Interactions between Mycorrhizal Fungi, Tea Wastes, and Algal Biomass Affecting the Microbial Community, Soil Structure, and Alleviating of Salinity Stress in Corn Yield (Zea mays L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Inoculation of Mycorrhiza Fungi
2.3. Collection of Algae
2.4. Tea Waste
2.5. Chemical Analysis
2.5.1. Soil Respiration
2.5.2. Organic Carbon
2.5.3. Bacterial and Fungal Population
2.5.4. Mean Weight Diameter
2.5.5. Estimation of Mycorrhizal Colonization Rate
2.5.6. Spores Numbers
2.6. Statistical Analysis
3. Result and Discussion
3.1. Effect of Treatments and Incubation Periods on Soil Respiration
3.2. Effect of Treatments on Mean Weight Diameter (MWD)
3.3. Effect of Treatments on Bacterial and Fungal Community
3.4. Effect of Treatments on Soil Organic Carbon
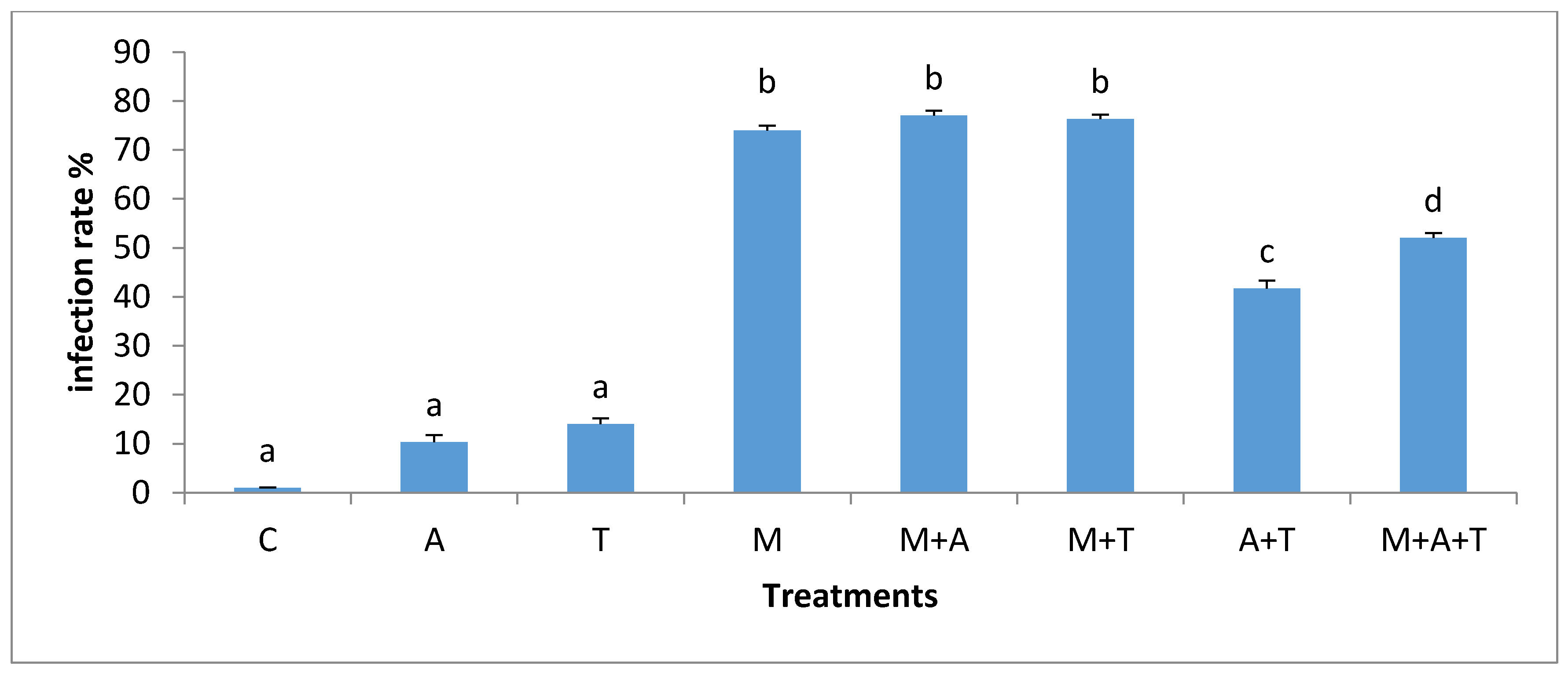
3.5. Effect of Treatments on the Infection Rate and Spores Density
3.6. Effect of Treatments on Corn Plant Yield
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pitman, M.G.; Läuchli, A. Global impact of salinity and agricultural ecosystems. In Salinity: Environment-Plants-Molecules; Springer: Berlin, Germany, 2002; pp. 3–20. Available online: https://link.springer.com/chapter/10.1007/0-306-48155-3_1 (accessed on 2 August 2018).
- Al-Karaki, G.N. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci. Hortic. 2006, 109, 1–7. [Google Scholar] [CrossRef]
- Cornillon, P.; Palloix, A. Influence of sodium chloride on the growth and mineral nutrition of pepper cultivars. J. Plant Nutr. 1997, 20, 1085–1094. [Google Scholar] [CrossRef]
- Miransari, M.; Smith, D. Overcoming the stressful effects of salinity and acidity on soybean nodulation and yields using signal molecule genistein under field conditions. J. Plant Nutr. 2007, 30, 1967–1992. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Rengasamy, P.; McDonald, G.K. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010, 61, 4449–4459. [Google Scholar] [CrossRef] [PubMed]
- Daei, G.; Ardekani, M.; Rejali, F.; Teimuri, S.; Miransari, M. Alleviation of salinity stress on wheat yield, yield components, and nutrient uptake using arbuscular mycorrhizal fungi under field conditions. J. Plant Physiol. 2009, 166, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Miransari, M.; Bahrami, H.; Rejali, F.; Malakouti, M. Using arbuscular mycorrhiza to alleviate the stress of soil compaction on wheat (Triticum aestivum L.) growth. Soil Biol. Biochem. 2008, 40, 1197–1206. [Google Scholar] [CrossRef]
- Asghari, H.; Marschner, P.; Smith, S.; Smith, F. Growth response of Atriplex nummularia to inoculation with arbuscular mycorrhizal fungi at different salinity levels. Plant Soil 2005, 273, 245–256. [Google Scholar] [CrossRef]
- Namdari, A.; Arani, A.B.; Moradi, A. Arbuscular mycorrhizal (Funneliformis mosseae) improves alfalfa (Medicago sativa L.) re-growth ability in saline soil through enhanced nitrogen remobilization and improved nutritional balance. J. Cent. Eur. Agric. 2018, 18, 30–40. [Google Scholar] [CrossRef]
- Giri, B.; Mukerji, K. Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: Evidence for reduced sodium and improved magnesium uptake. Mycorrhiza 2004, 14, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Wang, L.; Yin, N. Effects of AMF on soil enzyme activity and carbon sequestration capacity in reclaimed mine soil. Int. J. Min. Sci. Technol. 2012, 22, 553–557. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.; Azcón, R. Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol. Plant. 1995, 95, 472–478. [Google Scholar] [CrossRef]
- Leifheit, E.; Verbruggen, E.; Rillig, M. Arbuscular mycorrhizal fungi reduce decomposition of woody plant litter while increasing soil aggregation. Soil Biol. Biochem. 2015, 81, 323–328. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Talbot, J.; Allison, S.; Treseder, K. Decomposers in disguise: Mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct. Ecol. 2008, 22, 955–963. [Google Scholar] [CrossRef]
- Zhang, B.; Li, S.; Chen, S.; Ren, T.; Yang, Z.; Zhao, H.; Liang, Y.; Han, X. Arbuscular mycorrhizal fungi regulate soil respiration and its response to precipitation change in a semiarid steppe. Sci. Rep. 2016, 6, 19990. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Booker, F.L.; Tu, C.; Burkey, K.O.; Zhou, L.; Shew, H.D.; Rufty, T.W.; Hu, S. Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 2012, 337, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Campbell, C.D.; Fitter, A.H. An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 2001, 413, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, K.G.; Chamola, B.; Singh, J. Mycorrhizal Biology; Springer Science & Business Media: Berlin, Germany, 2012; Available online: https://books.google.iq/books?isbn=1461542650 (accessed on 2 August 2018).
- Leigh, J.; Fitter, A.H.; Hodge, A. Growth and symbiotic effectiveness of an arbuscular mycorrhizal fungus in organic matter in competition with soil bacteria. FEMS Microbiol. Ecol. 2011, 76, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Herman, D.J.; Firestone, M.K.; Nuccio, E.; Hodge, A. Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol. Ecol. 2012, 80, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Toljander, J.F.; Santos-González, J.C.; Tehler, A.; Finlay, R.D. Community analysis of arbuscular mycorrhizal fungi and bacteria in the maize mycorrhizosphere in a long-term fertilization trial. FEMS Microbiol. Ecol. 2008, 65, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Filion, M.; St-Arnaud, M.; Fortin, J. Direct interaction between the arbuscular mycorrhizal fungus Glomus intraradices and different rhizosphere microorganisms. New Phytol. 1999, 141, 525–533. [Google Scholar] [CrossRef]
- Welc, M.; Ravnskov, S.; Kieliszewska-Rokicka, B.; Larsen, J. Suppression of other soil microorganisms by mycelium of arbuscular mycorrhizal fungi in root-free soil. Soil Biol. Biochem. 2010, 42, 1534–1540. [Google Scholar] [CrossRef]
- Ravnskov, S.; Larsen, J.; Olsson, P.A.; Jakobsen, I. Effects of various organic compounds on growth and phosphorus uptake of an arbuscular mycorrhizal fungus. New Phytol. 1999, 141, 517–524. [Google Scholar] [CrossRef]
- Hodge, A. Microbial ecology of the arbuscular mycorrhiza. FEMS Microbiol. Ecol. 2000, 32, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Turgut, B.; Kose, B. Improvements in aggregate stability of sediments supplemented with tea waste and farmyard manure. Span. J. Soil Sci. 2016, 6, 98–106. [Google Scholar]
- Abdulghani, E.T. Effect of black tea wastes on some of soil properties and Barley (Hordium vugar L.) growth and yield. J. Tikrit Univ. Agric. Sci. 2012, 12, 29–37. [Google Scholar]
- Gougoulias, N.; Papapolymerou, G.; Karayannis, V.; Spiliotis, X.; Chouliaras, N. Effects of Manure Enriched with Algae Chlorella vulgaris on Soil Chemical Properties. Soil Water Res. 2018, 13, 70–80. [Google Scholar] [CrossRef]
- Crouch, I.J.; Van Staden, J. Commercial seaweed products as biostimulants in horticulture. J. Home Consum. Hortic. 1993, 1, 19–76. [Google Scholar] [CrossRef]
- Köppen, W.; Geiger, R. Klimate der Erde; Verlag Justus Perthes: Gotha, Germnay, 1928; Available online: http://koeppen-geiger.vu-wien.ac.at/pdf/Koppen_1936.pdf (accessed on 2 August 2018).
- Black, C.A. Methods of Soil Analysis Part 1 and 2; American Society of Agronomy, Inc.: Madison, WI, USA, 1965. Available online: www.env.go.jp/air/acidrain/man/soil_veget/refs.pdf (accessed on 2 August 2018).
- Gerdemann, J.; Nicolson, T.H. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963, 46, 235–244. [Google Scholar] [CrossRef]
- Prescott, G.W. Ecology of Panama canal algae. Trans. Am. Microsc. Soc. 1951, 70, 1–24. [Google Scholar] [CrossRef]
- Harborne, A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin, Germany, 1998; Available online: www.springer.com/gp/book/9780412572609 (accessed on 2 August 2018).
- Anderson, J.P. Soil Respiration. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1982; pp. 831–871. [Google Scholar]
- Ball, D. Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. Eur. J. Soil Sci. 1964, 15, 84–92. [Google Scholar] [CrossRef]
- Olsen, R.A.; Bakken, L.R. Viability of soil bacteria: Optimization of plate-counting technique and comparison between total counts and plate counts within different size groups. Microb. Ecol. 1987, 13, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Kemper, W.; Rosenau, R. Aggregate Stability and Size Distribution1. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; American Society of Agronomy: Madison, WI, USA, 1986; pp. 425–442. Available online: https://eprints.nwisrl.ars.usda.gov/732/3/585.pdf (accessed on 2 August 2018).
- Kormanik, P.P.; Bryan, W.C.; Schultz, R.C. Procedures and equipment for staining large numbers of plant root samples for endomycorrhizal assay. Can. J. Microbiol. 1980, 26, 536–538. [Google Scholar] [CrossRef] [PubMed]
- Abiven, S.; Menasseri, S.; Angers, D.; Leterme, P. Dynamics of aggregate stability and biological binding agents during decomposition of organic materials. Eur. J. Soil Sci. 2007, 58, 239–247. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Shaaban, M.M. Nutritional status and growth of maize plants as affected by green microalgae as soil additives. J. Biol. Sci. 2001, 6, 475–479. [Google Scholar]
- Hilal, Y.; Engelhardt, U. Characterisation of white tea—Comparison to green and black tea. J. Verbrauch. Lebensm. 2007, 2, 414–421. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Scullion, J. Interactions between earthworms and residues of differing quality affecting aggregate stability and microbial dynamics. Appl. Soil Ecol. 2013, 64, 56–62. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Kuroda, Y.; Ueno, H.; Kajiura, M.; Ae, N. Effect of phenolic acids on the formation and stabilization of soil aggregates. Soil Sci. Plant Nutr. 2018, 3, 1–12. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. Eur. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Johnson, D.; Leake, J.; Ostle, N.; Ineson, P.; Read, D. In situ 13CO2 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelia to the soil. New Phytol. 2002, 153, 327–334. [Google Scholar] [CrossRef]
- Ozdemir, N.; Yakupoglu, T.; Dengiz, O. The effects of bio-solid and tea waste application into different levels of eroded soil on N, P and K concentrations. Environ. Monit. Assess. 2009, 156, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Debode, J.; De Tender, C.; Soltaninejad, S.; Van Malderghem, C.; Haegeman, A.; Van der Linden, I.; Cottyn, B.; Heyndrickx, M.; Maes, M. Chitin mixed in potting soil alters lettuce growth, the survival of zoonotic bacteria on the leaves and associated rhizosphere microbiology. Front. Microbiol. 2016, 7, 565. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, P.D.; Rillig, M.C. Differential decomposition of arbuscular mycorrhizal fungal hyphae and glomalin. Soil Biol. Biochem. 2003, 35, 191–194. [Google Scholar] [CrossRef]
- Shrestha Vaidya, G.; Shrestha, K.; Khadge, B.R.; Johnson, N.C.; Wallander, H. Organic matter stimulates bacteria and arbuscular mycorrhizal fungi in Bauhinia purpurea and Leucaena diversifolia plantations on eroded slopes in Nepal. Restor. Ecol. 2008, 16, 79–87. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Porcel, R.; Calvo-Polanco, M.; Aroca, R. Improvement of Salt Tolerance in Rice Plants by Arbuscular Mycorrhizal Symbiosis. In Root Biology; Springer: Berlin, Germany, 2018; pp. 259–279. [Google Scholar]
- Al-Maliki, S.M.; Jones, D.L.; Godbold, D.L.; Gwynn-Jones, D.; Scullion, J. Elevated CO2 and Tree Species Affect Microbial Activity and Associated Aggregate Stability in Soil Amended with Litter. Forests 2017, 8, 70. [Google Scholar] [CrossRef]
- Safinaz, A.; Ragaa, A. Effect of some red marine algae as biofertilizers on growth of maize (Zea mays L.) plants. Int. Food Res. J. 2013, 20, 16–29. [Google Scholar]
- Kim, M.J.; Shim, C.K.; Kim, Y.K.; Hong, S.J.; Park, J.H.; Han, E.J.; Kim, J.H.; Kim, S.C. Effect of aerated compost tea on the growth promotion of lettuce, soybean, and sweet corn in organic cultivation. Plant Pathol. J. 2015, 31, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaliel, A. Effect of salinity stress on mycorrhizal association and growth response of peanut infected by Glomus mosseae. Plant Soil Environ. 2010, 56, 318–324. [Google Scholar] [CrossRef]
- Yanardağ, I.; Zornoza, R.; Bastida, F.; Büyükkiliç-Yanardağ, A.; García, C.; Faz, A.; Mermut, A. Native soil organic matter conditions the response of microbial communities to organic inputs with different stability. Geoderma 2017, 295, 1–9. [Google Scholar] [CrossRef]
- AL-Maliki, S.; AL-Mammory, H.; Scullion, J. Interactions between humic substances and organic amendments affecting soil biological properties and growth of Zea mays L. in the arid land region. Arid Land Res. Manag. 2018. [Google Scholar] [CrossRef]
- AL-Maliki, S. Response of microbial community in the arid land to wheat residues and soil compaction. J. Kerbala Univ. 2016, 14, 114–123. [Google Scholar]
- AL-Maliki, S. Abundance of bacterial and fungal community after amendments of organic residues in some soils from Babylon province. Karbala J. Agric. 2015, 2, 63–76. [Google Scholar]
- AL-Maliki, S. The Effect of Earthworms, Litter Quality and Atmospheric CO2 on Soil Aggregation and Associated Microbial Indices. Ph.D. Thesis, Aberystwyth University, Wales, UK, 2012. Available online: https://www.researchgate.net/.../317718215_THE_EFFECT_OF_EARTHWORMS_LITT (accessed on 2 August 2018).
- De Deyn, G.B.; Cornelissen, J.H.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Ghorchiani, M.; Etesami, H.; Alikhani, H.A. Improvement of growth and yield of maize under water stress by co-inoculating an arbuscular mycorrhizal fungus and a plant growth promoting rhizobacterium together with phosphate fertilizers. Agric. Ecosyst. Environ. 2018, 258, 59–70. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Azcón, R. Symbiotic efficiency and infectivity of an autochthonous arbuscular mycorrhizal Glomus sp. from saline soils and Glomus deserticola under salinity. Mycorrhiza 2000, 10, 137–143. [Google Scholar] [CrossRef]













| Properties | Units | Saline Soil | Non-Saline Soil |
|---|---|---|---|
| EC | (dS/m) | 7.9 | 3.6 |
| pH | 7.76 | 7.83 | |
| P | ppm | 2.26 | 1.81 |
| N | ppm | 0.071 | 0.057 |
| K | ppm | 35.2 | 94.8 |
| CaCo3 | % | 181.24 | 202.87 |
| CaSO4 | g/kg−1 | 8.24 | 2.21 |
| Texture | Sandy Clay | Clay Loam | |
| clay | g/kg−1 | 46 | 34 |
| silt | g/kg−1 | 7 | 37 |
| sand | g/kg−1 | 47 | 29 |
| Ca | Mg/L | 19 | 29 |
| Mg | Mg/L | 26 | 111 |
| Cl− | Mg/L | 78.96 | 25.38 |
| CO3−2 | Mg/L | ||
| HCO−3 | Mg/L | 4.2 | 5 |
| O.M | g/kg−1 | 1.41 | 1.13 |
| Bulk density | g/cm3 | 1.61 | 1.29 |
| Properties | Tea Waste | Algae |
|---|---|---|
| carbon | 30 | 28 |
| carbohydrates % | 1.3 | 0.85 |
| Zn ppm | 25 | 18 |
| Fe ppm | 33 | 37 |
| Total polyphenols % | 20 | 0.7 |
| Amino acids % | 2.0 | 18 |
| Protein % | 20 | 44 |
| N % | 1.5 | 1.6 |
| p % | 0.021 | 0.331 |
| K % | 1.1 | 1.45 |
| C/N | 19.3 | 20.8 |
| Treatments | Incubation Periods | |||||
|---|---|---|---|---|---|---|
| 2 Days | 4 Days | 8 Days | 12 Days | 30 Days | Mean | |
| C | 14.00 ± 0.57 b | 13.00 ± 0.57 b | 12.00 ± 0.57 b | 9.00 ± 0.57 b | 07.00 ± 0.57 b | 11.0 ± 0.73 b |
| A | 21.00 ± 0.57 a | 19.05 ± 0.53 a | 17.90 ± 0.58 a | 16.00 ± 0.57 a | 14.00 ± 0.57 a | 17.5 ± 0.68 a |
| T | 23.00 ± 0.57 d | 19.33 ± 0.88 d | 18.42 ± 0.81 ac | 16.17 ± 0.43 a | 14.66 ± 0.66 a | 18.3 ± 0.80 c |
| M | 18.46 ± 0.06 c | 18.00 ± 0.03 ca | 15.43 ± 0.40 a | 14.55 ± 0.51 a | 12.17 ± 0.61 a | 15.7 ± 0.63 d |
| M + A | 18.25 ± 0.06 c | 17.96 ± 0.18 c | 16.57 ± 0.47 a | 15.47 ± 0.03 a | 11.03 ± 0.24 c | 15.8 ± 0.70 d |
| M + T | 17.16 ± 0.06 c | 17.52 ± 0.03 c | 13.62 ± 0.47 ab | 13.62 ± 0.95 a | 11.51 ± 0.03 c | 15.3 ± 0.65 d |
| A + T | 19.38 ± 0.30 ca | 18.70 ± 0.51 ca | 19.66 ± 0.88 c | 15.83 ± 0.44 a | 13.50 ± 0.28 a | 17.4 ± 0.66 a |
| M + A + T | 19.03 ± 0.06 ca | 17.74 ± 0.16 c | 15.91 ± 0.37 a | 14.66 ± 0.54 a | 11.07 ± 0.67 c | 15.6 ± 0.75 d |
| mean | 18.78 ± 0.52 e | 17.66 ± 0.41 e | 16.57 ± 0.48 f | 14.41 ± 0.48 g | 11.87 ± 0.49 h | |
| Treatments | Incubation Periods | |||||
|---|---|---|---|---|---|---|
| 2 Days | 4 Days | 8 Days | 12 Days | 30 Days | Means | |
| C | 13.33 ± 0.88 a | 13.00 ± 0.57 a | 13.66 ± 0.33 a | 12.00 ± 0.57 a | 06.00 ± 0.57 a | 11.60 ± 0.79 a |
| A | 21.66 ± 0.88 bd | 21.66 ± 0.88 d | 17.50 ± 0.28 d | 15.83 ± 0.16 b | 13.29 ± 0.59 b | 17.99 ± 0.91 b |
| T | 23.00 ± 0.57 d | 24.00 ± 0.57 d | 18.01 ± 0.32 d | 17.15 ± 0.51 d | 14.40 ± 0.35 d | 19.31 ± 0.98 c |
| M | 18.25 ± 0.57 c | 18.00 ± 0.35 c | 16.85 ± 0.90 c | 14.65 ± 0.16 c | 11.77 ± 0.16 cb | 15.90 ± 0.67 ed |
| M + A | 17.71 ± 0.73 c | 17.32 ± 0.47 c | 15.19 ± 0.69 ca | 14.92 ± 0.48 c | 11.77 ± 1.25 c | 14.96 ± 0.82 e |
| M + T | 19.35 ± 0.06 c | 17.25 ± 0.28 c | 17.38 ± 0.35 c | 14.74 ± 0.06 c | 09.86 ± 1.20 c | 15.71 ± 0.90 ed |
| A + T | 19.50 ± 0.28 cb | 18.93 ± 0.52 c | 18.00 ± 0.28 c | 15.06 ± 0.52 c | 12.29 ± 0.35 cb | 16.75 ± 0.73 d |
| M + A + T | 18.92 ± 0.06 c | 17.65 ± 0.17 c | 16.75 ± 0.19 c | 14.19 ± 0.80 c | 10.60 ± 0.80 cb | 15.62 ± 0.79 e |
| mean | 18.96 ± 0.58 a | 18.48 ± 0.74 a | 16.67 ± 0.33 b | 14.81 ± 0.32 c | 10.98 ± 0.52 d | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Maliki, S.; AL-Masoudi, M. Interactions between Mycorrhizal Fungi, Tea Wastes, and Algal Biomass Affecting the Microbial Community, Soil Structure, and Alleviating of Salinity Stress in Corn Yield (Zea mays L.). Plants 2018, 7, 63. https://doi.org/10.3390/plants7030063
Al-Maliki S, AL-Masoudi M. Interactions between Mycorrhizal Fungi, Tea Wastes, and Algal Biomass Affecting the Microbial Community, Soil Structure, and Alleviating of Salinity Stress in Corn Yield (Zea mays L.). Plants. 2018; 7(3):63. https://doi.org/10.3390/plants7030063
Chicago/Turabian StyleAl-Maliki, Salwan, and Mugtaba AL-Masoudi. 2018. "Interactions between Mycorrhizal Fungi, Tea Wastes, and Algal Biomass Affecting the Microbial Community, Soil Structure, and Alleviating of Salinity Stress in Corn Yield (Zea mays L.)" Plants 7, no. 3: 63. https://doi.org/10.3390/plants7030063
APA StyleAl-Maliki, S., & AL-Masoudi, M. (2018). Interactions between Mycorrhizal Fungi, Tea Wastes, and Algal Biomass Affecting the Microbial Community, Soil Structure, and Alleviating of Salinity Stress in Corn Yield (Zea mays L.). Plants, 7(3), 63. https://doi.org/10.3390/plants7030063





