese milk vetch (Astragalus sinicus L.) residue incorporation on soil physicochemical, microbial properties and rice yields in yellow-mud paddy field. Acta Ecol. Sin. 2023, 11, 1–16. [Google Scholar]
- Liu, C.; Zhang, C.; Li, B.; Lyu, Y.; Nie, L.; Zhang, L. Effects of Astragalus sinicus combined with chemical fertilizer on nitrogen absorption and utilization of rice and nitrogen distribution and residue of Astragalus sinicus in rice-soil system. J. Appl. Ecol. 2021, 32, 1791–1798. [Google Scholar]
- Zhang, S.; Lu, J.; Cong, R.; Ren, T.; Li, X.; Liao, S.; Zhang, Y.; Guo, S.; Zhou, M.; Huang, Y. Effect of rapeseed rotation on the yield of next-stubble crops. Sci. Agric. Sin. 2020, 53, 2852–2858. [Google Scholar]
- Yang, T.; Wang, Y.-Q.; Wu, H.-L.; Yu, Y.; Chen, J.-R.; Chen, X.-F.; Qin, W.-J.; Liu, J.; Xu, C.-X. Analysis of seasonal distribution characteristics and pollution risk of nitrogen and phosphorus losses in double-cropping rice fields in the Poyang Lake Plain. J. Agric. Environ. Sci. 2023, 42, 852–860. [Google Scholar]
- Liu, Z.; Rong, Q.; Zhou, W.; Liang, G. Effects of inorganic and organic amendment on soil chemical properties, enzyme activities, microbial community and soil quality in yellow clayey soil. PLoS ONE 2017, 12, e0172767. [Google Scholar] [CrossRef]
- Lin, Y.; Ye, G.; Kuzyakov, Y.; Liu, D.; Fan, J.; Ding, W. Long-term manure application increases soil organic matter and aggregation, and alters microbial community structure and keystone taxa. Soil Biol. Biochem. 2019, 134, 187–196. [Google Scholar] [CrossRef]
- Liming, Z.; Xiaohua, D.; Miliang, Z.; Feng, T.; Jiongping, Z.; Zhimin, J.; Panfeng, J.; Minghua, Z. Effects of different green manures on microbial biomass and enzyme activities of tobacco-planting soil. Chin. Tob. Sci. 2016, 37, 13–18. [Google Scholar]
- Yang, Z.; XU, M.; Zheng, S.; Jun, N.; Gao, J.; Liao, Y.; Jian, X. Effects of long-term winter planted green manure on physical properties of reddish paddy soil under a double-rice cropping system. J. Integr. Agric. 2012, 11, 655–664. [Google Scholar] [CrossRef]
- Jin, Y.; Li, X.; Cai, Y.; Hu, H.; Liu, Y.; Fu, S.; Zhang, B. Effects of straw returning with chemical fertilizer on soil enzyme activities and microbial community structure in rice-rape rotation. Environ. Sci. 2021, 42, 3985–3996. [Google Scholar]
- Liu, W.; Zhang, J.; Qiu, C.; Bao, Y.; Feng, Y.; Lin, X. Study on community assembly processes under paddy-upland rotation. Soils 2020, 52, 710–717. [Google Scholar]
- Wang, X.T.; Chen, R.R.; Jin, Z.W.; Feng, Y.Z.; Yao, T.Y.; Lin, X.G. Comparative study on rhizosphere effects and bacterial communities in the rhizospheres of rice and wheat. Acta Pedol. Sin. 2019, 56, 443–453. [Google Scholar]
- Wang, Q.; Jiang, X.; Guan, D.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Li, L.; Cao, F.; Li, J. Long-term fertilization changes bacterial diversity and bacterial communities in the maize rhizosphere of Chinese Mollisols. Appl. Soil Ecol. 2018, 125, 88–96. [Google Scholar] [CrossRef]
- Pu, J.; Li, Z.; Zhong, J.; Jin, Z.; Tang, H.; Wei, C.; Dong, W.; He, T.; Li, Q. Effect of the combination of green manure with nitrogen fertilizer on microbial community in karst paddy soil. Acta Microbiol. Sin. 2022, 62, 2417–2432. [Google Scholar]
- Lin, X.; Shi, H.; Wu, L.; Cheng, Y.; Cai, S.; Huang, S.; He, S.; Huang, Q.; Zhang, K. Effects of cultivation methods on soil microbial community structure and diversity in Red Paddy. Ecol. Environ. 2020, 29, 2206. [Google Scholar]
- Zhong, J.; Tang, H.-Q.; Li, Z.; Dong, W.; Wei, C.; Li, Q.; He, T. Effects of combining green manure with chemical fertilizer on the bacterial community structure in karst paddy soil. J. Plant Nutr. Fertil. 2021, 27, 1746–1756. [Google Scholar]
- Wang, G.; Liu, J.; Yu, Z.; Wang, X.; Jin, J.; Liu, X. Research progress of Acidobacteria ecology in soils. Biotechnol. Bull. 2016, 32, 14. [Google Scholar]
- Ouchari, L.; Boukeskasse, A.; Bouizgarne, B.; Ouhdouch, Y. Antimicrobial potential of actinomycetes isolated from the unexplored hot Merzouga desert and their taxonomic diversity. Biol. Open 2019, 8, 035410. [Google Scholar] [CrossRef]
- Nie, S.; Wang, Y.; Lei, X.; Zhao, L.; Lin, R.; Wang, F.; Xing, S. Responses of fungal community structure and functional group to fertilization in yellow clayey soil. Chin. J. Appl. Ecol. 2018, 29, 2721–2729. [Google Scholar]
- Xu, H.F.; Wang, Z.W.; Sheng, R. Effects of farming patterns on abundance and community structure of fungi in paddy soils. J. Huazhong Agric. 2022, 41, 35–41. [Google Scholar]
- Zhang, L.; Shao, J.; Lin, Y.; Kuang, X.; Zhang, H.; Qing, C.; Ma, L.; Yao, B. Influence of microbial diversity and activity of soil on the rice-rice-rape rotation. Ecol. Environ. Sci. 2017, 26, 204–210. [Google Scholar]
- Li, Y.; Sheng, H.; Yin, Z.; Xiao, H.; Xue, Y.; Zhou, P. Response of microbial community in purple mud of double-cropped rice fields to 5-year continuous application of organic amendment and liming. Chin. J. Soil Sci. 2022, 53, 482–491. [Google Scholar]
- Yang, X.; Huang, X.; Wang, C.; Wang, X.; Yin, X.; Lin, S.; Wang, W. Comparison of fungal community structure and diversity in typical paddy fields. China Environ. Sci. 2020, 40, 4549–4556. [Google Scholar]
- Zhao, C.M.; Wang, W.B.; Zhang, Y.F. Characterization of soil microbial community structure among different parent materials in rubber plantation. J. South. Agric. 2021, 52, 1869–1876. [Google Scholar]
- Wang, Y.Y.; Wang, Z.F.; Huang, R.; Lv, S.; Gao, M. Characterization of soil microbial community structure as affected by vegetation in Jinyun Mountain. Acta Pedol. Sin. 2019, 56, 1210–1220. [Google Scholar]
- Qingyuan, L.; Chunbo, H. AMF helps in carbon sequestration in soil. Mar. Geol. Front. 2016, 32, 41–46. [Google Scholar]
- Bao, S.D. Soil Agricultural Chemical Analysis; China Agricultural Press: Beijing, China, 2000; pp. 265–267. [Google Scholar]
- Olsen, S.; Sommers, L.; Page, A. Methods of soil analysis: Part 2 chemical and microbiological properties of phosphorus. In ASA Monograph 9; Wiley: New York, NY, USA, 1982; pp. 403–430. [Google Scholar]
- Helmke, P.A.; Sparks, D.L. Lithium, sodium, potassium, rubidium, and cesium. In Methods of Soil Analysis: Part 3 Book Series No. 5. Soil Science Society of America; Madison, Ed.; Wiley: New York, NY, USA, 1996; pp. 551–573. [Google Scholar]
- Cambardella, C.A.; Elliott, E.T. Particulate soil organic matter changes across a grassland cultivation sequence. Soil Sci. Soc. Am. J. 2007, 56, 777–783. [Google Scholar] [CrossRef]
- Bowles, T.; Acosta-martinez, M.; Calderon, V.; Louise, F. Soil enzyme activities, microbial communities, and carbon and nitrogen availability in organic agroecosystems across an inten sively-managed agricultural landscape. Soil Biol. Biochem. 2014, 68, 252–262. [Google Scholar] [CrossRef]
- Wear, E.K.; Wilbanks, E.G.; Nelson, C.E.; Carlson, C.A. Primer selection impacts specific population abundances but not community dynamics in a monthly time-series 16S rRNA gene amplicon analysis of coastal marine bacterioplankton. Environ. Microbiol. 2018, 20, 2709–2726. [Google Scholar] [CrossRef]



| Year | Treatment | Period | pH | Organic Matter (g kg−1) | Total N (g kg−1) | Available P (mg kg−1) | Available K (mg kg−1) | NO3-N (mg kg−1) | NH4+-N (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| 2021 | Winter crop | 5.10 ± 0.04 a | 29.38 ± 1.44 a | 1.94 ± 0.09 a | 37.01 ± 3.07 ab | 20.00 ± 2.52 a | 30.25 ± 1.83 b | 15.50 ± 1.59 a | |
| CRR(CK) | Early rice | 4.78 ± 0.14 ab | 31.66 ± 1.96 a | 1.88 ± 0.10 a | 36.31 ± 2.65 ab | 46.67 ± 2.33 b | 17.10 ± 3.50 b | 9.97 ± 1.47 a | |
| Late rice | 5.78 ± 0.06 a | 27.51 ± 1.12 a | 1.81 ± 0.10 a | 22.84 ± 2.62 a | 28.33 ± 4.18 a | 13.30 ± 3.50 b | 10.63 ± 2.11 a | ||
| Winter crop | 5.20 ± 0.15 a | 29.83 ± 0.93 a | 1.88 ± 0.06 a | 36.88 ± 2.59 ab | 19.33 ± 2.91 a | 48.46 ± 1.69 a | 13.01 ± 3.24 a | ||
| CRI | Early rice | 4.60 ± 0.11 b | 32.12 ± 1.97 a | 1.93 ± 0.11 a | 29.97 ± 4.81 b | 42.67 ± 2.67 b | 32.62 ± 6.21 a | 7.59 ± 1.14 a | |
| Late rice | 5.61 ± 0.07 a | 26.96 ± 0.94 a | 1.97 ± 0.12 a | 20.52 ± 1.34 a | 31.00 ± 3.06 a | 27.12 ± 4.37 a | 12.75 ± 2.31 a | ||
| Winter crop | 4.85 ± 0.10 a | 27.93 ± 1.99 a | 1.79 ± 0.11 a | 33.56 ± 2.81 ab | 21.67 ± 4.33 a | 32.84 ± 3.06 b | 16.00 ± 0.46 a | ||
| RRR | Early rice | 4.97 ± 0.09 a | 28.96 ± 2.27 a | 1.68 ± 0.14 a | 31.90 ± 1.21 ab | 39.67 ± 1.86 b | 6.39 ± 0.22 c | 10.24 ± 2.55 a | |
| Late rice | 5.82 ± 0.04 a | 27.00 ± 0.46 a | 1.69 ± 0.10 a | 21.48 ± 1.68 a | 28.33 ± 1.86 a | 14.53 ± 1.98 ab | 11.28 ± 4.90 a | ||
| Winter crop | 4.89 ± 0.10 a | 27.84 ± 1.39 a | 1.77 ± 0.07 a | 30.10 ± 3.08 b | 14.67 ± 1.45 a | 21.15 ± 1.19 c | 16.93 ± 3.08 a | ||
| RRI | Early rice | 4.70 ± 0.11 ab | 29.92 ± 1.78 a | 1.79 ± 0.11 a | 32.29 ± 4.94 ab | 47.00 ± 3.00 b | 5.03 ± 1.02 c | 13.53 ± 3.70 a | |
| Late rice | 5.65 ± 0.08 a | 28.34 ± 0.58 a | 1.92 ± 0.08 a | 26.65 ± 7.76 a | 32.67 ± 5.04 a | 19.61 ± 6.76 ab | 10.62 ± 3.12 a | ||
| Winter crop | 4.81 ± 0.14 a | 28.50 ± 1.72 a | 1.78 ± 0.14 a | 41.65 ± 1.11 a | 23.00 ± 2.31 a | 20.82 ± 3.17 c | 14.57 ± 2.51 a | ||
| PRR | Early rice | 4.89 ± 0.07 ab | 32.23 ± 1.48 a | 1.75 ± 0.18 a | 42.96 ± 1.84 a | 60.33 ± 5.24 a | 4.35 ± 0.39 c | 6.91 ± 1.71 a | |
| Late rice | 5.77 ± 0.11 a | 26.57 ± 1.08 a | 1.62 ± 0.18 a | 26.16 ± 0.45 a | 36.67 ± 2.19 a | 9.37 ± 1.58 b | 6.23 ± 1.37 a | ||
| 2022 | Winter crop | 5.18 ± 0.13 a | 34.36 ± 0.62 a | 1.60 ± 0.06 c | 28.18 ± 1.11 b | 31.00 ± 0.58 a | 3.29 ± 0.61 b | 18.40 ± 1.31 ab | |
| CRR (CK) | Early rice | 5.13 ± 0.12 a | 26.92 ± 1.12 c | 1.62 ± 0.07 c | 29.32 ± 1.38 bc | 33.67 ± 2.03 c | 11.23 ± 1.60 b | 19.26 ± 2.24 a | |
| Late rice | 5.36 ± 0.02 a | 32.10 ± 2.32 ab | 1.82 ± 0.15 ab | 25.81 ± 0.38 d | 27.67 ± 2.60 c | 14.00 ± 1.59 b | 10.92 ± 1.21 b | ||
| Winter crop | 5.38 ± 0.12 a | 29.34 ± 0.26 b | 1.73 ± 0.01 bc | 31.37 ± 0.95 b | 32.67 ± 3.28 a | 9.82 ± 0.42 a | 18.54 ± 1.03 ab | ||
| CRI | Early rice | 5.17 ± 0.07 a | 33.37 ± 1.52 a | 1.81 ± 0.04 bc | 31.32 ± 1.69 bc | 51.67 ± 1.76 b | 22.55 ± 3.24 a | 17.86 ± 0.27 a | |
| Late rice | 5.20 ± 0.12 a | 34.66 ± 1.38 a | 1.94 ± 0.03 a | 39.55 ± 1.64 a | 52.33 ± 4.33 a | 24.80 ± 0.11 a | 23.48 ± 1.86 a | ||
| Winter crop | 5.40 ± 0.10 a | 35.04 ± 1.06 a | 1.95 ± 0.05 ab | 28.92 ± 1.66 b | 32.00 ± 0.58 a | 3.48 ± 0.16 b | 19.68 ± 1.29 a | ||
| RRR | Early rice | 5.27 ± 0.02 a | 31.50 ± 1.48 ab | 1.96 ± 0.05 ab | 27.78 ± 0.70 c | 41.00 ± 2.65 c | 10.62 ± 2.31 b | 19.90 ± 1.15 a | |
| Late rice | 5.14 ± 0.23 a | 29.78 ± 1.55 abc | 1.76 ± 0.10 ab | 30.45 ± 1.62 cd | 29.67 ± 2.33 bc | 9.83 ± 1.18 c | 9.08 ± 0.57 b | ||
| Winter crop | 5.32 ± 0.08 a | 28.01 ± 1.51 b | 2.17 ± 0.08 a | 29.22 ± 2.82 b | 31.67 ± 2.91 a | 2.47 ± 0.26 b | 13.67 ± 0.86 c | ||
| RRI | Early rice | 5.21 ± 0.06 a | 27.24 ± 0.88 c | 2.12 ± 0.10 a | 33.25 ± 1.75 b | 41.00 ± 2.89 c | 18.86 ± 2.46 a | 7.92 ± 1.24 b | |
| Late rice | 5.04 ± 0.17 a | 27.10 ± 0.52 bc | 1.69 ± 0.10 ab | 35.48 ± 0.60 ab | 39.33 ± 4.26 b | 14.22 ± 1.47 b | 21.03 ± 1.79 a | ||
| Winter crop | 5.17 ± 0.04 a | 31.60 ± 2.10 ab | 1.81 ± 0.11 bc | 38.15 ± 1.49 a | 34.33 ± 1.86 a | 9.56 ± 0.78 a | 14.97 ± 1.23 bc | ||
| PRR | Early rice | 5.20 ± 0.10 a | 28.58 ± 1.26 bc | 1.75 ± 0.13 bc | 41.12 ± 1.30 a | 63.00 ± 4.16 a | 17.78 ± 1.18 ab | 9.31 ± 1.29 b | |
| Late rice | 5.45 ± 0.03 a | 25.67 ± 1.78 c | 1.53 ± 0.09 b | 33.42 ± 2.31 bc | 62.67 ± 2.96 a | 10.29 ± 1.35 bc | 8.00 ± 0.24 b |
| Microorganism | Treatment | Alpha Diversity Index of Species | |||
|---|---|---|---|---|---|
| Sobs | Shannon | Simpson | Coverage | ||
| Bacteria | CRR(CK) | 45.67 ± 0.33 a | 2.38 ± 0.01 a | 0.14 ± 0.00 b | 1.00 ± 0.00 a |
| CRI | 42.00 ± 1.53 b | 2.20 ± 0.06 b | 0.16 ± 0.01 a | 1.00 ± 0.00 a | |
| RRR | 45.67 ± 0.67 a | 2.41 ± 0.00 a | 0.14 ± 0.00 b | 1.00 ± 0.00 a | |
| RRI | 42.33 ± 0.33 b | 2.25 ± 0.03 b | 0.16 ± 0.01 ab | 1.00 ± 0.00 a | |
| PRR | 46.67 ± 0.33 a | 2.37 ± 0.01 a | 0.14 ± 0.01 ab | 1.00 ± 0.00 a | |
| Fungi | CRR(CK) | 20.67 ± 0.67 a | 0.85 ± 0.14 a | 0.67 ± 0.07 b | 1.00 ± 0.00 a |
| CRI | 19.00 ± 0.58 a | 0.44 ± 0.06 b | 0.83 ± 0.03 a | 1.00 ± 0.00 a | |
| RRR | 20.33 ± 1.33 a | 0.98 ± 0.04 a | 0.61 ± 0.03 b | 1.00 ± 0.00 a | |
| RRI | 18.33 ± 0.67 a | 0.40 ± 0.06 b | 0.84 ± 0.03 a | 1.00 ± 0.00 a | |
| PRR | 20.00 ± 1.15 a | 0.86 ± 0.05 a | 0.63 ± 0.03 b | 1.00 ± 0.00 a | |
| Treatment | Cropping Pattern |
|---|---|
| CRR(CK) | Chinese milk vetch–early rice–late rice |
| CRI | Chinese milk vetch–early rice–sweet potato || late soybean |
| RRR | Rapeseed–early rice–late rice (RRR), rapeseed–early rice–sweet potato || late soybean |
| RRI | Rapeseed–early rice–sweet potato || late soybean |
| PRR | Potato–early rice–late rice |
| Crop | Variety | Seeding and Harvest Time | Fertilizing Amount |
|---|---|---|---|
| Chinese milk vetch | Yujiang big leaf seed | 2.10.2020~6.04.2021 1.10.2021~7.04.2022 | No fertilizer |
| rapeseed | Zhongyou821 | 4.12.2020~6.04.2021 10.11.2021~7.04.2022 | No fertilizer |
| early rice | Zhongjia early17 | 2.05.2021~22.07.2021 2.05.2022~23.7.2022 | N 180 kg/hm2, P2O590 kg/hm2, K2O 120 kg/hm2 |
| late rice | Tianyou Huazhan | 29.07.2021~24.10.2021 30.07.2022~30.10.2022 | N 180 kg/hm2, P2O590 kg/hm2, K2O 120 kg/hm2 |
| soybean | Fengyuan No.1 | 13.08.2021~8.11.2021 4.08.2022~18.11.2022 | N 150 kg/hm2, P2O5150 kg/hm2, K2O 375 kg/hm2 |
| sweet potato | Guangshu 87 | 13.08.2021~8.11.2021 4.08.2022~18.11.2022 | N 80 kg/hm2, P2O5375 kg/hm2, K2O 80 kg/hm2 |
| potato | Dongnong 303 | 6.12.2020~10.04.2021 1.12.2021~7.04.2022 | No fertilizer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Zhou, J.; Liu, N.; Huang, Y.; Liu, Q.; Altihani, F.A.; Yang, B. Response of Soil Microbial Diversity to Triple-Cropping System in Paddy Fields in Middle Reaches of Yangtze River. Plants 2025, 14, 1292. https://doi.org/10.3390/plants14091292
Tang H, Zhou J, Liu N, Huang Y, Liu Q, Altihani FA, Yang B. Response of Soil Microbial Diversity to Triple-Cropping System in Paddy Fields in Middle Reaches of Yangtze River. Plants. 2025; 14(9):1292. https://doi.org/10.3390/plants14091292
Chicago/Turabian StyleTang, Haiying, Junlin Zhou, Ning Liu, Yao Huang, Qin Liu, Faizah Amer Altihani, and Binjuan Yang. 2025. "Response of Soil Microbial Diversity to Triple-Cropping System in Paddy Fields in Middle Reaches of Yangtze River" Plants 14, no. 9: 1292. https://doi.org/10.3390/plants14091292
APA StyleTang, H., Zhou, J., Liu, N., Huang, Y., Liu, Q., Altihani, F. A., & Yang, B. (2025). Response of Soil Microbial Diversity to Triple-Cropping System in Paddy Fields in Middle Reaches of Yangtze River. Plants, 14(9), 1292. https://doi.org/10.3390/plants14091292






