Effect of Selenium on the Responses Induced by Heat Stress in Plant Cell Cultures
Abstract
1. Introduction
2. Results
2.1. HS and Se Effects on Cell Viability and Cytoplasmic Shrinkage
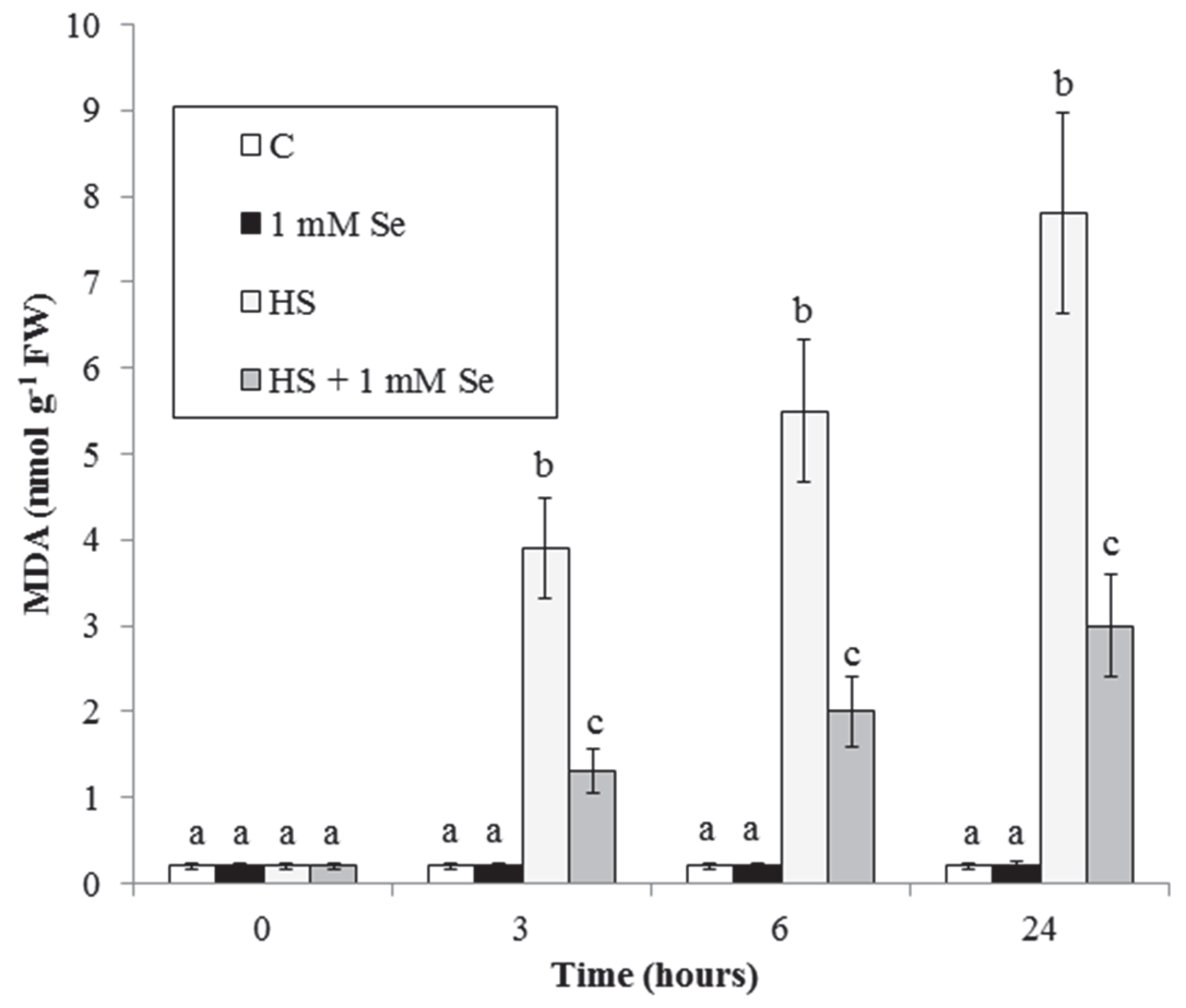
2.2. HS and Se Effects on Accumulation of O2.− and MDA and on Caspase-3-like Activity
2.3. HS and Se Effects on Stress-related Proteins
3. Discussion
3.1. HS and Se Effects on Cell Viability and Cytoplasmic Shrinkage.
3.2. HS and Se Effects on O2−. and Malondialdehyde Accumulations.
3.3. HS and Se Effects on Caspase-3-like Activity and on Cytochrome c Release
3.4. HS and Se Effects on the Levels of Hsp90, BiP and 14-3-3s.
4. Material and Methods
4.1. Cell Culture Growth and Experimental Conditions
4.2. Cell Death and Cytoplasmic Shrinkage Assays
4.3. O2.− Assay
4.4. Proteases Activity and Membrane Lipid Peroxidation
4.5. SDS-PAGE and Protein Gel Blots
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nejat, N.; Mantri, N. Plant immune system: Crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Curr. Issues Mol. Biol. 2017, 23, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Gielen, B.; Naudts, K.; D’Haese, D.; Lemmens, C.M.H.M.; De Boeck, H.J.; Biebaut, E.; Serneels, L.; Valcke, R.; Nijs, I.; Ceulemans, R. Effects of climate warming and species richness on photochemistry of grasslands. Physiol. Plant 2007, 131, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Vacca, R.A.; de Pinto, M.C.; Valenti, D.; Passarella, S.; Marra, E.; De Gara, L. Production of reactive oxygen species, alteration of cytosolic ascorbate peroxidase, and impairment of mitochondrial metabolism are early events in heat shock-induced programmed cell death in tobacco Bright-Yellow 2 cells. Plant Physiol. 2004, 134, 1100–1112. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Katano, K. Coordination between ROS regulatory systems and other pathways under heat stress and pathogen attack. Front. Plant Sci. 2018, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Crosti, P.; Cerana, R. Effect of heat stress on actin cytoskeleton and endoplasmic reticulum of tobacco BY-2 cultured cells and its inhibition by Co2+. Protoplasma 2010, 239, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 70, 3292–3293. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 2003, 57, 134–144. [Google Scholar] [CrossRef]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 6, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An overview of selenium uptake, metabolism, and toxicity in plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef] [PubMed]
- Galeas, M.L.; Zhang, L.H.; Freeman, J.L.; Wegner, M.; Pilon-Smits, E.A.H. Seasonal fluctuations of selenium and sulfur accumulation in selenium hyperaccumulators and related non-accumulators. New Phytol. 2007, 173, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium enrichment of horticultural crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Pilon-Smits, E.A.H. Selenium biofortification and phytoremediation phytotechnologies: A review. J. Environ. Qual. 2017, 46, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Feng, R.W.; Wei, C.Y. Antioxidative mechanisms on selenium accumulation in Pteris vittata L., a potential phytoremediation plant. Plant Soil Environ. 2012, 58, 105–110. [Google Scholar] [CrossRef]
- Cartes, P.; Jara, A.A.; Pinilla, L.; Rosas, A.; Mora, M.L. Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann. Appl. Biol. 2010, 156, 297–307. [Google Scholar] [CrossRef]
- Xue, T.L.; Hou, S.F.; Tan, J.A.; Liu, G.L. The antioxidative function of selenium in higher plants: II. Non-enzymatic mechanisms. Chin. Sci. Bull. 1993, 38, 356–358. [Google Scholar]
- Wang, J.D.; Wang, X.; Wong, J.S. Proteomics analysis reveals multiple regulatory mechanisms in response to selenium in rice. J. Proteom. 2012, 75, 1849–1866. [Google Scholar] [CrossRef] [PubMed]
- Hussey, P.J.; Ketelaar, T.; Deeks, M.J. Control of actin cytoskeleton in plant cell growth. Annu. Rev. Plant Biol. 2006, 57, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Role of peroxynitrite in the responses induced by heat stress in tobacco BY-2 cultured cells. Protoplasma 2018, 255, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Balal, R.M.; Shahid, M.A.; Javaid, M.M.; Iqbal, Z.; Anjum, M.A.; Garcia-Sanchez, F.; Mattson, N.S. The role of selenium in amelioration of heat-induced oxidative damage in cucumber under high temperature stress. Acta Physiol. Plant 2016, 38, 158–172. [Google Scholar] [CrossRef]
- Bocchini, M.; D’Amato, R.; Ciancaleoni, S.; Fontanella, M.C.; Palmerini, C.A.; Beone, G.M.; Onofri, A.; Negri, V.; Marconi, G.; Albertini, E.; et al. Soil selenium (Se) biofortification changes the physiological, biochemical and epigenetic responses to water stress in Zea mays L. by inducing a higher drought tolerance. Front. Plant Sci. 2018, 9, 389. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Mahabub Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Dai, H.; Wang, X.; Wang, G. Physiological and proteomic analysis of selenium-mediated tolerance to Cd stress in cucumber (Cucumis sativus L.). Ecotox. Environ. Safe. 2016, 133, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.L.; Tamaoki, M.; Stushnoff, C.; Quinn, C.F.; Cappa, J.J.; Devonshire, J.; Fakra, S.C.; Marcus, M.A.; McGrath, S.P.; Hoewyk, D.V.; et al. Molecular mechanisms of selenium tolerance and hyperaccumulation in Stanleya pinnata. Plant Physiol. 2010, 153, 1630–1652. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malerba, M.; Cerana, R. Effect of Selenium on the Responses Induced by Heat Stress in Plant Cell Cultures. Plants 2018, 7, 64. https://doi.org/10.3390/plants7030064
Malerba M, Cerana R. Effect of Selenium on the Responses Induced by Heat Stress in Plant Cell Cultures. Plants. 2018; 7(3):64. https://doi.org/10.3390/plants7030064
Chicago/Turabian StyleMalerba, Massimo, and Raffaella Cerana. 2018. "Effect of Selenium on the Responses Induced by Heat Stress in Plant Cell Cultures" Plants 7, no. 3: 64. https://doi.org/10.3390/plants7030064
APA StyleMalerba, M., & Cerana, R. (2018). Effect of Selenium on the Responses Induced by Heat Stress in Plant Cell Cultures. Plants, 7(3), 64. https://doi.org/10.3390/plants7030064




