Effects of Different Calcium Preparations on Fresh-Cut Quality and Storage Quality of Starkrimson Apple
Abstract
1. Introduction
2. Results
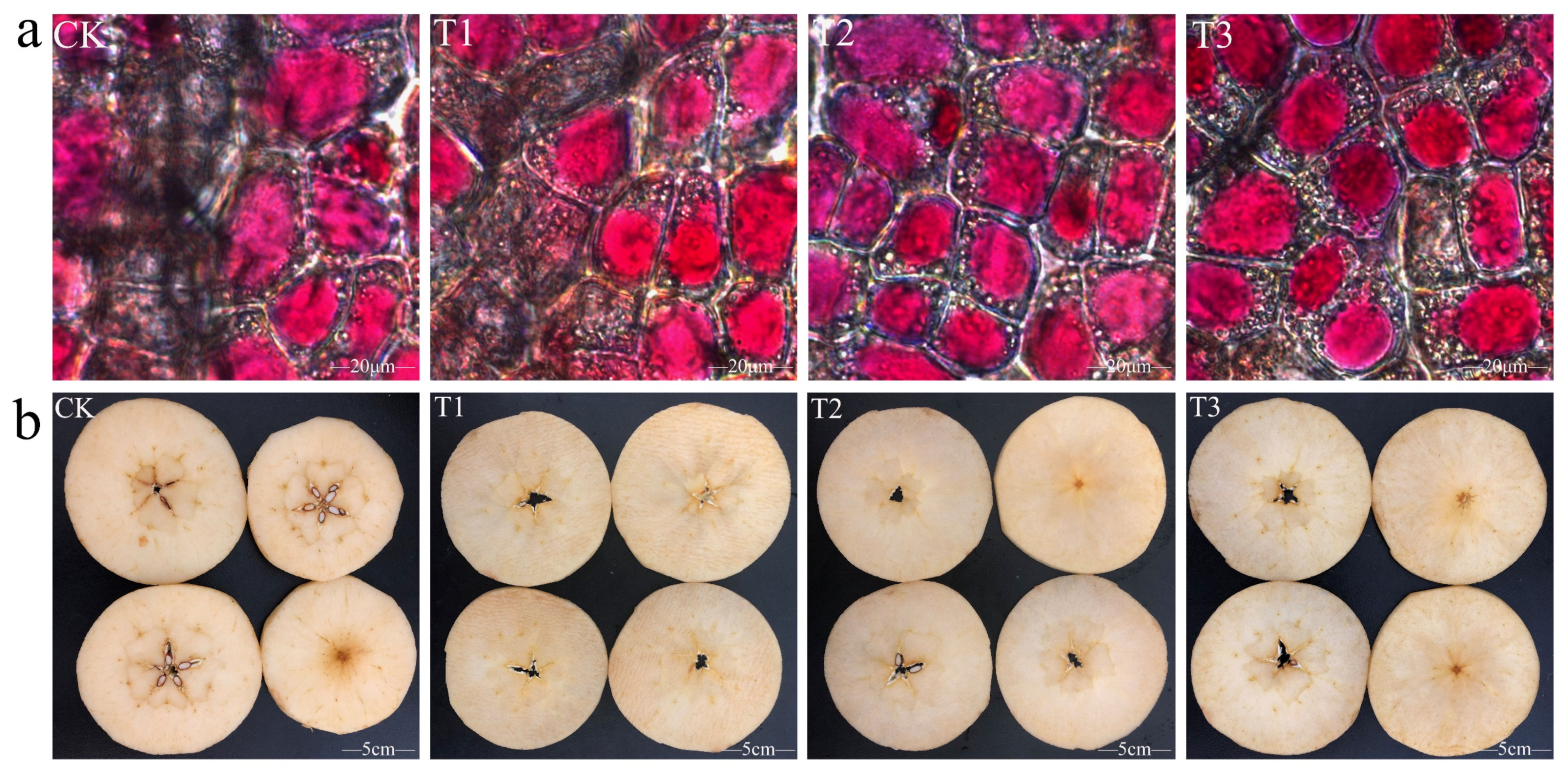
2.1. Effects of Different Calcium Preparation Treatments on Browning of Fresh-Cut Apples
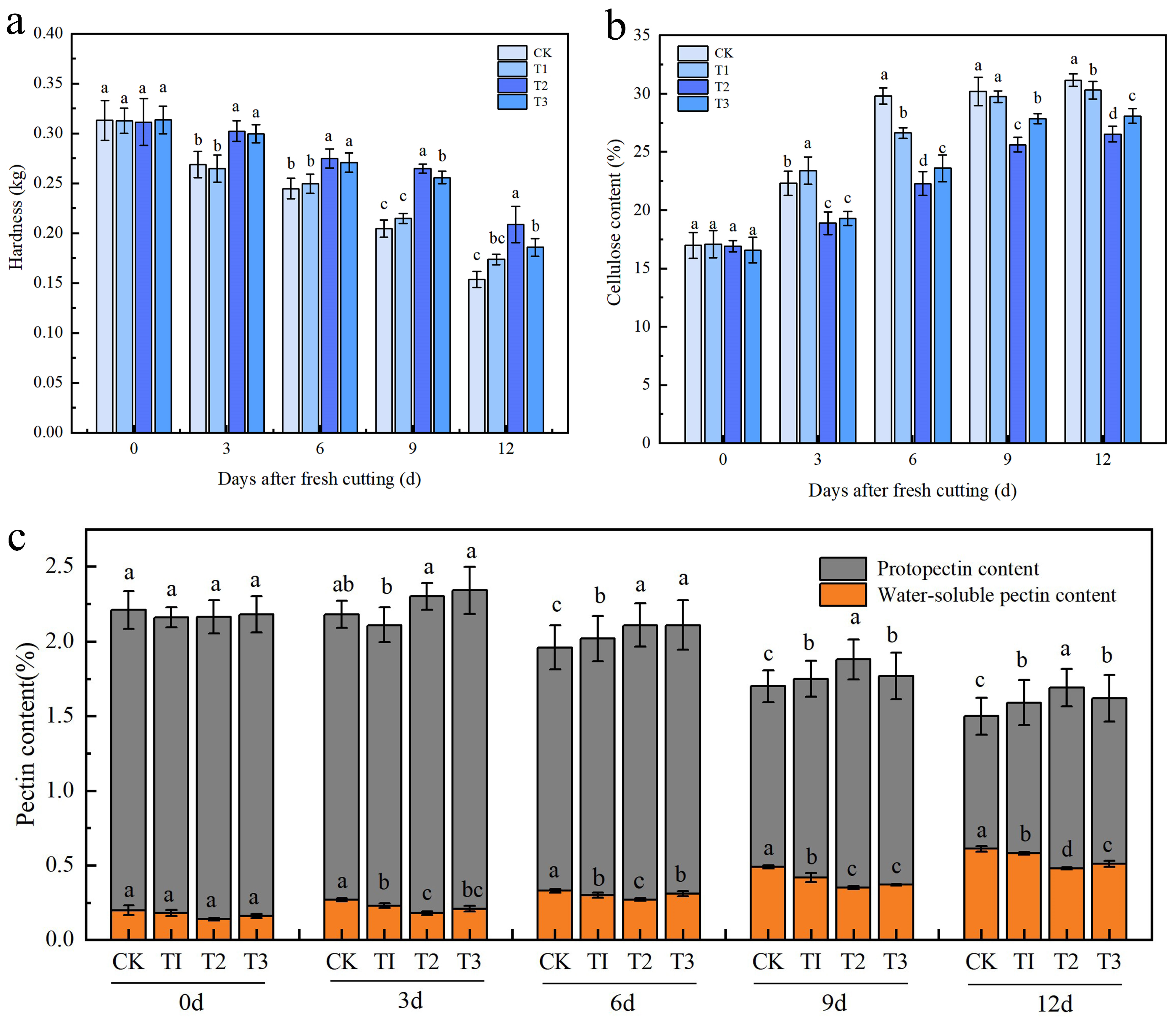
2.2. Effects of Different Calcium Preparation Treatments on Hardness, Cellulose Content, and Pectin Content of Fresh-Cut Apples
2.3. Effects of Different Calcium Preparation Treatments on the Ultrastructure of Fresh-Cut Apple Cell Wall
2.4. Effects of Different Calcium Preparation Treatments on Spoilage Microorganisms of Fresh-Cut Apples
2.5. Effects of Different Postharvest Calcium on Metabolomics of Fresh-Cut Apples During Storage
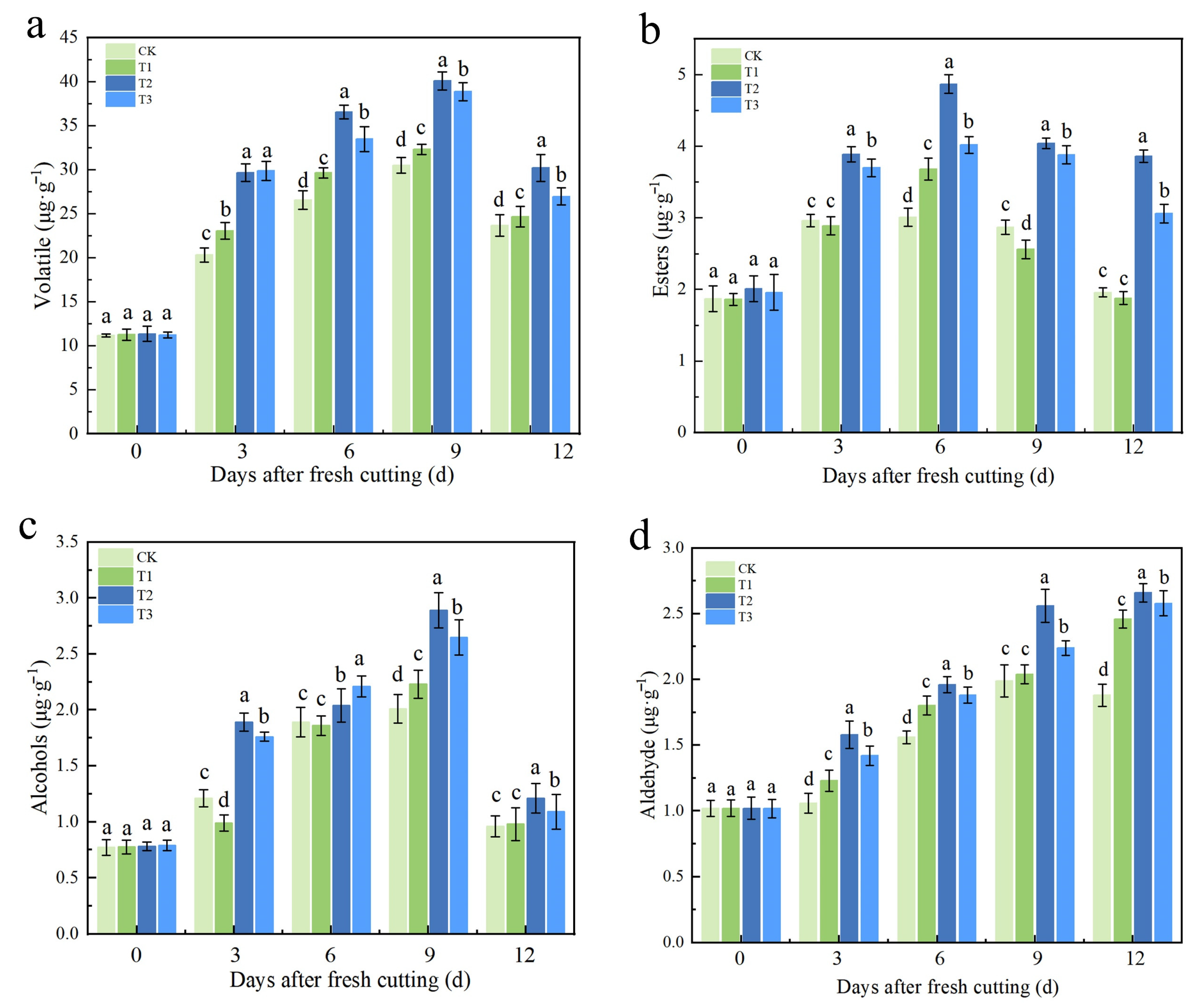
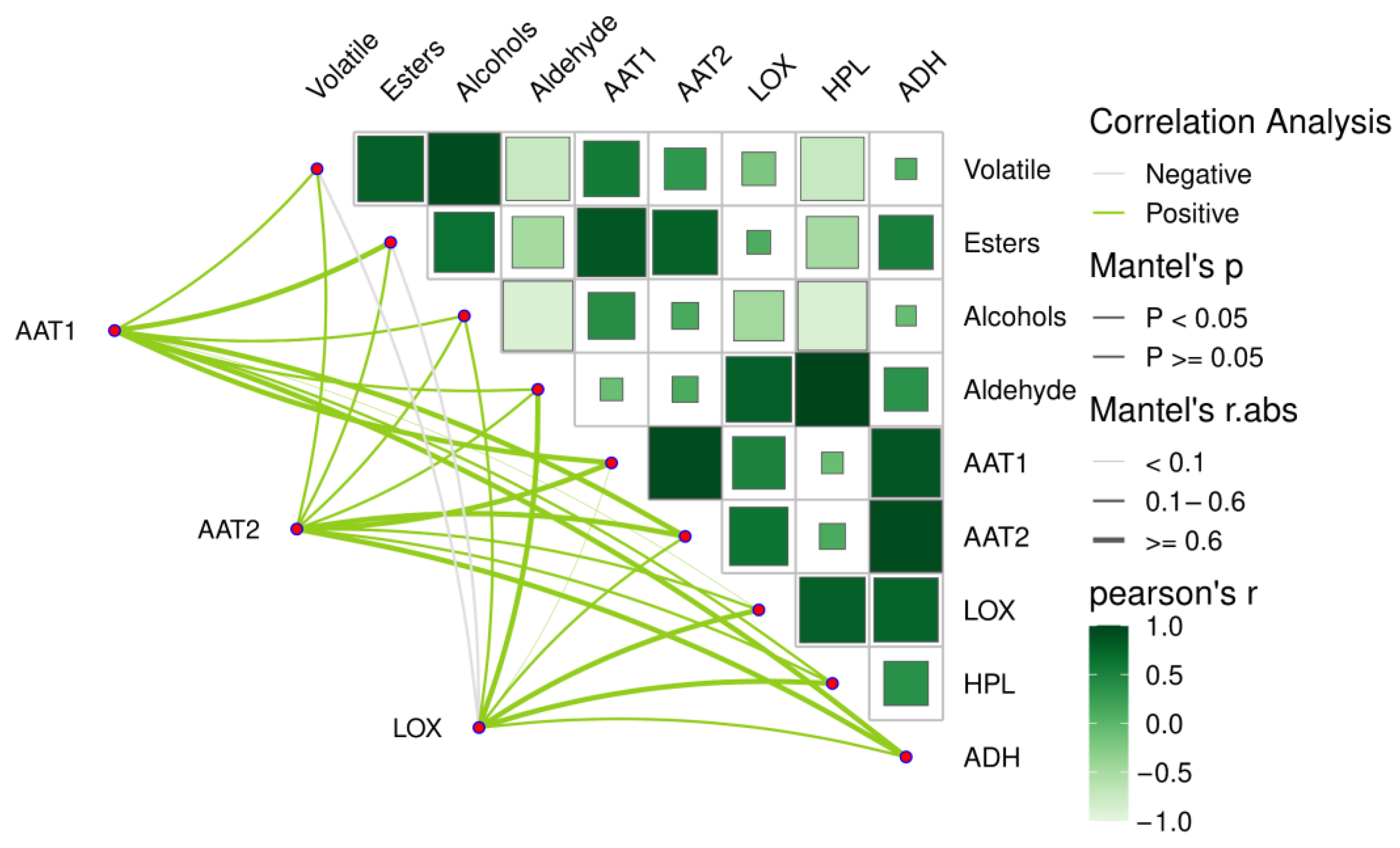
2.6. Effects of Different Calcium Treatments After Harvest on Aroma and Volatile Components of Fruit During Storage
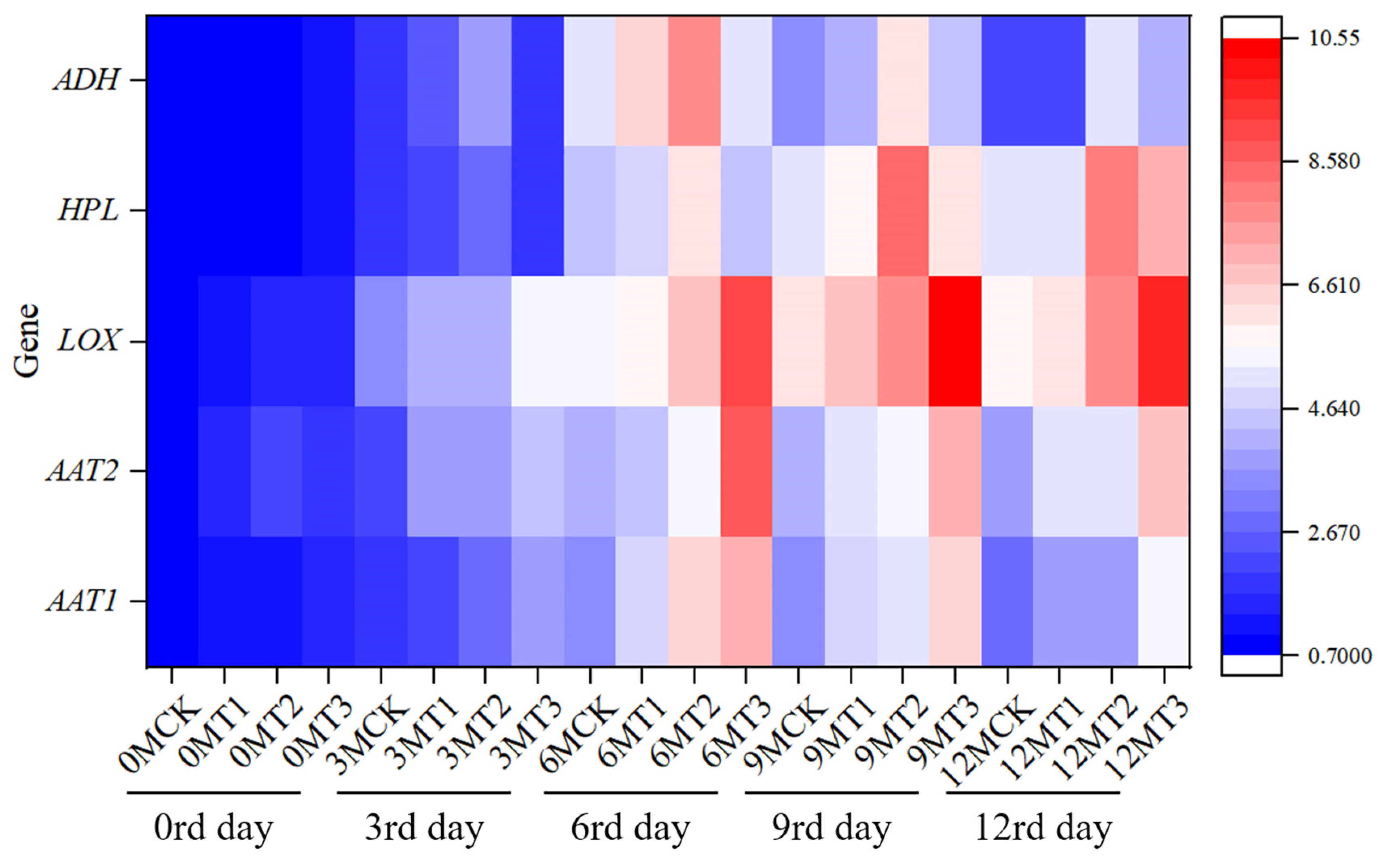
2.7. Effects of Different Calcium Preparations After Harvest on the Expression of Genes Related to Aroma Metabolism in Fruit During Storage
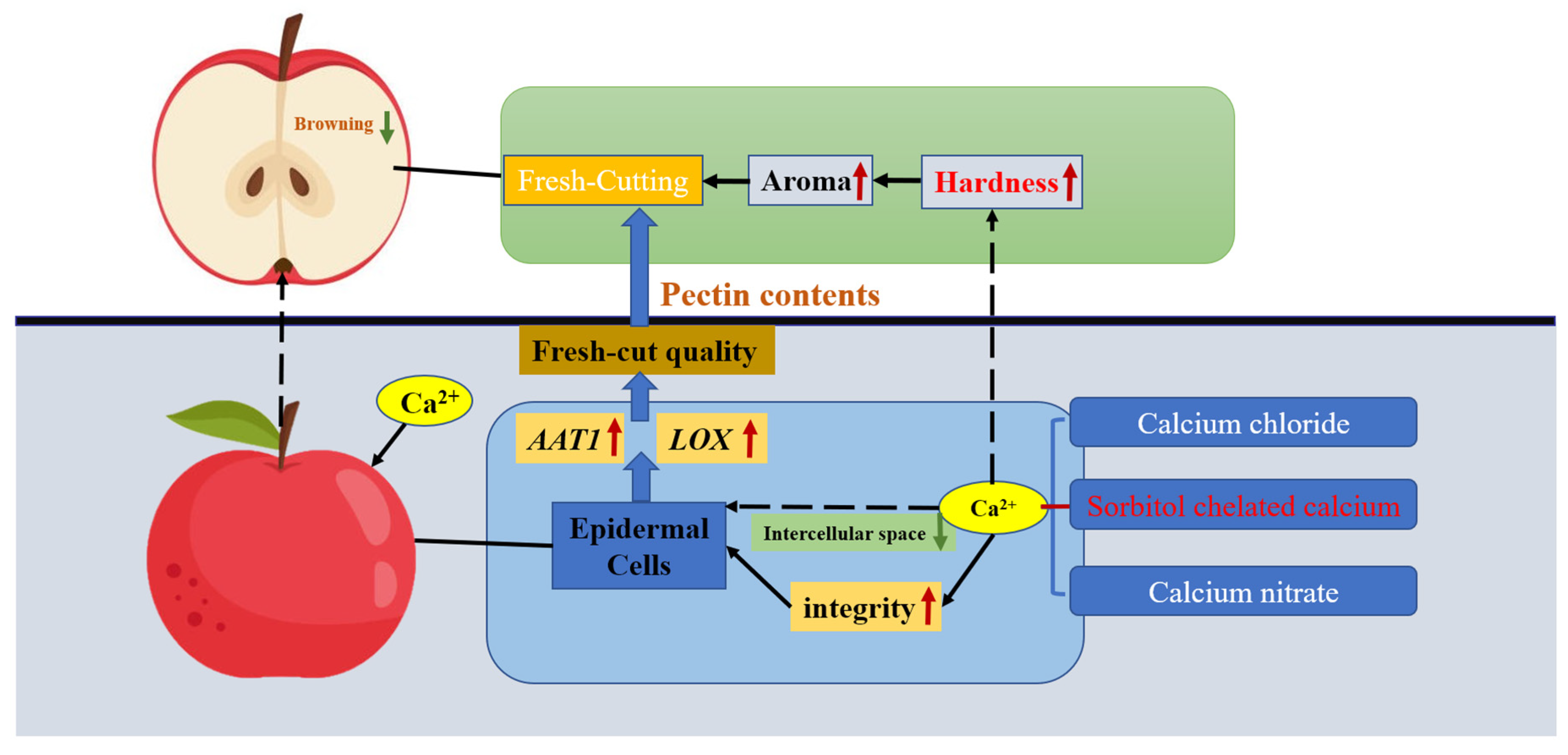
3. Discussion
4. Materials and Methods
4.1. Experimental Site and Materials
4.2. Color Measurement
4.3. Hardness Measurement
4.4. Cellulose Content Determination
4.5. Pectin Content Determination
4.6. Ultrastructural Observation
4.7. Methods for Microbial Enumeration in Apples
4.8. Volatile Extraction and Concentration
4.9. GC–MS Conditions and Metabolomics Detection
4.10. Determination of Aroma-Metabolism-Related Genes
4.11. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferreira, C.; Ribeiro, C.; Nunes, F.M. Effect of storage conditions on phenolic composition, vitamin C and antioxidant activity of ‘Golden Delicious’ and ‘Red Delicious’ apples. Postharvest Biol. Technol. 2024, 210, 112754. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Phenolic compounds from apples: From natural fruits to the Beneficial effects in the Digestive System. Molecules 2024, 29, 568. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhao, X.; Guo, C.; Chen, L.; Li, K. Spinach 14-3-3 protein interacts with the plasma membrane H+-ATPase and nitrate reductase in response to excess nitrate stress. Plant Physiol. Biochem. 2016, 106, 187–197. [Google Scholar] [CrossRef]
- Xiao, Q.; Ye, S.; Wang, H.; Xing, S.; Zhu, W.; Zhang, H.; Lv, X. Soluble sugar, organic acid and phenolic composition and flavor evaluation of plum fruits. Food Chem. X 2024, 24, 101790. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, X.; Cao, Y.; Gao, P.; Xu, T.; Xiong, D.; Zhao, Z. Lactiplantibacillus plantarum exerts strain-specific effects on malolactic fermentation, antioxidant activity, and aroma profile of apple cider. Food Chem. X 2024, 23, 101575. [Google Scholar] [CrossRef]
- Feng, Z.Q.; Li, T.; Wang, X.; Sun, W.J.; Zhang, T.T.; You, C.X.; Wang, X.F. Identification and characterization of apple MdNLP7 transcription factor in the nitrate response. Plant Sci. 2022, 316, 111158. [Google Scholar] [CrossRef]
- Moussa, A.Y.; Siddiqui, S.A.; Elhawary, E.A.; Guo, K.; Anwar, S.; Xu, B. Phytochemical constituents, bioactivities, and applications of custard apple (Annona squamosa L.): A narrative review. Food Chem. 2024, 459, 140363. [Google Scholar] [CrossRef]
- Chavan, P.; Lata, K.; Kaur, T.; Jambrak, A.R.; Sharma, S.; Roy, S.; Rout, A. Recent advances in the preservation of postharvest fruits using edible films and coatings: A comprehensive review. Food Chem. 2023, 418, 135916. [Google Scholar] [CrossRef]
- Park, J.; Oh, S.M.; Park, M.K.; Park, J.D.; Ahn, J.H.; Sung, J.M. Enhancing quality and shelf life of fresh-cut Paprika (Capsicum annuum L.) using insulated packaging with ice packs. Postharvest Biol. Technol. 2025, 222, 113360. [Google Scholar] [CrossRef]
- Singla, G.; Chaturvedi, K.; Sandhu, P.P. Status and recent trends in fresh-cut fruits and vegetables. In Fresh-Cut Fruits and Vegetables; Academic Press: Cambridge, MA, USA, 2020; pp. 17–49. [Google Scholar] [CrossRef]
- Ansah, F.A.; Amodio, M.L.; Colelli, G. Quality of fresh-cut products as affected by harvest and postharvest operations. J. Sci. Food Agric. 2018, 98, 3614–3626. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, Q.; Niu, B.; Liu, R.; Chen, H.; Xiao, S.; Gao, H. The dual function of calcium ion in fruit edible coating: Regulating polymer internal crosslinking state and improving fruit postharvest quality. Food Chem. 2024, 447, 138952. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ye, F.; Wu, Z.; Zhou, Y.; Lei, L.; Zhou, S.; Zhao, G. Sucrose and Ca2+ synergistically regulate the rheological properties of apple high-methoxyl pectin. Int. J. Biol. Macromol. 2024, 271, 132397. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, C.; Jin, Y.; Zhang, S.; Wang, M.; Ge, Y. Repression of cell wall metabolism by calcium lactate enhances the postharvest quality maintenance of Jinfeng pear fruit. Sci. Hortic. 2023, 322, 112460. [Google Scholar] [CrossRef]
- Mohebbi, S.; Babalar, M.; Zamani, Z.; Askari, M.A. Influence of early season boron spraying and postharvest calcium dip treatment on cell-wall degrading enzymes and fruit firmness in ‘Starking Delicious’ apple during storage. Sci. Hortic. 2020, 259, 108822. [Google Scholar] [CrossRef]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Kaur, J.; Woodward, A. Water loss: A postharvest quality marker in apple storage. Food Bioprocess Technol. 2024, 17, 2155–2180. [Google Scholar] [CrossRef]
- Li, R.; Rosado-Souza, L.; Sampathkumar, A.; Fernie, A.R. The relationship between cell wall and postharvest physiological deterioration of fresh produce. Plant Physiol. Biochem. 2024, 210, 108568. [Google Scholar] [CrossRef]
- Hocq, L.; Habrylo, O.; Sénéchal, F.; Voxeur, A.; Pau-Roblot, C.; Safran, J.; Lefebvre, V. Mutation of AtPME2, a pH-dependent pectin methylesterase, affects cell wall structure and hypocotyl elongation. Plant Cell Physiol. 2024, 65, 301–318. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Wu, C.; Kou, X.; Xue, Z. Storage quality prediction of winter jujube based on particle swarm optimization-backpropagation-artificial neural network (PSO-BP-ANN). Sci. Hortic. 2024, 331, 112789. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Han, X.; Chen, R.; Xue, X. Effects of spraying calcium fertilizer on photosynthesis, mineral content, sugar–acid metabolism and fruit quality of Fuji apples. Agronomy 2022, 12, 2563. [Google Scholar] [CrossRef]
- Ji, J.; Zhuang, H.; Zhou, L.; Zhang, Y. The single atomic Pt promoted CoMo heterostructure catalyst for efficient sorbitol hydrogenolysis to ethanol. Appl. Catal. B Environ. Energy 2024, 350, 123890. [Google Scholar] [CrossRef]
- Liu, X.; Hao, N.; Feng, R.; Meng, Z.; Li, Y.; Zhao, Z. Transcriptome and metabolite profiling analyses provide insight into volatile compounds of the apple cultivar ‘Ruixue’ and its parents during fruit development. BMC Plant Biol. 2021, 21, 231. [Google Scholar] [CrossRef] [PubMed]
- Schiller, D.; Contreras, C.; Vogt, J.; Dunemann, F.; Defilippi, B.G.; Beaudry, R.; Schwab, W. A dual positional specific lipoxygenase functions in the generation of flavor compounds during climacteric ripening of apple. Hortic. Res. 2015, 2, 15003. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, C.; Guo, M.; Liu, J.; Qu, L.; Fan, Y.; Ge, Y. Caffeic acid enhances storage ability of apple fruit by regulating fatty acid metabolism. Postharvest Biol. Technol. 2022, 192, 112012. [Google Scholar] [CrossRef]
- Brizzolara, S.; Santucci, C.; Tenori, L.; Hertog, M.; Nicolai, B.; Stürz, S.; Tonutti, P. A metabolomics approach to elucidate apple fruit responses to static and dynamic controlled atmosphere storage. Postharvest Biol. Technol. 2017, 127, 76–87. [Google Scholar] [CrossRef]
- Rasane, P.; Singh, J.; Kaur, S.; Bakshi, M.; Gunjal, M.; Kaur, J.; Mahato, D.K. Strategic advances in the management of browning in fruits and vegetables. Food Bioprocess Technol. 2024, 17, 325–350. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, L.; Luo, H.; Yu, Z. Establishment of a statistical model for browning of fresh-cut lotus root during storage. Postharvest Biol. Technol. 2014, 92, 164–171. [Google Scholar] [CrossRef]
- Zulli, R.; Chen, Z.; Santi, F.; Trych, U.; Szczepańska-Stolarczyk, J.; Cywińska-Antonik, M.; Spilimbergo, S. Effect of high-pressure carbon dioxide combined with modified atmosphere packaging on the quality of fresh-cut squash during storage. Food Chem. 2025, 472, 142882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zuo, C.; Wang, M.; Song, S.; Hu, Y.; Song, J.; Pan, L. Optical properties related to cell wall pectin contribute to determine the firmness and microstructural changes during apple softening. Postharvest Biol. Technol. 2024, 218, 113150. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Wang, H.; Yuan, J.; Zheng, Y.; Duan, L.; Jiang, Y. Heat shock pretreatment and low temperature fluctuation cold storage maintains flesh quality and retards watercore dissipation of watercored ‘Fuji’ apples. Sci. Hortic. 2024, 323, 112492. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Wang, Z.; Zhang, Y.; Guo, M.; Chen, G. Epidermal wax damage on Hami melon (Cucumis melo L.) caused by postharvest commercialization induced fruit physiological softening and degradation of pectin polysaccharide nanostructure. Food Hydrocoll. 2024, 151, 109841. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, W.; Wang, P.; Zhao, S.; Wang, D.; Zhao, X. Transcriptome analysis integrated with changes in cell wall polysaccharides of different fresh-cut chili pepper cultivars during storage reveals the softening mechanism. Food Chem. 2024, 452, 139445. [Google Scholar] [CrossRef] [PubMed]
- Francis, G.A.; Gallone, A.; Nychas, G.J.; Sofos, J.N.; Colelli, G.; Amodio, M.L.; Spano, G. Factors affecting quality and safety of fresh-cut produce. Crit. Rev. Food Sci. Nutr. 2012, 52, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Ndayambaje, J.P.; Liu, X.; Xia, X. Microbial spoilage of fruits: A review on causes and prevention methods. Food Rev. Int. 2022, 38 (Suppl. S1), 225–246. [Google Scholar] [CrossRef]
- Gao, H.; Wu, S.; Zeng, Q.; Li, P.; Guan, W. Effects of exogenous γ-aminobutyric acid treatment on browning and food-borne pathogens in fresh-cut apples. Postharvest Biol. Technol. 2018, 146, 1–8. [Google Scholar] [CrossRef]
- Cifuentes, M.; Vahid, F.; Devaux, Y.; Bohn, T. Biomarkers of food intake and their relevance to metabolic syndrome. Food Funct. 2024, 15, 7271–7304. [Google Scholar] [CrossRef]
- Zhou, J.W.; Ji, P.C.; Wang, C.Y.; Yang, Y.J.; Zhao, X.Y.; Tang, H.Z. Anti-virulence activity of dihydrocuminyl aldehyde and nisin against spoilage bacterium Pseudomonas aeruginosa XZ01. LWT 2023, 177, 114573. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Yi, C.; Chen, F.; Liu, Y.; Liao, Y.; Lv, J. Integrative analysis of non-targeted metabolome and transcriptome reveals the mechanism of volatile formation in pepper fruit. Front. Genet. 2023, 14, 1290492. [Google Scholar] [CrossRef]
- Zhang, B.; Shen, J.Y.; Wei, W.W.; Xi, W.P.; Xu, C.J.; Ferguson, I.; Chen, K. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef]
- Lu, H.; Li, L.; Xu, Y.; Li, D.; Li, G.; Yan, Y.; Luo, Z. FaLEC2 repressing FaLOX2 promoter involved in the metabolism of LOX-derived volatiles during strawberry ripening. Sci. Hortic. 2022, 303, 111188. [Google Scholar] [CrossRef]
- Li, T.; Wei, Q.; Sun, W.; Tan, H.; Cui, Y.; Han, C.; Yan, D. Spraying sorbitol-chelated calcium affected foliar calcium absorption and promoted the yield of peanut (Arachis hypogaea L.). Front. Plant Sci. 2022, 13, 1075488. [Google Scholar] [CrossRef]
- Dang, D.S.; Buhler, J.F.; Stafford, C.D.; Taylor, M.J.; Shippen, J.E.; Dai, X.; Matarneh, S.K. Nix Pro 2 and Color Muse as potential colorimeters for evaluating color in foods. LWT 2021, 147, 111648. [Google Scholar] [CrossRef]
- Cheng, X.; Li, R.; Xie, P.; Wang, X.; Yu, L.; Wu, R.; Bi, Y. Predictive modeling of patulin accumulation in apple lesions infected by Penicillium expansum using machine learning. Postharvest Biol. Technol. 2024, 217, 113115. [Google Scholar] [CrossRef]
- Wang, Q.Q.; He, Z.; Zhu, Z.; Zhang, Y.H.; Ni, Y.; Luo, X.L.; Zhu, J.Y. Evaluations of cellulose accessibilities of lignocelluloses by solute exclusion and protein adsorption techniques. Biotechnol. Bioeng. 2012, 109, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, D.; Wang, P.; Zhao, W.; Zhao, S.; Ma, Y.; Zhao, X. Microbiota response of pectin determined by its structural characteristics during in vitro fecal fermentation: A comparative study of various pectin sources. Food Hydrocoll. 2024, 150, 109730. [Google Scholar] [CrossRef]
- Li, S.; Li, Q.; Qu, G.; Cao, J.; Jiang, W. Fractionation and characterization of sodium carbonate-soluble fractions of cell wall pectic polysaccharides involved in the rapid mealiness of ‘Hongjiangjun’ apple fruit. Food Chem. 2024, 455, 139961. [Google Scholar] [CrossRef]
- Decadt, H.; Weckx, S.; De Vuyst, L. The microbial and metabolite composition of Gouda cheese made from pasteurized milk is determined by the processing chain. Int. J. Food Microbiol. 2024, 412, 110557. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Cui, J.; Gao, S.; Bai, S.; You, L.; Wang, S. Dynamic changes in the water and volatile compounds of chicken breast during the frying process. Food Res. Int. 2024, 175, 113715. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qin, W.; Guo, Z.; Li, R.; Ding, C.; Zhang, S.; Tan, Z. Metabonomics study of fresh bruises on an apple using the gas chromatography–mass spectrometry (GC–MS) method. Eur. Food Res. Technol. 2020, 246, 201–212. [Google Scholar] [CrossRef]
- Gao, W.; Meng, Q.; Luo, H.; Chen, F.; Zhou, Y.; He, M. Transcriptional responses for biosynthesis of flavor volatiles in methyl jasmonate-treated Chrysanthemum indicum var. aromaticum leaves. Ind. Crops Prod. 2020, 147, 112254. [Google Scholar] [CrossRef]


| Color Hues | Treatment | Days After Fresh-Cutting (d) | ||||
|---|---|---|---|---|---|---|
| 0 d | 3 d | 6 d | 9 d | 12 d | ||
| a* value | CK | −5.59 ± 0.31 a | −4.91 ± 0.18 c | −4.62 ± 0.34 c | −4.47 ± 0.27 c | −4.29 ± 0.21 c |
| T1 | −5.41 ± 0.31 a | −4.57 ± 0.23 b | −4.33 ± 0.35 b | −4.2 ± 0.25 b | −3.42 ± 0.23 b | |
| T2 | −5.37 ± 0.38 a | −4.23 ± 0.26 a | −3.37 ± 0.42 a | −2.96 ± 0.10 a | −2.90 ± 0.15 a | |
| T3 | −5.43 ± 0.23 a | −4.54 ± 0.25 b | −4.35 ± 0.23 b | −4.25 ± 0.39 b | −3.45 ± 0.17 b | |
| b* value | CK | 26.91 ± 0.32 a | 25.67 ± 0.52 b | 25.34 ± 0.55 b | 24.76 ± 0.62 b | 23.87 ± 0.62 c |
| T1 | 27.01 ± 0.24 a | 26.29 ± 0.43 a | 25.34 ± 0.32 b | 24.86 ± 0.55 b | 24.25 ± 0.32 b | |
| T2 | 27.21 ± 0.85 a | 26.44 ± 0.22 a | 26.17 ± 0.22 a | 25.59 ± 0.13 a | 25.34 ± 0.23 a | |
| T3 | 26.91 ± 0.12 a | 25.34 ± 0.27 b | 24.49 ± 0.27 b | 24.69 ± 0.81 b | 23.86 ± 0.14 c | |
| L* value | CK | 79.01 ± 1.08 a | 73.54 ± 0.75 c | 69.7 ± 0.66 d | 68.28 ± 0.51 c | 65.05 ± 0.55 d |
| T1 | 79.37 ± 0.66 a | 74.03 ± 0.66 b | 71.85 ± 0.42 c | 70.47 ± 0.84 b | 66.81 ± 0.61 c | |
| T2 | 79.78 ± 0.61 a | 79.18 ± 0.83 a | 78.40 ± 0.93 a | 77.48 ± 0.82 a | 72.03 ± 0.98 a | |
| T3 | 78.58 ± 0.67 a | 75.17 ± 0.55 b | 73.71 ± 0.61 b | 71.03 ± 0.64 b | 68.12 ± 0.65 b | |
| Microbial Species | Different Calcium Preparation Treatment Groups | |||
|---|---|---|---|---|
| CK | T1 | T2 | T3 | |
| Penicillium | 2.34 ± 0.09 a | 1.89 ± 0.08 b | 1.13 ± 0.09 d | 1.77 ± 0.02 c |
| Aspergillus | 1.43 ± 0.06 a | 1.33 ± 0.06 b | 1.07 ± 0.06 c | 1.12 ± 0.04 bc |
| Alternaria | 2.89± 0.11 a | 1.87 ± 0.13 b | 1.61 ± 0.07 c | 1.76 ± 0.04 b |
| Erwinia carotovora | 4.43 ± 0.12 a | 3.34 ± 0.13 b | 2.97 ± 0.15 c | 3.03 ± 0.12 c |
| Total colonies | 5.68 ± 0.18 a | 4.80 ± 0.12 b | 3.70 ± 0.09 d | 4.19 ± 0.13 c |
| Volatile Compound | Retention Index | Markers | Mass Concentration (mg·kg−1) | |||
|---|---|---|---|---|---|---|
| CK | T1 | T2 | T3 | |||
| Ethanol | 1.66 | 0.01 ± 0.001 c | 0.02 ± 0.001 b | 0.02 ± 0.002 b | 1.59 ± 0.311 a | |
| Silanol, trimethyl- | 1.792 | V | 1.51 ± 0.123 a | 0.78 ± 0.074 c | 1.2 ± 0.083 b | 0.3 ± 0.023 d |
| Pentanoic acid, 3-methyl-4-oxo- | 1.996 | V | 0.35 ± 0.025 b | 0.92 ± 0.08 a | 0.36 ± 0.02 b | 0.22 ± 0.014 c |
| Silanediol, dimethyl- | 2.335 | V | 0.26 ± 0.019 b | 0.12 ± 0.006 d | 0.19 ± 0.012 c | 0.62 ± 0.044 a |
| Hexanal | 3.339 | V | 1.49 ± 0.081 c | 0.27 ± 0.019 b | 0.38 ± 0.025 b | 5.66 ± 0.471 a |
| Cyclotrisiloxane, hexamethyl- | 5.136 | V | 7.39 ± 0.548 a | 0.77 ± 0.073 b | 0.18 ± 0.009 d | 0.43 ± 0.027 c |
| kaempferol- | 5.606 | V | 0.03 ± 0.002 d | 3.94 ± 0.27 b | 5.04 ± 0.436 a | 0.82 ± 0.041 c |
| 2-Hexenal | 5.817 | V | 0.03 ± 0.002 c | 0.26 ± 0.015 b | 0.41 ± 0.031 a | 0 ± 0 d |
| 2-Hexenal | 6.333 | V | 0.01 ± 0.001 c | 0.91 ± 0.047 a | 0.73 ± 0.055 b | 0.01 ± 0.001 c |
| 4-Hexen-1-ol, acetate | 6.454 | V | 0.25 ± 0.015 a | 0.15 ± 0.012 b | 0.02 ± 0.002 c | 0.24 ± 0.016 a |
| 2-Hexen-1-ol, (E)- | 6.543 | 1.15 ± 0.096 a | 0.1 ± 0.007 d | 0.69 ± 0.052 b | 0.16 ± 0.009 c | |
| Cyclopropane, propyl- | 6.779 | 0.1 ± 0.006 d | 0.45 ± 0.031 b | 0.24 ± 0.019 c | 0.76 ± 0.044 a | |
| 1-Butanol, 3-methyl-, acetate | 6.817 | V | 0.05 ± 0.003 d | 0.18 ± 0.009 c | 1.1 ± 0.056 a | 0.83 ± 0.061 b |
| Oxime-, methoxy-phenyl-_ | 7.01 | SV | 0.28 ± 0.026 b | 0.84 ± 0.044 a | 0.98 ± 0.084 a | 0.13 ± 0.012 c |
| Acetic acid, pentyl ester | 7.838 | T | 0.14 ± 0.007 b | 0.15 ± 0.009 b | 0.12 ± 0.008 c | 0.64 ± 0.054 a |
| 2-Hexen-1-ol, acetate, (Z)- | 8.016 | V | 0.78 ± 0.067 a | 0.04 ± 0.003 c | 0.02 ± 0.001 c | 0.14 ± 0.007 b |
| 4′,6′-Dimethoxy-2′,3′-dimethylacetop | 8.21 | V | 0.14 ± 0.008 b | 0.11 ± 0.006 b | 0.7 ± 0.05 a | 0.03 ± 0.003 c |
| 3-Hydroxy-4-methoxybenzaldehyde | 9.106 | V | 0.25 ± 0.013 a | 0.14 ± 0.011 c | 0.2 ± 0.014 b | 0.06 ± 0.004 d |
| Cyclotrisiloxane, hexamethyl- | 9.215 | V | 0.02 ± 0.001 c | 0.01 ± 0.001 d | 0.07 ± 0.004 b | 0.17 ± 0.011 a |
| 5-Hepten-2-one, 6-methyl- | 9.833 | V | 0.15 ± 0.014 a | 0.02 ± 0.002 c | 0.05 ± 0.004 b | 0.01 ± 0.001c |
| 2-Octanone | 9.954 | 1.98 ± 0.127 a | 0.13 ± 0.011 c | 0.18 ± 0.011 b | 0.16 ± 0.011 b | |
| Cyclotetrasiloxane, octamethyl- | 10.029 | V | 1.02 ± 0.055 c | 2.22 ± 0.117 a | 0.01 ± 0.001 d | 1.88 ± 0.183 b |
| 2-Octanol, (S)- | 10.215 | SV | 15.29 ± 0.882 a | 0.72 ± 0.06 b | 0.06 ± 0.003 c | 0.59 ± 0.057 b |
| 3-Hexen-1-ol, acetate, (E)- | 10.335 | T | 11.39 ± 0.765 a | 10.69 ± 0.568 b | 1.61 ± 0.138 c | 12.45 ± 0.72 a |
| Acetic acid, hexyl ester | 10.48 | S | 15.31 ± 1.205 a | 12.91 ± 1.26 b | 0.45 ± 0.032 c | 16.89 ± 0.951 a |
| 2-Hexen-1-ol, acetate, (Z)- | 10.664 | T | 17.64 ± 0.965 b | 21.05 ± 1.263 a | 2.35 ± 0.143 c | 21.6 ± 1.153 a |
| Nonane, 5-butyl- | 10.758 | 0.02 ± 0.001 c | 24.24 ± 1.268 a | 10.17 ± 0.705 b | 23.47 ± 1.389 a | |
| Quercetin | 11.575 | 0.01 ± 0.001 b | 0.02 ± 0.001 b | 16.32 ± 0.867 a | 0.01 ± 0.001 b | |
| Decane, 3,7-dimethyl- | 11.683 | 0.13 ± 0.008 b | 0.01 ± 0.001 c | 22.2 ± 1.737 a | 0.01 ± 0.001 c | |
| hydroxytyrosol- | 11.761 | SV | 0.06 ± 0.003 c | 0.25 ± 0.024 b | 24.04 ± 1.202 a | 0.24 ± 0.014 b |
| Nonane, 5-(2-methylpropyl)- | 11.842 | T | 0.02 ± 0.002 a | 0.02 ± 0.001 a | 0.01 ± 0.001 b | 0.02 ± 0.001 a |
| 4H-3,1-Benzoxazin-2-amine, 4-ethyl-N- | 11.904 | V | 0.92 ± 0.087 a | 0.1 ± 0.007 b | 0.01 ± 0.001 c | 0.11 ± 0.009 b |
| 1-Hexene, 3,3,5-trimethyl- | 12.142 | V | 0.17 ± 0.009 b | 0.02 ± 0.002 c | 0.24 ± 0.013 a | 0.02 ± 0.001 c |
| 3-Nonanone | 12.333 | V | 0.12 ± 0.007 a | 0.03 ± 0.002 b | 0.02 ± 0.002 c | 0.01 ± 0.001 d |
| Octane, 2,3,6,7-tetramethyl- | 12.592 | V | 0.36 ± 0.032 a | 0.32 ± 0.021 a | 0.11 ± 0.006 b | 0.01 ± 0.001 c |
| Nonane, 2-methyl- | 12.739 | V | 0.27 ± 0.017 a | 0.15 ± 0.009 b | 0.01 ± 0.001 c | 0.03 ± 0.002 c |
| Dodecane, 2,6,11-trimethyl- | 12.867 | V | 0.07 ± 0.004 b | 0.09 ± 0.005 a | 0.02 ± 0.002 d | 0.04 ± 0.003 c |
| Nonane, 4,5-dimethyl- | 12.921 | V | 0.78 ± 0.041 a | 0.29 ± 0.028 b | 0.03 ± 0.003 c | 0.27 ± 0.017 b |
| Tyrosol- | 13.06 | 0.03 ± 0.002 c | 0.01 ± 0.001 c | 0.3 ± 0.027 a | 0.17 ± 0.012 b | |
| 5,6,6,7-Tetramethyl-13-oxa-5,7- | 13.125 | 0.02 ± 0.002 c | 0.33 ± 0.018 a | 0.16 ± 0.008 b | 0.07 ± 0.006 c | |
| trans-2-Heptenyl acetate | 13.232 | V | 0.14 ± 0.008 b | 0.07 ± 0.004 c | 0.06 ± 0.003 c | 0.27 ± 0.026 a |
| 2-Octanol, acetate | 13.333 | V | 0.03 ± 0.002 d | 0.71 ± 0.045 a | 0.28 ± 0.017 b | 0.07 ± 0.007 c |
| Phosphonoacetic acid, 3TMS derivative | 13.89 | V | 0.05 ± 0.003 b | 0.01 ± 0.001 b | 0.1 ± 0.001 b | 0.23 ± 0.018 a |
| Cyclopentasiloxane, decamethyl- | 14.054 | V | 0.01 ± 0.001 c | 0.04 ± 0.003 b | 0.26 ± 0.015 a | 0.06 ± 0.005 b |
| Undecane, 3,4-dimethyl- | 14.225 | V | 0.17 ± 0.015 b | 0.01 ± 0.001 d | 0.05 ± 0.003 c | 0.62 ± 0.044 a |
| Isoflavones | 14.475 | 0.04 ± 0.002 b | 0.02 ± 0.001 c | 0.57 ± 0.034 a | 0.01 ± 0.001 c | |
| Pentadecane | 15.374 | V | 0.05 ± 0.003 a | 0.01 ± 0.001 b | 0.01 ± 0.001 b | 0.04 ± 0.002 a |
| 3-Isopropoxy-1,1,1,5,5,5-hexamethyl-3- | 15.483 | V | 0.05 ± 0.003 a | 0.03 ± 0.002 b | 0.05 ± 0.003 a | 0.01 ± 0.001 c |
| Decanal | 15.549 | V | 0.05 ± 0.003 a | 0.02 ± 0.001 b | 0.02 ± 0.002 b | 0.02 ± 0.001 b |
| Undecane, 4,6-dimethyl- | 15.625 | V | 0.31 ± 0.022 a | 0.01 ± 0.001 d | 0.02 ± 0.001 c | 0.06 ± 0.004 b |
| Dodecane, 4-methyl- | 15.692 | V | 0.01 ± 0.001 c | 0.01 ± 0.001 c | 0.02 ± 0.001 b | 0.05 ± 0.003 a |
| Decane, 2,4,6-trimethyl- | 15.879 | V | 0.86 ± 0.062 a | 0.12 ± 0.007 b | 0.03 ± 0.002 c | 0.02 ± 0.001 c |
| Volatile | Esters | Alcohols | Aldehyde | AAT1 | AAT2 | LOX | HPL | ADH | |
|---|---|---|---|---|---|---|---|---|---|
| Volatile | 1 | ||||||||
| Esters | 0.884 * | 1 | |||||||
| Alcohols | 0.909 * | 0.735 | 1 | ||||||
| Aldehyde | 0.588 | 0.554 | 0.297 | 1 | |||||
| AAT1 | 0.898 * | 0.921 * | 0.777 | 0.740 | 1 | ||||
| AAT2 | 0.825 | 0.934 * | 0.672 | 0.736 | 0.983 ** | 1 | |||
| LOX | 0.874 | 0.830 | 0.671 | 0.895 * | 0.950 * | 0.921 * | 1 | ||
| HPL | 0.605 | 0.555 | 0.341 | 0.997 ** | 0.762 | 0.750 | 0.906 * | 1 | |
| ADH | 0.783 | 0.848 | 0.668 | 0.770 | 0.976 ** | 0.978 ** | 0.923 * | 0.797 | 1 |
| Treatment | Control | Calcium Chloride | Sorbitol-Chelated Calcium | Calcium Nitrate |
|---|---|---|---|---|
| CK | Water | — | — | — |
| T1 | — | 4% | — | — |
| T2 | — | — | 4% | — |
| T3 | — | — | — | 4% |
| Gene Name | Forward Sequence of the Primers (5′–3′) | Reverse Sequence of the Primers (5′–3′) |
|---|---|---|
| AAT1 | GCTGGATTGCTCTTGTTC | TGGTTACTGGATGCGTAT |
| AAT2 | GGATTACTCAGGAACCTAA | GACACAACTCTACATTGC |
| LOX | GATGGTCTCCTCGTATGG | CTTCGTGTCCCTTATTCTTG |
| ADH | CCACCACAAGCAAATGAA | ACCAACACTCTCCACAAT |
| HPL | TAGGAGGGAAGTGAGAGG | AGAGAAACAAAGCGAGGT |
| Actin | TGACCGAATGAGCAAGGAAATTACT | TACTCAGCTTTGGCAATCCACATC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Wang, F.; Ci, J.; Liu, Y.; Li, K.; Wang, D.; Yu, W.; Zhuang, Y.; Xiao, Y. Effects of Different Calcium Preparations on Fresh-Cut Quality and Storage Quality of Starkrimson Apple. Plants 2025, 14, 1293. https://doi.org/10.3390/plants14091293
Sun M, Wang F, Ci J, Liu Y, Li K, Wang D, Yu W, Zhuang Y, Xiao Y. Effects of Different Calcium Preparations on Fresh-Cut Quality and Storage Quality of Starkrimson Apple. Plants. 2025; 14(9):1293. https://doi.org/10.3390/plants14091293
Chicago/Turabian StyleSun, Maoxiang, Fen Wang, Jianchao Ci, Yangyang Liu, Keyi Li, Dong Wang, Wen Yu, Yu Zhuang, and Yuansong Xiao. 2025. "Effects of Different Calcium Preparations on Fresh-Cut Quality and Storage Quality of Starkrimson Apple" Plants 14, no. 9: 1293. https://doi.org/10.3390/plants14091293
APA StyleSun, M., Wang, F., Ci, J., Liu, Y., Li, K., Wang, D., Yu, W., Zhuang, Y., & Xiao, Y. (2025). Effects of Different Calcium Preparations on Fresh-Cut Quality and Storage Quality of Starkrimson Apple. Plants, 14(9), 1293. https://doi.org/10.3390/plants14091293


_Ambaw.png)





