Leaf Senescence by Magnesium Deficiency
Abstract
:1. Introduction


2. Alterations to Mg Distribution and the Potential Strategies to Recycle Mg during Deficiency


3. Initial Physiological Responses to Mg Deficiency
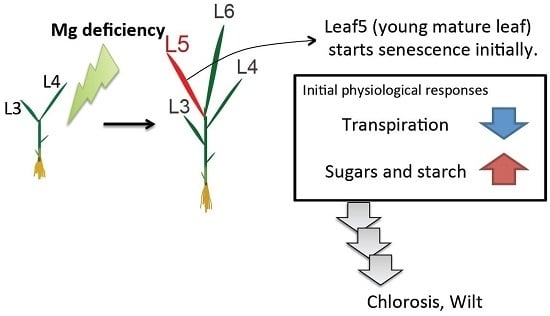
3.1. Young Mature Leaves are the Initial Sites of Mg-Deficiency Symptoms

3.2. The Decline in Transpiration
3.3. Sugar and Starch Accumulation
3.4. The Influence on the Photosynthetic Apparatus by Mg-Deficiency Associated with Chlorophyll
4. The Mechanism of Chlorosis and Necrosis of Leaves Induced by Mg Deficiency

5. Responses of Mg Transporters to Mg Deficiency

6. Summary and Perspective

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buchanan, B.B. Role of light in the regulation of chloroplast enzymes. Annu. Rev. Plant Physiol. 1980, 31, 341–374. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition in Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Takata, J.; Takamatsu, T.; Satake, K.; Sase, H. Data on Elemental Concentration in Land Plants by Neutron Activation Analysis (No.1); Takata, J., Takamatsu, T., Satake, K., Sase, H., Eds.; National Institute for Environmental Studies: Tsukuba, Japan, 1994. [Google Scholar]
- Lahner, B.; Gong, J.; Mahmoudian, M.; Smith, E.L.; Abid, K.B.; Rogers, E.E.; Guerinot, M.L.; Harper, J.F.; Ward, J.M.; McIntyre, L.; et al. Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat. Biotechnol. 2003, 21, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.I.; Iwata, N.; Saito, T.; Suzuki, H.; Iwata, R.; Tanoi, K.; Nakanishi, T.M. Application of 28Mg for characterization of Mg uptake in rice seedling under different pH conditions. J. Radioanal. Nucl. Chem. 2013, 296, 531–534. [Google Scholar] [CrossRef]
- Jacoby, B. Calcium-magnesium ratios in the root medium as related to magnesium uptake by citrus seedlings. Plant Soil 1961, 15, 74–80. [Google Scholar] [CrossRef]
- Tanaka, H.; Ougimoto, T.; Sahashi, H. Effects of calcium and magnesium of nutrient solution on their composition in leaves of tomato seedlings. Jpn. J. Soil Sci. Plant Nutr. 1991, 62, 507–511. [Google Scholar]
- Bennett, W.F. Nutrient Deficiencies and Toxicities in Crop Plants; Bennett, W.F., Ed.; APS Press: St. Paul, MN, USA, 1993. [Google Scholar]
- Uchida, R. Essential Nutrients for Plant Growth: Nutrient Functions and Deficiency Symptoms; Silva, J.A., Uchida, R., Eds.; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Hawaii, HI, USA, 2000. [Google Scholar]
- Teuscher, H.; Adler, R. The Soil and Its Fertility; Teuscher, H., Adler, R., Eds.; Reinhold Publishing Corporation: New York, NY, USA, 1960. [Google Scholar]
- Aitken, R.L.; Dickson, T.; Hailes, K.J.; Moody, P.W. Response of field-grown maize to applied magnesium in acidic soils in north-eastern Australia. Aust. J. Agr. Res. 1999, 50, 191–198. [Google Scholar]
- Moss, G.I.; Higgins, M.L. Magnesium influences on the fruit quality of sweet orange (Citrus sinensis L. osbeck). Plant Soil 1974, 41, 103–112. [Google Scholar] [CrossRef]
- Hariadi, Y.; Shabala, S. Screening broad beans (Vicia faba) for magnesium deficiency. I. Growth characteristics, visual deficiency symptoms and plant nutritional status. Funct. Plant Biol. 2004, 31, 529–537. [Google Scholar] [CrossRef]
- Hermans, C.; Johnson, G.N.; Strasser, R.J.; Verbruggen, N. Physiological characterisation of magnesium deficiency in sugar beet: Acclimation to low magnesium differentially affects photosystems I and II. Planta 2004, 220, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Verbruggen, N. Physiological characterization of Mg deficiency in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.I.; Saito, T.; Iwata, N.; Ohmae, Y.; Iwata, R.; Tanoi, K.; Nakanishi, T.M. Leaf senescence in rice due to magnesium deficiency mediated defect in transpiration rate before sugar accumulation and chlorosis. Physiol. Plant 2013, 148, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.C.; Bourgis, F.F.; Faucher, M.M.; Strasser, R.J.R.; Delrot, S.S.; Verbruggen, N.N. Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Luo, W.; Xu, G. Characterisation of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Zhong, W.; Schobert, C.; Komor, E. Transport of magnesium ions in the phloem of Ricinus communis L. seedlings. Planta 1993, 190, 114–119. [Google Scholar] [CrossRef]
- Shaul, O.; Hilgemann, D.W.; de-Almeida-Engler, J.; van Montagu, M.; Inz, D.; Galili, G. Cloning and characterization of a novel Mg2+/H+ exchanger. EMBO J. 1999, 18, 3973–3980. [Google Scholar] [CrossRef] [PubMed]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 309–323. [Google Scholar] [CrossRef] [PubMed]
- David-Assael, O.; Saul, H.; Saul, V.; Mizrachy-Dagri, T.; Berezin, I.; Brook, E.; Shaul, O. Expression of AtMHX, an Arabidopsis vacuolar metal transporter, is repressed by the 5′ untranslated region of its gene. J. Exp. Bot. 2005, 56, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Berezin, I.; Mizrachy-Dagry, T.; Brook, E.; Mizrahi, K.; Elazar, M.; Zhuo, S.; Saul-Tcherkas, V.; Shaul, O. Overexpression of AtMHX in tobacco causes increased sensitivity to Mg2+, Zn2+, and Cd2+ ions, induction of V-ATPase expression, and a reduction in plant size. Plant Cell Rep. 2008, 27, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Thimann, K.V. Senescence in Plants; Thimann, K.V., Ed.; CRC Press, Inc: Florida, FL, USA, 1986. [Google Scholar]
- Hayashi, H.; Chino, M. Collection of pure phloem sap from wheat and its chemical composition. Plant Cell Physiol. 1986, 27, 1387–1393. [Google Scholar]
- Hermans, C.; Vuylsteke, M.; Coppens, F.; Craciun, A.; Inzé, D.; Verbruggen, N. Early transcriptomic changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol. 2010, 187, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Conn, S.J.; Chen, J.; Xiao, Q.; Verbruggen, N. An update on magnesium homeostasis mechanisms in plants. Metallomics 2013, 5, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Nobori, T.; Kobayashi, N.I.; Tanoi, K.; Nakanishi, T.M. Effects of potassium in reducing the radiocesium translocation to grain in rice. Soil Sci. Plant Nutr. 2014, 60, 772–781. [Google Scholar] [CrossRef]
- Gebert, M.; Meschenmoser, K.; Svidovà, S.; Weghuber, J.; Schweyen, R.; Eifler, K.; Lenz, H.; Weyand, K.; Knoop, V. A root-expressed magnesium transporter of the MRS2/MGT gene family in Arabidopsis thaliana allows for growth in low-Mg2+ environments. Plant Cell 2009, 21, 4018–4030. [Google Scholar] [CrossRef] [PubMed]
- Ai, P.; Sun, S.; Zhao, J.; Fan, X.; Xin, W.; Guo, Q.; Yu, L.; Shen, Q.; Wu, P.; Miller, A.J.; Xu, G. Two rice phosphate transporters, OsPht1;2 and OsPht1;6, have different functions and kinetic properties in uptake and translocation. Plant J. 2009, 57, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Drummond, R.S.M.; Tutone, A.; Li, Y.-C.; Gardner, R.C. A putative magnesium transporter AtMRS2–11 is localized to the plant chloroplast envelope membrane system. Plant Sci. 2006, 170, 78–89. [Google Scholar] [CrossRef]
- Saito, T.; Kobayashi, N.I.; Tanoi, K.; Iwata, N.; Suzuki, H.; Iwata, R.; Nakanishi, T.M. Expression and functional analysis of the CorA-MRS2-ALR-type magnesium transporter family in rice. Plant Cell Physiol. 2013, 54, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tutone, A.F.; Drummond, R.S.; Gardner, R.C.; Luan, S. A novel family of magnesium transport genes in Arabidopsis. Plant Cell 2001, 13, 2761–2775. [Google Scholar] [CrossRef] [PubMed]
- Schock, I.; Gregan, J.; Steinhauser, S.; Schweyen, R.; Brennicke, A.; Knoop, V. A member of a novel Arabidopsis thaliana gene family of candidate Mg2+ ion transporters complements a yeast mitochondrial group II intron-splicing mutant. Plant J. 2000, 24, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Vuylsteke, M.; Coppens, F.; Cristescu, S.M.; Harren, F.J.M.; Inzé, D.; Verbruggen, N. Systems analysis of the responses to long-term magnesium deficiency and restoration in Arabidopsis thaliana. New Phytol. 2010, 187, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C. Physiological and molecular responses to magnesium nutritional imbalance in plants. Plant Soil 2013, 368, 87–99. [Google Scholar] [CrossRef]
- Wüthrich, K.L.; Bovet, L.; Hunziker, P.E.; Donnison, I.S.; Hörtensteiner, S. Molecular cloning, functional expression and characterisation of RCC reductase involved in chlorophyll catabolism. Plant J. 2000, 21, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Hörtensteiner, S. Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends Plant Sci. 2009, 14, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Hörtensteiner, S. Update on the biochemistry of chlorophyll breakdown. Plant Mol. Biol. 2013, 82, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Duff, S.M.; Plaxton, W.C.; Lefebvre, D.D. Phosphate-starvation response in plant cells: De novo synthesis and degradation of acid phosphatases. Proc. Natl. Acad. Sci. USA 1991, 88, 9538–9542. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, W.D.; Kirkby, E.A.; Peuke, A.D.; Pate, J.S.; Hartung, W. Effects of P deficiency on assimilation and transport of nitrate and phosphate in intact plants of castor bean (Ricinus communis L.). J. Exp. Bot. 1997, 48, 75–91. [Google Scholar] [CrossRef]
- Himelblau, E.; Amasino, R.M. Nutrients mobilized from leaves of Arabidopsis thaliana during leaf senescence. J. Plant Physiol. 2001, 158, 1317–1323. [Google Scholar] [CrossRef]
- Peng, H.-Y.; Qi, Y.-P.; Lee, J.; Yang, L.-T.; Guo, P.; Jiang, H.-X.; Chen, L.-S. Proteomic analysis of Citrus sinensis roots and leaves in response to long-term magnesium-deficiency. BMC Genom. 2015, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Geilfus, C.-M.; Bayer, A.; Mühling, K.-H. Photosynthetic capacity, nutrient status, and growth of maize (Zea mays L.) upon MgSO4 leaf-application. Front. Plant Sci. 2014, 5, 781. [Google Scholar] [CrossRef] [PubMed]
- Jabnoune, M.; Espeout, S.; Mieulet, D.; Fizames, C.; Verdeil, J.-L.; Conéjéro, G.; Rodríguez-Navarro, A.; Sentenac, H.; Guiderdoni, E.; Abdelly, C.; et al. Diversity in expression patterns and functional properties in the rice HKT transporter family. Plant Physiol. 2009, 150, 1955–1971. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, G.; Biricolti, S.; Locatelli, F.; Baldoni, E.; Mattana, M. Osmyb4 expression improves adaptive responses to drought and cold stress in transgenic apples. Plant Cell Rep. 2008, 27, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Dubouzet, J.G.; Sakuma, Y.; Ito, Y.; Kasuga, M.; Dubouzet, E.G.; Miura, S.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J. 2003, 33, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.S.; Bremer, E. Influence of magnesium deficiency on rates of leaf expansion, starch and sucrose accumulation, and net assimilation in Phaseolus vulgaris. Physiol. Plant 1993, 89, 271–276. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J. Exp. Bot. 1994, 45, 1251–1257. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, L.; Qu, H.; Lian, J.; Liu, W.; Hu, Y.; Xu, G. Alteration of nutrient allocation and transporter genes expression in rice under N, P, K, and Mg deficiencies. Acta Physiol. Plant 2012, 34, 939–946. [Google Scholar] [CrossRef]
- Lavon, R.; Goldschmidt, E.E.; Salomon, R.; Frank, A. Effect of potassium, magnesium, and calcium deficiencies on carbohydrate pools and metabolism in Citrus Leaves. J. Am. Soc. Hortic. Sci. 1995, 120, 54–58. [Google Scholar]
- Marschner, H.; Kirkby, E.A.; Cakmak, I. Effect of mineral nutritional status on shoot-root partitioning of photoassimilates and cycling of mineral nutrients. J. Exp. Bot. 1996, 47, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Ballicora, M.A.; Iglesias, A.A.; Preiss, J. ADP-Glucose Pyrophosphorylase: A regulatory enzyme for plant starch synthesis. Photosynth. Res. 2004, 79, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Zeeman, S.C. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Caspar, T.; Huber, S.C.; Somerville, C. Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol. 1985, 79, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sanz, A.; Brenner, M.L.; Smith, A. Sucrose synthase, starch accumulation, and tomato fruit sink strength. Plant Physiol. 1993, 101, 321–327. [Google Scholar] [PubMed]
- Muñoz, F.J.; Baroja-Fernández, E.; Morán-Zorzano, M.T.; Viale, A.M.; Etxeberria, E.; Alonso-Casajús, N.; Pozueta-Romero, J. Sucrose synthase controls both intracellular ADP glucose levels and transitory starch biosynthesis in source leaves. Plant Cell Physiol. 2005, 46, 1366–1376. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Fernández, E.; Muñoz, F.J.; Montero, M.; Etxeberria, E.; Sesma, M.T.; Ovecka, M.; Bahaji, A.; Ezquer, I.; Li, J.; Prat, S.; et al. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol. 2009, 50, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Barratt, D.H.P.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA 2009, 106, 13124–13129. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farhat, N.; Ivanov, A.G.; Krol, M.; Rabhi, M.; Smaoui, A.; Abdelly, C.; Hüner, N.P.A. Preferential damaging effects of limited magnesium bioavailability on photosystem I in Sulla carnosa plants. Planta 2015, 241, 1189–1206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-M.; Yu, H.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Xia, X.-J. Photoprotective roles of anthocyanins in Begonia semperflorens. Plant Sci. 2010, 179, 202–208. [Google Scholar] [CrossRef]
- Chou, T.-S.; Chao, Y.-Y.; Huang, W.-D.; Hong, C.-Y.; Kao, C.H. Effect of magnesium deficiency on antioxidant status and cadmium toxicity in rice seedlings. J. Plant Physiol. 2011, 168, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-H.; Yang, L.-T.; Jiang, H.-X.; Li, Y.; Wang, P.; Chen, L.-S. Physiological impacts of magnesium-deficiency in Citrus seedlings: Photosynthesis, antioxidant system and carbohydrates. Trees 2012, 26, 1237–1250. [Google Scholar] [CrossRef]
- Sobeih, W.Y.; Dodd, I.C.; Bacon, M.A.; Grierson, D.; Davies, W.J. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. J. Exp. Bot. 2004, 55, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Davies, W.J. Drought, ozone, ABA and ethylene: New insights from cell to plant to community. Plant Cell Environ. 2010, 33, 510–525. [Google Scholar] [CrossRef] [PubMed]
- Timpa, J.D.; Burke, J.J.; Quisenberry, J.E.; Wendt, C.W. Effects of water stress on the organic Acid and carbohydrate compositions of cotton plants. Plant Physiol. 1986, 82, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Semel, Y.; Schauer, N.; Roessner, U.; Zamir, D.; Fernie, A.R. Metabolite analysis for the comparison of irrigated and non-irrigated field grown tomato of varying genotype. Metabolomics 2007, 3, 289–295. [Google Scholar] [CrossRef]
- Levi, A.; Paterson, A.H.; Cakmak, I.; Saranga, Y. Metabolite and mineral analyses of cotton near-isogenic lines introgressed with QTLs for productivity and drought-related traits. Physiol. Plant 2011, 141, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Matsukura, C.; Ban, Y.; Shoji, K.; Sugiyama, M.; Fukuda, N.; Nishimura, S. Salinity stress affects assimilate metabolism at the gene-expression level during fruit development and improves fruit quality in tomato (Solanum lycopersicum L.). J. Jpn. Soc. Hortic. Sci. 2008, 77, 61–68. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, F.; Li, M.; Liang, D.; Zou, J. Physiological responses of kiwifruit plants to exogenous ABA under drought conditions. Plant Growth Regul. 2011, 64, 63–74. [Google Scholar] [CrossRef]
- Munnik, T.; Vermeer, J.E.M. Osmotic stress-induced phosphoinositide and inositol phosphate signalling in plants. Plant Cell Environ. 2010, 33, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci. Hortic. 2006, 108, 7–14. [Google Scholar] [CrossRef]
- Muchhal, U.S.U.; Pardo, J.M.J.; Raghothama, K.G.K. Phosphate transporters from the higher plant Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 1996, 93, 10519–10523. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Vidmar, J.; Glass, A. Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: Responses to nitrate provision. Plant Cell Physiol. 2003, 44, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Gansel, X.; Muños, S.; Tillard, P.; Gojon, A. Differential regulation of the NO3− and NH4+ transporter genes AtNrt2.1 and AtAmt1.1 in Arabidopsis: Relation with long-distance and local controls by N status of the plant. Plant J. 2001, 26, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Gierth, M.; Mäser, P.; Schroeder, J.I. The potassium transporter AtHAK5 functions in K+ deprivation-induced high-affinity K+ uptake and AKT1 K+ channel contribution to K+ uptake kinetics in Arabidopsis roots. Plant Physiol. 2005, 137, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, N.; Takahashi, H.; Smith, F.W.; Yamaya, T.; Saito, K. Two distinct high-affinity sulfate transporters with different inducibilities mediate uptake of sulfate in Arabidopsis roots. Plant J. 2002, 29, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E. Iron stress in plants. Genome Biol. 2002, 3, reviews1024.1–reviews1024.4. [Google Scholar] [CrossRef] [PubMed]
- Wintz, H.; Fox, T.; Wu, Y.-Y.; Feng, V.; Chen, W.; Chang, H.-S.; Zhu, T.; Vulpe, C. Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J. Biol. Chem. 2003, 278, 47644–47653. [Google Scholar] [CrossRef] [PubMed]
- Socha, A.L.; Guerinot, M.L. Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Grotz, N.; Fox, T.; Connolly, E.; Park, W.; Guerinot, M.L.; Eide, D. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proc. Natl. Acad. Sci. USA 1998, 95, 7220–7224. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Wada, M.; Ludewig, U.; Schaaf, G.; von Wirén, N.; Fujiwara, T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 2006, 18, 1498–1509. [Google Scholar] [CrossRef] [PubMed]
- Snavely, M.D.; Gravina, S.A.; Cheung, T.T.; Miller, C.G.; Maguire, M.E. Magnesium transport in Salmonella typhimurium. Regulation of mgtA and mgtB expression. J. Biol. Chem. 1991, 266, 824–829. [Google Scholar] [PubMed]
- Chamnongpol, S.; Groisman, E.A. Mg2+ homeostasis and avoidance of metal toxicity. Mol. Microbiol. 2002, 44, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Lejona, S.; Aguirre, A.; Cabeza, M.L.; García Véscovi, E.; Soncini, F.C. Molecular characterization of the Mg2+-responsive PhoP-PhoQ regulon in Salmonella enterica. J. Bacteriol. 2003, 185, 6287–6294. [Google Scholar] [CrossRef] [PubMed]
- Soncini, F.C.; García Véscovi, E.; Solomon, F.; Groisman, E.A. Molecular basis of the magnesium deprivation response in Salmonella typhimurium: Identification of PhoP-regulated genes. J. Bacteriol. 1996, 178, 5092–5099. [Google Scholar] [PubMed]
- Mao, D.; Chen, J.; Tian, L.; Liu, Z.; Yang, L.; Tang, R.; Li, J.; Lu, C.; Yang, Y.; Shi, J.; et al. Arabidopsis transporter MGT6 mediates magnesium uptake and is required for growth under magnesium limitation. Plant Cell 2014, 26, 2234–2248. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Tanaka, Y.; Fukai, S.; Ishitani, R.; Nureki, O. Crystal structure of the MgtE Mg2+ transporter. Nature 2007, 448, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Dalmas, O.; Sandtner, W.; Medovoy, D.; Frezza, L.; Bezanilla, F.; Perozo, E. A repulsion mechanism explains magnesium permeation and selectivity in CorA. Proc. Natl. Acad. Sci. USA 2014, 111, 3002–3007. [Google Scholar] [CrossRef] [PubMed]
- Dalmas, O.; Sompornpisut, P.; Bezanilla, F.; Perozo, E. Molecular mechanism of Mg2+-dependent gating in CorA. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Philip, D.; Neuhaeuser, B.; Schulze, W.X.; Ludewig, U. Protein dynamics in young maize root hairs in response to macro- and micro-nutrient deprivation. J. Proteome Res. 2015, 14, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Yamagami, M.; Hirai, M.Y.; Fujiwara, T. Establishment of an in planta magnesium monitoring system using CAX3 promoter-luciferase in Arabidopsis. J. Exp. Bot. 2012, 63, 355–363. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanoi, K.; Kobayashi, N.I. Leaf Senescence by Magnesium Deficiency. Plants 2015, 4, 756-772. https://doi.org/10.3390/plants4040756
Tanoi K, Kobayashi NI. Leaf Senescence by Magnesium Deficiency. Plants. 2015; 4(4):756-772. https://doi.org/10.3390/plants4040756
Chicago/Turabian StyleTanoi, Keitaro, and Natsuko I. Kobayashi. 2015. "Leaf Senescence by Magnesium Deficiency" Plants 4, no. 4: 756-772. https://doi.org/10.3390/plants4040756
APA StyleTanoi, K., & Kobayashi, N. I. (2015). Leaf Senescence by Magnesium Deficiency. Plants, 4(4), 756-772. https://doi.org/10.3390/plants4040756