Postharvest Flavor Quality Changes and Preservation Strategies for Peach Fruits: A Comprehensive Review
Abstract
1. Introduction
2. Progress in the Study of Postharvest Quality Changes in Peach
2.1. Amino Acids
2.2. Phenolic Compounds
2.3. Sugar–Acid Contents
2.4. Volatile Substances
| Species | Representative | Odor | Reference |
|---|---|---|---|
| Ester | Hexyl acetate | Spicy, banana, fruity aroma | [39,48] |
| Ethyl hexanoate | Flower fragrance, greenness | ||
| (Z)-3-Hexenyl acetate | Candy fragrance | ||
| Ethyl caproate | Fruity aroma, sweet | ||
| Ethyl octanoate | Fruity, sweet | ||
| Lactone | γ-Hexalactone | Coconut, vanilla, peach, sweet fragrance | [48,49] |
| δ-Decalactone | Fruity, sweet | [50] | |
| γ-Decalactone | Peach-like, fruity, sweet | [39] | |
| γ-Octalactone | Coconut, fruity | [39] | |
| δ-Octalactone | Coconut, peach-like | [39] | |
| Aldehyde | 2-Hexenal | Grassy, almondy | [44,48,51] |
| Benzaldehyde | Grassy | [48] | |
| Hexanal | Grassy | [44,48] | |
| Terpene | β-Myrcene | Resin, fruity fragrance | [39,52] |
| linalool | Flowery, fruity, woody | [43] | |
| Limonene | Orange, lemon | [39] |
3. Advances in Freshness Preservation Technologies
3.1. Temperature-Controlled Preservation
3.2. Gas-Conditioning Preservation Technology
3.3. Packaging Technology
3.4. Exogenous Hormone Treatment
3.5. Combined Treatment
4. Summary and Prospects
Funding
Conflicts of Interest
References
- Zhang, Y.; Zhang, B.; Cai, Z.; Shen, Z.; Yu, M.; Ma, R. Elucidating the influence of volatile compounds on aroma profiles across peach (Prunus persica L.) cultivars and offspring exhibiting diverse flesh colors. Curr. Res. Food Sci. 2024, 9, 100901. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, Y.; Jiang, S.; Wang, X.; Xu, F.; Wang, H.; Shao, X. Flavor development in peach fruit treated with 1-methylcyclopropene during shelf storage. Food Res. Int. 2020, 137, 109653. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Xu, Y.; Jiang, L.; Huan, C.; Yu, Z. Effects of postharvest temperature on apoptosis-related enzyme activity and gene expression in peach fruits (Prunus persica L. cv. Xiahui 8). Sci. Hortic. 2019, 245, 178–184. [Google Scholar] [CrossRef]
- Hanif, M.; Azam, M.; Khan, M.A.; Zahid, T.; Ali, B.; Akram, M.T.; Naveed, K.; Ilahy, R.; Hussain, I.; Liu, H.; et al. Investigating the effect of marigold flower extract on bioactive compounds, antioxidants and postharvest quality and shelf life of peach during storage. J. Food Meas. Charact. 2025, 19, 1837–1850. [Google Scholar] [CrossRef]
- Guo, T.; Li, J.; Guo, M.; Yang, Q.; Dai, X.; Qiao, X.; Song, Z.; Tian, C.; Li, Y.; Ge, H.; et al. Low temperature inhibits pectin degradation by PpCBFs to prolong peach storage time. J. Food Sci. 2023, 88, 3725–3736. [Google Scholar] [CrossRef] [PubMed]
- Ai, S.; Xu, S.; Wu, C.; Grierson, D.; Chen, K.; Xu, C. Novel insights into modified atmosphere mediated cold tolerance in peach fruit during postharvest storage. Postharvest Biol. Technol. 2024, 218, 113187. [Google Scholar] [CrossRef]
- Dias, C.; Ribeiro, T.; Rodrigues, A.C.; Ferrante, A.; Vasconcelos, M.W.; Pintado, M. Improving the ripening process after 1-MCP application: Implications and strategies. Trends Food Sci. Technol. 2021, 113, 382–396. [Google Scholar] [CrossRef]
- Feng, B.-S.; Kang, D.-C.; Sun, J.; Leng, P.; Liu, L.-X.; Wang, L.; Ma, C.; Liu, Y.-G. Research on melatonin in fruits and vegetables and the mechanism of exogenous melatonin on postharvest preservation. Food Biosci. 2022, 50, 102196. [Google Scholar] [CrossRef]
- Cai, H.; Han, S.; Yu, M.; Ma, R.; Yu, Z. The alleviation of methyl jasmonate on loss of aroma lactones correlated with ethylene biosynthesis in peaches. J. Food Sci. 2020, 85, 2389–2397. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Kou, X.; Li, J.; Luo, D.; Huang, T.; Wang, X.; Cao, S. Salicylic acid mitigates chilling injury to peaches by improving antioxidant capacity and energy metabolism. Sci. Hortic. 2024, 338, 113841. [Google Scholar] [CrossRef]
- Hou, T.; Ma, S.; Wang, F.; Wang, L. A comprehensive review of intelligent controlled release antimicrobial packaging in food preservation. Food Sci. Biotechnol. 2023, 32, 1459–1478. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Li, S.; Tu, T.; Li, Y.; Niu, M.; Tong, Y.; Yang, Y.; Xu, T.; Zhao, J.; Shen, C.; et al. Free amino acids identification and process optimization in greengage wine fermentation and flavor formation. J. Food Sci. 2023, 88, 988–1003. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Zhao, B.; Cai, Z.; Yan, J.; Ma, R.; Yu, M. Amino Acid Profiles in Peach (Prunus persica L.) Fruit. Foods 2022, 11, 1718. [Google Scholar] [CrossRef]
- Bachmanov, A.A.; Bosak, N.P.; Glendinning, J.I.; Inoue, M.; Li, X.; Manita, S.; McCaughey, S.A.; Murata, Y.; Reed, D.R.; Tordoff, M.G.; et al. Genetics of Amino Acid Taste and Appetite. Adv. Nutr. 2023, 7, 806S–822S. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, W.; Tao, L.; Wei, X.; Gao, L.; Gao, Y.; Suo, J.; Yu, W.; Hu, Y.; Yang, B.; et al. Ethylene treatment promotes umami taste-active amino acids accumulation of Torreya grandis nuts post-harvest by comparative chemical and transcript analyses. Food Chem. 2022, 408, 135214. [Google Scholar] [CrossRef]
- Guo, C.; Bi, J.; Li, X.; Lyu, J.; Xu, Y.; Hu, J. Investigation on the phenolic composition, related oxidation and antioxidant activity of thinned peach dried by different methods. LWT-Food Sci. Technol. 2021, 147, 111573. [Google Scholar] [CrossRef]
- Maatallah, S.; Dabbou, S.; Castagna, A.; Guizani, M.; Hajlaoui, H.; Ranieri, A.M.; Flamini, G. Prunus persica by-products: A source of minerals, phenols and volatile compounds. Sci. Hortic. 2020, 261, 109016. [Google Scholar] [CrossRef]
- Mrázová, M.; Rampáčková, E.; Šnurkovič, P.; Ondrášek, I.; Nečas, T.; Ercisli, S. Determination of Selected Beneficial Substances in Peach Fruits. Sustainability 2021, 13, 14028. [Google Scholar] [CrossRef]
- Reig, G.; Iglesias, I.; Gatius, F.; Alegre, S. Antioxidant Capacity, Quality, and Anthocyanin and Nutrient Contents of Several Peach Cultivars [Prunus persica (L.) Batsch] Grown in Spain. J. Agric. Food Chem. 2013, 61, 6344–6357. [Google Scholar] [CrossRef]
- Botoran, O.R.; Ionete, R.E.; Miricioiu, M.G.; Costinel, D.; Radu, G.L.; Popescu, R. Amino Acid Profile of Fruits as Potential Fingerprints of Varietal Origin. Molecules 2019, 24, 4500. [Google Scholar] [CrossRef]
- He, Y.; Qin, H.; Wen, J.; Wang, L.; Cao, W.; Fan, S.; Lu, W.; Li, J.; Li, C. Characterization of Amino Acid Composition, Nutritional Value, and Taste of Fruits from Different Actinidia arguta Resources. J. Food Qual. 2024, 2024, 1005194. [Google Scholar] [CrossRef]
- Kim, H.-R.; Kim, I.-D.; Dhungana, S.K.; Kim, M.-O.; Shin, D.-H. Comparative assessment of physicochemical properties of unripe peach (Prunus persica) and Japanese apricot (Prunus mume). Asian Pac. J. Trop. Biomed. 2014, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Monti, L.L.; Bustamante, C.A.; Osorio, S.; Gabilondo, J.; Borsani, J.; Lauxmann, M.A.; Maulión, E.; Valentini, G.; Budde, C.O.; Fernie, A.R.; et al. Metabolic profiling of a range of peach fruit varieties reveals high metabolic diversity and commonalities and differences during ripening. Food Chem. 2016, 190, 879–888. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Falcão, A.; Alves, G.; Silva, L.R.; Flores-Félix, J.D. Antioxidant activity of the main phenolics found in red fruits: An in vitro and in silico study. Food Chem. 2024, 452, 139459. [Google Scholar] [CrossRef]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, Q.-G.; Wang, W.-Q.; Grierson, D.; Yin, X.-R. Molecular basis of the formation and removal of fruit astringency. Food Chem. 2021, 372, 131234. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Anwar, F.; Mahmood, Z.; Rashid, U.; Ashraf, M. Variation in Minerals, Phenolics and Antioxidant Activity of Peel and Pulp of Different Varieties of Peach (Prunus persica L.) Fruit from Pakistan. Molecules 2012, 17, 6491–6506. [Google Scholar] [CrossRef]
- Stojanovic, B.T.; Mitic, S.S.; Stojanovic, G.S.; Mitic, M.N.; Kostic, D.A.; Paunovic, D.D.; Arsic, B.B. Phenolic Profile and Antioxidant Activity of Pulp and Peel from Peach and Nectarine Fruits. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 175–182. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, J.; Liu, Y.; Zhang, X.; Cao, Y.; Zheng, B.; Zhang, R.-X.; Zhao, C.; Ai, X.; He, H.; et al. Metabolic basis for superior antioxidant capacity of red-fleshed peaches. Food Chem. X 2024, 23, 101698. [Google Scholar] [CrossRef]
- Dong, X.; He, Y.; Yuan, C.; Cheng, X.; Li, G.; Shan, Y.; Zhu, X. Controlled Atmosphere Improves the Quality, Antioxidant Activity and Phenolic Content of Yellow Peach during the Shelf Life. Antioxidants 2022, 11, 2278. [Google Scholar] [CrossRef] [PubMed]
- Saidani, F.; Giménez, R.; Aubert, C.; Chalot, G.; Betrán, J.A.; Gogorcena, Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J. Food Compos. Anal. 2017, 62, 126–133. [Google Scholar] [CrossRef]
- Baccichet, I.; Chiozzotto, R.; Bassi, D.; Gardana, C.; Cirilli, M.; Spinardi, A. Characterization of fruit quality traits for organic acids content and profile in a large peach germplasm collection. Sci. Hortic. 2020, 278, 109865. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J. Sci. Food Agric. 2005, 85, 2611–2616. [Google Scholar] [CrossRef]
- Versari, A.; Castellari, M.; Parpinello, G.P.; Riponi, C.; Galassi, S. Characterisation of peach juices obtained from cultivars Redhaven, Suncrest and Maria Marta grown in Italy. Food Chem. 2002, 76, 181–185. [Google Scholar] [CrossRef]
- Wu, H.; Xu, Y.; Wang, H.; Miao, Y.; Li, C.; Zhao, R.; Shi, X.; Wang, B. Physicochemical Characteristics, Antioxidant Activities, and Aroma Compound Analysis of Seven Peach Cultivars (Prunus persica L. Batsch) in Shihezi, Xinjiang. Foods 2022, 11, 2944. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, S.; Ye, Z.; Zhou, H.; Shen, S.; Zheng, X.; Huan, C. The effects of chilling (4 °C) and non-chilling (12 °C) temperatures on storage quality and flavor development of yellow peach fruit. J. Food Compos. Anal. 2024, 139, 1005194. [Google Scholar] [CrossRef]
- Zhou, H.; Su, M.; Du, J.; Zhang, X.; Li, X.; Zhang, M.; Hu, Y.; Huan, C.; Ye, Z. Crucial roles of sorbitol metabolism and energy status in the chilling tolerance of yellow peach. Plant Physiol. Biochem. 2023, 204, 108092. [Google Scholar] [CrossRef]
- Liu, G.; Bi, J.; Chen, Q. Impact of postharvest ripening on peach quality: Aroma release and formation mechanism. Food Chem. 2025, 479, 143743. [Google Scholar] [CrossRef]
- Zhou, D.; Liu, Q.; Zhu, T.; Li, T.; Fan, G.; Li, X.; Wu, C. Effects of ultraviolet C on the quality and aroma volatile in peach fruit during postharvest storage. Food Chem. 2024, 456, 139906. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Li, S.; Yang, L.; Wang, Y.; Zhao, J.; Jiang, Q. Volatile characteristics of 50 peaches and nectarines evaluated by HP–SPME with GC–MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Aubert, C.; Chalot, G.; Lurol, S.; Ronjon, A.; Cottet, V. Relationship between fruit density and quality parameters, levels of sugars, organic acids, bioactive compounds and volatiles of two nectarine cultivars, at harvest and after ripening. Food Chem. 2019, 297, 124954. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, P.; Zhang, C.; Xiao, X.; Chen, C.; Song, F. Aroma of peach fruit: A review on aroma volatile compounds and underlying regulatory mechanisms. Int. J. Food Sci. Technol. 2023, 58, 4965–4979. [Google Scholar] [CrossRef]
- Xin, R.; Liu, X.; Wei, C.; Yang, C.; Liu, H.; Cao, X.; Wu, D.; Zhang, B.; Chen, K. E-Nose and GC-MS Reveal a Difference in the Volatile Profiles of White- and Red-Fleshed Peach Fruit. Sensors 2018, 18, 765. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, G.; Besada, C.; Badenes, M.L.; Monforte, A.J.; Granell, A. A Non-Targeted Approach Unravels the Volatile Network in Peach Fruit. PLoS ONE 2012, 7, e38992. [Google Scholar] [CrossRef]
- Leng, P.; Hu, H.-W.; Cui, A.-H.; Tang, H.-J.; Liu, Y.-G. HS-GC-IMS with PCA to analyze volatile flavor compounds of honey peach packaged with different preservation methods during storage. LWT—Food Sci. Technol. 2021, 149, 111963. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Su, M.; Zeng, W.; Wang, S.; Du, J.; Zhou, H.; Yang, X.; Zhang, X.; Li, X.; et al. Multidimensional analysis of the flavor characteristics of yellow peach at different ripening stages: Chemical composition profiling and sensory evaluation. Food Chem. 2025, 471, 142772. [Google Scholar] [CrossRef]
- Feng, C.; Ni, Y.; Zhang, Y.; Yang, J.; Xiong, R. Characteristic aroma identification of differentially colored peach fruits based on HS-SPME-GC–MS. Food Chem. 2024, 467, 142280. [Google Scholar] [CrossRef]
- Cao, X.; Su, Y.; Zhao, T.; Zhang, Y.; Cheng, B.; Xie, K.; Yu, M.; Allan, A.; Klee, H.; Chen, K.; et al. Multi-omics analysis unravels chemical roadmap and genetic basis for peach fruit aroma improvement. Cell Rep. 2024, 43, 114623. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Wu, J.; Xiao, X.; Zhang, L.; Chen, C.; Wilson, A.S.; Song, F. Ester-related volatile compounds reveal the diversity and commonalities of different types of late-ripening peaches. J. Food Sci. 2024, 89, 1485–1497. [Google Scholar] [CrossRef]
- Wang, J.-R.; Wu, X.-Y.; Cui, C.-B.; Bi, J.-F. Effect of osmotic dehydration combined with vacuum freeze-drying treatment on characteristic aroma components of peach slices. Food Chem. X 2024, 22, 101337. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, Y.; Ma, R.; Yu, M. Comparison of Aroma Trait of the White-Fleshed Peach ‘Hu Jing Mi Lu’ and the Yellow-Fleshed Peach ‘Jin Yuan’ Based on Odor Activity Value and Odor Characteristics. Horticulturae 2022, 8, 245. [Google Scholar] [CrossRef]
- Song, C.; Wang, K.; Xiao, X.; Liu, Q.; Yang, M.; Li, X.; Feng, Y.; Li, S.; Shi, L.; Chen, W.; et al. Membrane lipid metabolism influences chilling injury during cold storage of peach fruit. Food Res. Int. 2022, 157, 111249. [Google Scholar] [CrossRef]
- Liu, H.; He, H.; Wang, Q.; Li, S.; Guo, F.; Zhang, C.; Wang, K.; Liu, C.; Wang, C.; Wang, X.; et al. Near-freezing temperature alters ROS metabolism and gene transcription to enhance peach chilling tolerance and aroma quality. Food Res. Int. 2025, 209, 116297. [Google Scholar] [CrossRef] [PubMed]
- Farcuh, M.; Hopfer, H. Aroma volatiles as predictors of chilling injury development during peach (Prunus persica (L.) Batsch) cold storage and subsequent shelf-life. Postharvest Biol. Technol. 2022, 195, 112137. [Google Scholar] [CrossRef]
- Rodrigues, C.; Gaspar, P.D.; Simões, M.P.; Silva, P.D.; Andrade, L.P. Review on techniques and treatments toward the mitigation of the chilling injury of peaches. J. Food Process. Preserv. 2020, 46, e14358. [Google Scholar] [CrossRef]
- Yang, Q.; Hou, J.; Wang, F.; Qi, Y.; Zhao, Q. Cold shock treatment alleviates pitting in sweet cherry fruit by enhancing antioxidant enzymes activity and regulating membrane lipid metabolism. J. Sci. Food Agric. 2024, 105, 54–64. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, S.; Chen, G.; Zheng, Y.; Jin, P. Cold shock treatment alleviates chilling injury in peach fruit by regulating antioxidant capacity and membrane lipid metabolism. Food Qual. Saf. 2022, 6, fyab026. [Google Scholar] [CrossRef]
- Jia, Z.; Bao, Y.; Zhao, Y.; Liu, Y.; Zheng, Y.; Feng, Z.; Jin, P. Cold shock treatment enhances cold tolerance in peach fruit through modulating PpbZIP9 and PpVIP1-mediated respiratory metabolism. Postharvest Biol. Technol. 2023, 204, 112421. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, M.; Mei, J.; Xie, J. Effects of Different Postharvest Precooling Treatments on Cold-Storage Quality of Yellow Peach (Amygdalus persica). Plants 2022, 11, 2334. [Google Scholar] [CrossRef]
- Fang, Y.; Wakisaka, M. A Review on the Modified Atmosphere Preservation of Fruits and Vegetables with Cutting-Edge Technologies. Agriculture 2021, 11, 992. [Google Scholar] [CrossRef]
- Liu, H.; He, H.; Liu, C.; Wang, C.; Qiao, Y.; Zhang, B. Changes of Sensory Quality, Flavor-Related Metabolites and Gene Expression in Peach Fruit Treated by Controlled Atmosphere (CA) under Cold Storage. Int. J. Mol. Sci. 2022, 23, 7141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, X.; Su, M.; Du, J.; Li, X.; Zhang, M.; Hu, Y.; Huan, C.; Ye, Z. Controlled atmosphere storage alleviates internal browning in flat peach fruit by regulating energy and sugar metabolisms. Plant Physiol. Biochem. 2022, 186, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Akbudak, B.; Eris, A. Physical and chemical changes in peaches and nectarines during the modified atmosphere storage. Food Control 2004, 15, 307–313. [Google Scholar] [CrossRef]
- Veloso, A.; Gaspar, P.D.; Andrade, L.P.; Santo, C.E.; Resende, M.; Silva, P.D.; Canavarro, C.; Simões, M.P. The effect of controlled atmosphere storage on fruit quality and postharvest performance of ‘Sweet Henry’ peach cultivar. Acta Hortic. 2022, 1352, 635–642. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, T.; Zhao, Y.; Cheng, C.; Yin, H.; Yue, J. The developments and trends of electrospinning active food packaging: A review and bibliometrics analysis. Food Control. 2024, 160, 110291. [Google Scholar] [CrossRef]
- Cheng, C.; Min, T.; Luo, Y.; Zhang, Y.; Yue, J. Electrospun polyvinyl alcohol/chitosan nanofibers incorporated with 1,8-cineole/cyclodextrin inclusion complexes: Characterization, release kinetics and application in strawberry preservation. Food Chem. 2023, 418, 135652. [Google Scholar] [CrossRef]
- Aaqil, M.; Peng, C.; Kamal, A.; Nawaz, T.; Gong, J. Recent Approaches to the Formulation, Uses, and Impact of Edible Coatings on Fresh Peach Fruit. Foods 2024, 13, 267. [Google Scholar] [CrossRef]
- Sortino, G.; Saletta, F.; Puccio, S.; Scuderi, D.; Allegra, A.; Inglese, P.; Farina, V. Extending the Shelf Life of White Peach Fruit with 1-Methylcyclopropene and Aloe arborescens Edible Coating. Agriculture 2020, 10, 151. [Google Scholar] [CrossRef]
- Jiao, W.; Shu, C.; Li, X.; Cao, J.; Fan, X.; Jiang, W. Preparation of a chitosan-chlorogenic acid conjugate and its application as edible coating in postharvest preservation of peach fruit. Postharvest Biol. Technol. 2019, 154, 129–136. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, L.; Xia, Q.; Yi, K.; Li, Y. Novel Post-Harvest Preservation Techniques for Edible Fungi: A Review. Foods 2024, 13, 1554. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Hu, P.; Wang, Y.; Jin, X.; Chen, R.; Zhang, W.; Ni, Y.; Wang, J. Multifunctional packaging film with sustained release behavior triggered by pH microenvironment for efficient preservation of pork. Food Chem. 2023, 438, 138007. [Google Scholar] [CrossRef]
- Du, L.; Huang, X.; Li, Z.; Qin, Z.; Zhang, N.; Zhai, X.; Shi, J.; Zhang, J.; Shen, T.; Zhang, R.; et al. Application of Smart Packaging in Fruit and Vegetable Preservation: A Review. Foods 2025, 14, 447. [Google Scholar] [CrossRef]
- Cai, H.; Han, S.; Wang, Q.; Liu, X.; Yu, Z. Transcriptomic and metabolite analyses provided a new sight of 1-MCP on organic acid metabolism in peach during storage. J. Food Sci. 2023, 88, 3323–3331. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, L.; Su, M.; Zhang, X.; Du, J.; Li, X.; Zhang, M.; Hu, Y.; Ye, Z.; Huan, C. Comparative network analysis reveals the regulatory mechanism of 1-methylcyclopropene on sugar and acid metabolisms in yellow peach stored at non-chilling temperatures. Plant Physiol. Biochem. 2024, 216, 109100. [Google Scholar] [CrossRef]
- Huan, C.; Zhang, J.; Jia, Y.; Li, S.; Jiang, T.; Shen, S.; Zheng, X. Effect of 1-methylcyclopropene treatment on quality, volatile production and ethanol metabolism in kiwifruit during storage at room temperature. Sci. Hortic. 2020, 265, 109266. [Google Scholar] [CrossRef]
- Cai, H.; An, X.; Han, S.; Jiang, L.; Yu, M.; Ma, R.; Yu, Z. Effect of 1-MCP on the production of volatiles and biosynthesis-related gene expression in peach fruit during cold storage. Postharvest Biol. Technol. 2018, 141, 50–57. [Google Scholar] [CrossRef]
- Cai, H.; Han, S.; Jiang, L.; Yu, M.; Ma, R.; Yu, Z. 1-MCP treatment affects peach fruit aroma metabolism as revealed by transcriptomics and metabolite analyses. Food Res. Int. 2019, 122, 573–584. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Li, X.; Wang, Z.; Wang, H.; Li, W.; Liu, T.; Li, X.; Jiang, Y.; Tang, Y. Combination of 1-methylcyclopropene and phytic acid inhibits surface browning and maintains texture and aroma of fresh-cut peaches. Postharvest Biol. Technol. 2023, 200, 112328. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using Exogenous Melatonin, Glutathione, Proline, and Glycine Betaine Treatments to Combat Abiotic Stresses in Crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, J.; Xiang, M.; Zeng, J.; Chen, J.; Chen, M. Exogenous melatonin treatment affects ascorbic acid metabolism in postharvest ‘Jinyan’ kiwifruit. Front. Nutr. 2022, 9, 1081476. [Google Scholar] [CrossRef]
- Cao, S.; Shao, J.; Shi, L.; Xu, L.; Shen, Z.; Chen, W.; Yang, Z. Melatonin increases chilling tolerance in postharvest peach fruit by alleviating oxidative damage. Sci. Rep. 2018, 8, 806. [Google Scholar] [CrossRef]
- Wu, C.; Hao, W.; Yan, L.; Zhang, H.; Zhang, J.; Liu, C.; Zheng, L. Postharvest melatonin treatment enhanced antioxidant activity and promoted GABA biosynthesis in yellow-flesh peach. Food Chem. 2023, 419, 136088. [Google Scholar] [CrossRef]
- Bao, Z.; Zhou, Q.; Yu, Y.; Chen, W.; Yang, Z.; Cao, S.; Shi, L. Melatonin treatment induces DNA methylation to alleviate chilling induced-browning in cold stored peach fruit. Postharvest Biol. Technol. 2023, 208, 112686. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, C.; Brummell, D.A.; Qi, S.; Lin, Q.; Duan, Y. Jasmonic acid treatment alleviates chilling injury in peach fruit by promoting sugar and ethylene metabolism. Food Chem. 2021, 338, 128005. [Google Scholar] [CrossRef]
- Duan, W.; Yang, C.; Cao, X.; Zhang, C.; Liu, H.; Chen, K.; Li, X.; Zhang, B. Transcriptome and DNA methylome analysis reveal new insights into methyl jasmonate-alleviated chilling injury of peach fruit after cold storage. Postharvest Biol. Technol. 2022, 189, 111915. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, P.; Liu, W.; Zhang, Q.; Li, G.; Shan, Y.; Zhu, X. Understanding the volatile organic compounds of 1-methylcyclopropylene fumigation and packaging on yellow-fleshed peach via headspace-gas chromatography-ion mobility spectrometry and chemometric analyses. J. Food Sci. 2022, 87, 4009–4026. [Google Scholar] [CrossRef]
- Li, Y.-L.; Liu, S.; Chen, S.-Q.; Rashid, A.; Wang, L.; Wang, K. Physicochemical changes in fresh-cut peaches with the combined treatment of UV-B irradiation and 1-MCP. Postharvest Biol. Technol. 2022, 184, 111755. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Yin, J. Effects of hot air treatment in combination with Pichia guilliermondii on postharvest preservation of peach fruit. J. Sci. Food Agric. 2018, 99, 647–655. [Google Scholar] [CrossRef]
- Wu, X.; Yuan, J.; Wang, X.; Yu, M.; Ma, R.; Yu, Z. Synergy of Nitric Oxide and 1-Methylcyclopropene Treatment in Prolong Ripening and Senescence of Peach Fruit. Foods 2021, 10, 2956. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, H.; Wang, Q.; Su, C.; Sun, Y.; Qiu, C.; Pang, J. Research progress and future trends in smart response packaging for food preservation. J. Stored Prod. Res. 2025, 112, 102597. [Google Scholar] [CrossRef]
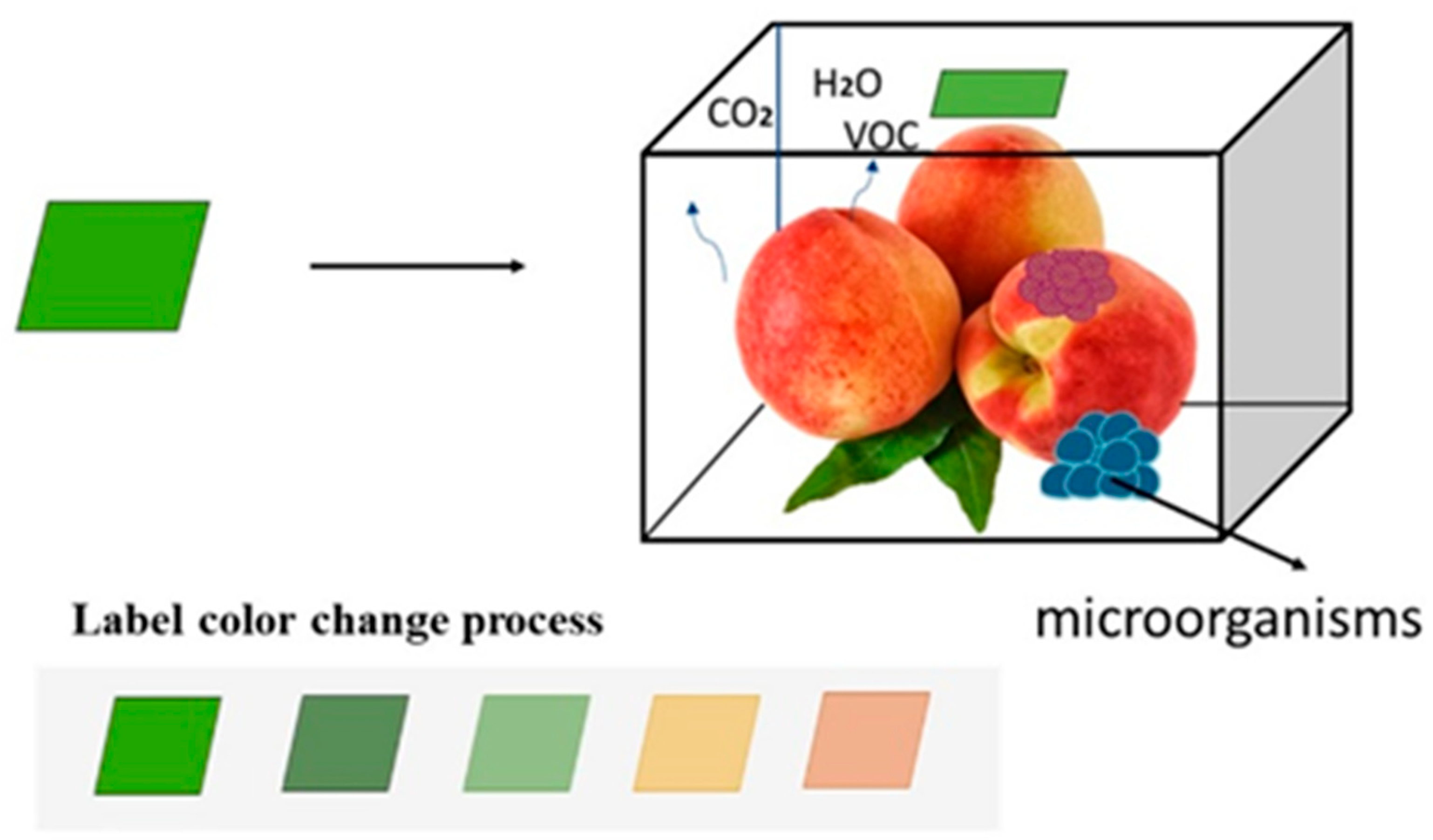

| Peach Non-Volatile Flavor Components | Main Representative Substances | Affects Flavor | Species Examples | Reference |
|---|---|---|---|---|
| Amino acids | Pro | Sweet | Golden Honey Peach | [12,13,14,15] |
| Glu | Umami | Yangshan Honey Peach | ||
| Asp | Sweet, sour | Feicheng Peach | ||
| Asn | Bitter, astringent | Yellow-fleshed Peach | ||
| Ala | Pure and sweet | Yulu Flat Peach | ||
| Arg | Slightly bitter | Qingzhou Green Peach | ||
| Phenolics | Chlorogenic acid | Slightly bitter | Jjubao Peach | [16,17] |
| Neochlorogenic acid | Bitter | Golden Peach | ||
| Epicatechins | Acerbic | Red-fleshed Peach | ||
| Anthocyanins | Acerbic | Red-fleshed Peach, Yellow-fleshed Peach | ||
| RutinProanthocyanidins | Acerbic | Blood peach | ||
| Sugars | Sucrose | Sweet | Jinxia | [13,18] |
| Glucose | Sweet | Yixianghong | ||
| Fructose | Sweet | Touxinhong | ||
| Sorbitol | Sweet | Yixianghong | ||
| Acids | Quinic acid | Sour | Yixianghong | [13,19] |
| Malic acid | Sour | NJN76 | ||
| Citric acid | Sour, acerbic | Tropic Prince |
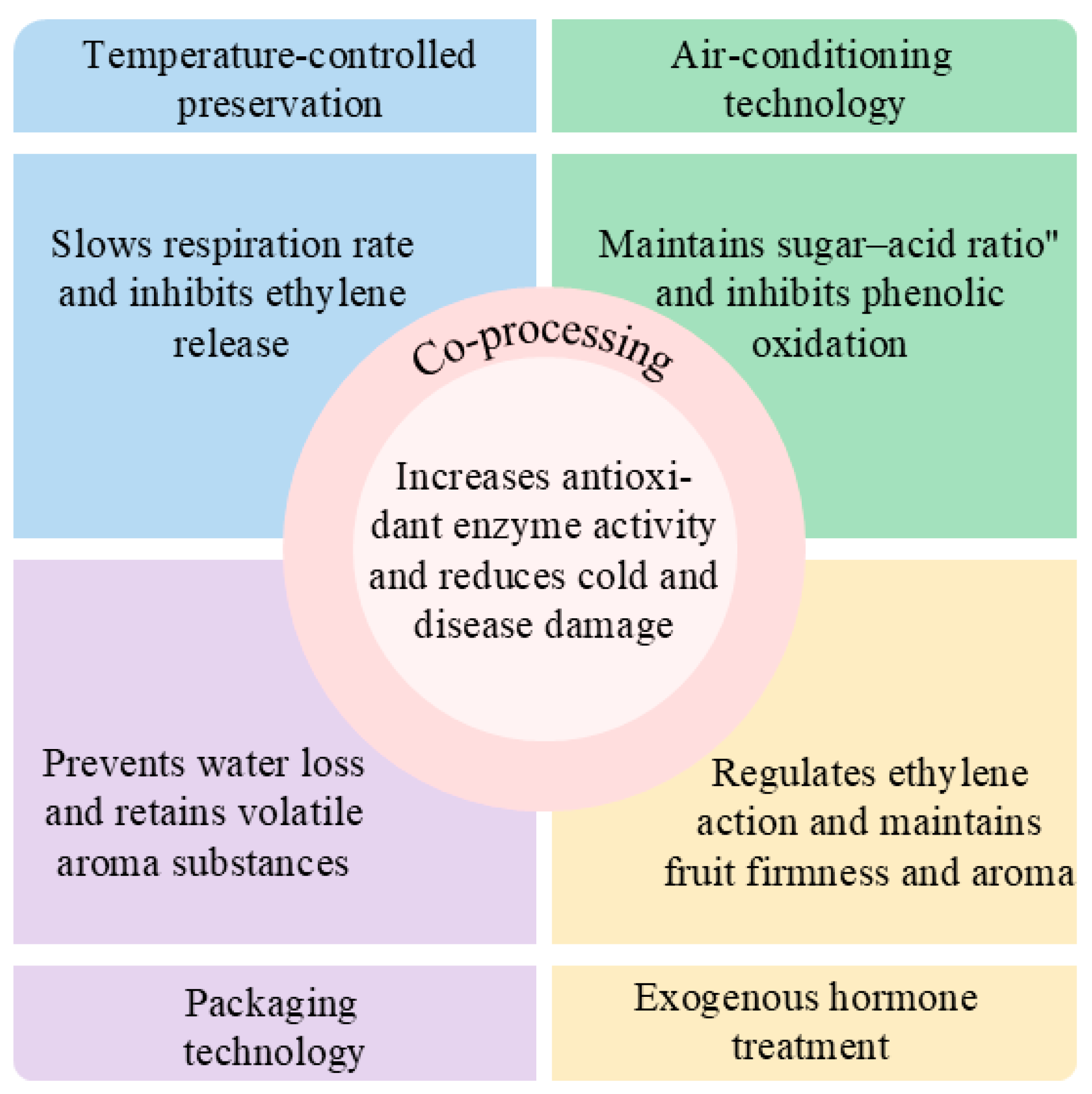
| Preservation Technology | Main Methods | Dominance | Limitations | Reference |
|---|---|---|---|---|
| Temperature-controlled preservation | Low-temperature storage, near-freezing temperature, CS, heat treatment | Low cost, easy to operate, widely applicable | Low temperatures are prone to cold damage and heat treatment may affect flavor | [57,58] |
| Air-conditioning technology | MA, CA | Effectively extended shelf life, maintained nutrition and flavor | High equipment costs and precise control of gas ratios | [61] |
| Packaging technology | Active packaging, smart packaging, coating technology | Extended shelf life and increased market value | Certain materials may affect the natural flavor and cost more | [11,74,92] |
| Exogenous hormone treatment | Ethylene/1-MCP, MeJA, MT treatment | Regulation of the postharvest ripening process, specifically targeted | Certain hormones may inhibit aroma release and affect overall flavor | [7,80,85] |
| Combined treatment | Combined application of multiple technologies | Combined effect and extended shelf life | Requires optimized combination of solutions and is complex to operate | [87,88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Q.; Wang, L.; Wang, Q.; Wang, R.; Li, C.; Qiao, Y.; Liu, H. Postharvest Flavor Quality Changes and Preservation Strategies for Peach Fruits: A Comprehensive Review. Plants 2025, 14, 1310. https://doi.org/10.3390/plants14091310
Qin Q, Wang L, Wang Q, Wang R, Li C, Qiao Y, Liu H. Postharvest Flavor Quality Changes and Preservation Strategies for Peach Fruits: A Comprehensive Review. Plants. 2025; 14(9):1310. https://doi.org/10.3390/plants14091310
Chicago/Turabian StyleQin, Qiaoping, Lili Wang, Qiankun Wang, Rongshang Wang, Chunxi Li, Yongjin Qiao, and Hongru Liu. 2025. "Postharvest Flavor Quality Changes and Preservation Strategies for Peach Fruits: A Comprehensive Review" Plants 14, no. 9: 1310. https://doi.org/10.3390/plants14091310
APA StyleQin, Q., Wang, L., Wang, Q., Wang, R., Li, C., Qiao, Y., & Liu, H. (2025). Postharvest Flavor Quality Changes and Preservation Strategies for Peach Fruits: A Comprehensive Review. Plants, 14(9), 1310. https://doi.org/10.3390/plants14091310






