Germinative and Post-Germinative Behaviours of Brassica napus Seeds Are Impacted by the Severity of S Limitation Applied to the Parent Plants
Abstract
:1. Introduction
2. Results
2.1. Impacts of Suboptimal Levels of Sulphate Applied from the Beginning of the Plant Growth Cycle on S Status of Seeds and Its Consequence for Germination
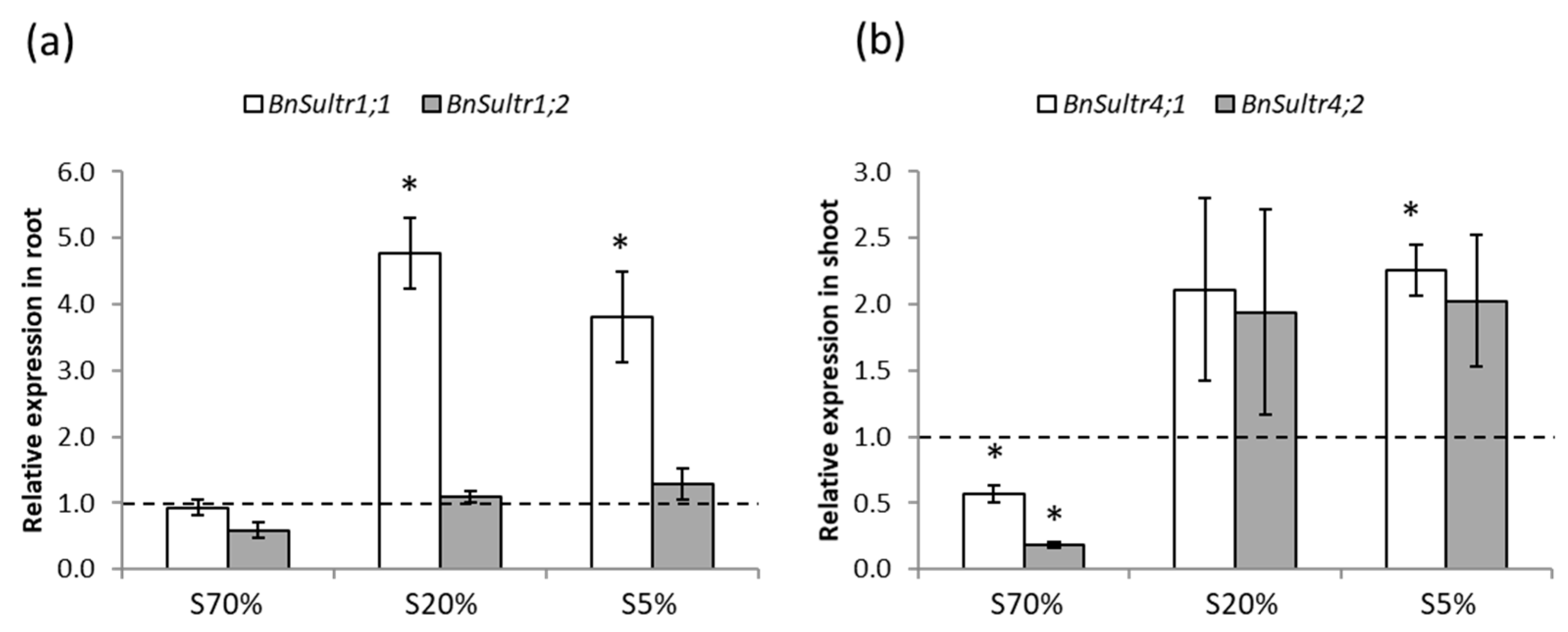
2.2. Development and S Management of Green Seedlings
3. Discussion
3.1. S-Limitation Impacts on Germination Sensu Stricto and on Post-Germinative Events
3.2. Seedling Sulphate Uptake Capacity Is Impacted by S Limitation Applied to the Parent Plant
4. Materials and Methods
4.1. Production of Seeds from Plants Supplied with Different Levels of Sulphate
4.2. Germination Tests and Indexes
4.3. Determination of Normal Seedling Rate
4.4. Protocol for Studying the Development and Sulphate Uptake Capacities of Seedlings Coming from Seeds Produced by Plants Supplied with Suboptimal Levels of Sulphate from the Beginning of the Plant Growth Cycle
4.5. Biochemical and Molecular Analyses
4.5.1. C, N, S and 34S Analysis
4.5.2. Determination of S-Sulphate Amount
4.5.3. Determination of S-Protein Amount
4.5.4. Determination of Glutathione (GSH) Amounts by HPLC
4.6. Relative Expression of BnSultr1 and BnSultr4 Genes Using Q-PCR
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubousset, L.; Etienne, P.; Avice, J.-C. Is the remobilization of S and N reserves for seed filling of winter oilseed rape modulated by sulphate restrictions occurring at different growth stages? J. Exp. Bot. 2010, 61, 4313–4324. [Google Scholar] [CrossRef] [Green Version]
- D’Hooghe, P.; Dubousset, L.; Gallardo, K.; Kopriva, S.; Avice, J.-C.; Trouverie, J. Evidence for proteomic and metabolic adaptations associated with alterations of seed yield and quality in sulfur-limited Brassica napus L. Mol. Cell. Proteomics 2014, 13, 1165–1183. [Google Scholar] [CrossRef] [PubMed]
- Bewley, J.D.; Black, M. Seeds: Physiology of Development and Germination, 2nd ed.; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef]
- Nonogaki, H.; Bassel, G.W.; Bewley, J.D. Germination—Still a mystery. Plant Sci. 2010, 179, 574–581. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant. Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- López-Granados, F.; Lutman, P. Effect of environmental conditions on the dormancy and germination of volunteer oilseed rape seed (Brassica napus). Weed Sci. 1998, 46, 419–423. [Google Scholar]
- Nakabayashi, K.; Okamoto, M.; Koshiba, T.; Kamiya, Y.; Nambara, E. Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: Epigenetic and genetic regulation of transcription in seed. Plant J. 2005, 41, 697–709. [Google Scholar] [CrossRef]
- Alban, C.; Job, D.; Douce, R. Biotin metabolism in plants. Annu. Rev. Plant. Biol. 2000, 51, 17–47. [Google Scholar] [CrossRef]
- Subbiah, V.; Reddy, K.J. Interactions between ethylene, abscisic acid and cytokinin during germination and seedling establishment in Arabidopsis. J. Biosci. 2010, 35, 451–458. [Google Scholar] [CrossRef]
- Davidian, J.-C.; Kopriva, S. Regulation of sulfate uptake and assimilation—The same or not the same? Mol. Plant 2010, 3, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant. Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef]
- Brown, R.F.; Mayer, D.G. Representing cumulative germination. 2. The use of the Weibull function and other empirically derived curves. Ann. Bot. 1988, 61, 127–138. [Google Scholar] [CrossRef]
- Bradbeer, J.W. Seed Dormancy and Germination; Blackie and Son Ltd.: Glasgow, Scotland; London, UK, 1988. [Google Scholar]
- Schopfer, P.; Plachy, C. Control of Seed Germination by Abscisic Acid: II. Effect on Embryo Water Uptake in Brassica napus L. Plant Physiol. 1984, 76, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Schopfer, P.; Plachy, C. Control of Seed Germination by Abscisic Acid: III. Effect on Embryo Growth Potential (Minimum Turgor Pressure) and Growth Coefficient (Cell Wall Extensibility) in Brassica napus L. Plant Physiol. 1985, 77, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.-J.; Wang, Z.; Zhao, Q.; Mao, J.-L.; Speiser, A.; Wirtz, M.; Hell, R.; Zhu, J.-K.; Xiang, C.-B. Sulfate availability affects ABA levels and germination response to ABA and salt stress in Arabidopsis thaliana. Plant J. 2014, 77, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Brunel-Muguet, S.; D’Hooghe, P.; Bataillé, M.-P.; Larré, C.; Kim, T.-H.; Trouverie, J.; Avice, J.-C.; Etienne, P.; Dürr, C. Heat stress during seed filling interferes with sulfur restriction on grain composition and seed germination in oilseed rape (Brassica napus L.). Front. Plant Sci. 2015, 6, 1236. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Linkies, A.; Muller, K.; Morris, K.; Tureckova, V.; Wenk, M.; Cadman, C.S.C.; Corbineau, F.; Strnad, M.; Lynn, J.R.; Finch-Savage, W.E.; et al. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: A comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell 2009, 21, 3803–3822. [Google Scholar] [CrossRef]
- Gallardo, K.; Job, C.; Groot, S.P.C.; Puype, M.; Demol, H.; Vandekerckhove, J.; Job, D. Importance of methionine biosynthesis for Arabidopsis seed germination and seedling growth. Physiol. Plant. 2002, 116, 238–247. [Google Scholar] [CrossRef]
- Hatzig, S.V.; Nuppenau, J.-N.; Snowdon, R.J.; Schießl, S.V. Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape (Brassica napus L.). BMC Plant Biol. 2018, 18, 297. [Google Scholar] [CrossRef] [PubMed]
- Groot, M.P.; Kooke, R.; Knoben, N.; Vergeer, P.; Keurentjes, J.J.B.; Ouborg, N.J.; Verhoeven, K.J.F. Effects of Multi-Generational Stress Exposure and Offspring Environment on the Expression and Persistence of Transgenerational Effects in Arabidopsis thaliana. PLoS ONE 2016, 11, e0151566. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, I.; Abdin, M.Z. Effect of sulfur fertilisation on oil accumulation, acetyl-CoA concentration, and acetyl-CoA carboxylase activity in the developing seeds of rapeseed (Brassica campestris L.). Aust. J. Agric. Res. 2000, 51, 1023. [Google Scholar] [CrossRef]
- Zhao, F.; Evans, E.J.; Bilsborrow, P.E.; Syers, J.K. Influence of nitrogen and sulphur on the glucosinolate profile of rapeseed (Brassica napus L). J. Sci. Food Agric. 1994, 64, 295–304. [Google Scholar] [CrossRef]
- Ceusters, J.; Van de Poel, B. Ethylene Exerts Species-Specific and Age-Dependent Control of Photosynthesis. Plant Physiol. 2018, 176, 2601–2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán, P.; Ecker, J.R. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 1990, 2, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Achard, P.; Vriezen, W.H.; Van Der Straeten, D.; Harberd, N.P. Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 2003, 15, 2816–2825. [Google Scholar] [CrossRef]
- Koralewska, A.; Buchner, P.; Stuiver, C.E.E.; Posthumus, F.S.; Kopriva, S.; Hawkesford, M.J.; De Kok, L.J. Expression and activity of sulfate transporters and APS reductase in curly kale in response to sulfate deprivation and re-supply. J. Plant Physiol. 2009, 166, 168–179. [Google Scholar] [CrossRef]
- Takahashi, H.; Watanabe-Takahashi, A.; Smith, F.W.; Blake-Kalff, M.M.A.; Hawkesford, M.J.; Saito, K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J. 2000, 23, 171–182. [Google Scholar] [CrossRef]
- Brunel-Muguet, S.; Mollier, A.; Kauffmann, F.; Avice, J.-C.; Goudier, D.; Sénécal, E.; Etienne, P. SuMoToRI, an Ecophysiological Model to Predict Growth and Sulfur Allocation and Partitioning in Oilseed Rape (Brassica napus L.) Until the Onset of Pod Formation. Front. Plant Sci. 2015, 6, 993. [Google Scholar] [CrossRef]
- Salon, C.; Bataillé, M.-P.; Gallardo, K.; Jeudy, C.; Santoni, A.-L.; Trouverie, J.; Voisin, A.-S.; Avice, J.-C. 34S and 15N labelling to model S and N flux in plants and determine the different components of N and S use efficiency. Methods Mol. Biol. 2014, 1090, 335–346. [Google Scholar] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Koprivova, A.; North, K.A.; Kopriva, S. Complex signaling network in regulation of adenosine 5′-phosphosulfate reductase by salt stress in Arabidopsis roots. Plant Physiol. 2008, 146, 1408–1420. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]



| Seed Lot | Seed Dry Weight (mg·seed−1) | C Amount (mg·seed−1) | N Amount (µg·seed−1) | S Amount (µg·seed−1) | S-Sulphate Amount (µg·seed−1) | S-Protein Amount (µg·seed−1) | S-Glutathione Amount (ng·seed−1) |
|---|---|---|---|---|---|---|---|
| S400% | 4.02 ± 0.2 | 2.62 ± 0.1 | 220.35 ± 13.61 | 58.95 ± 5.43 | 11.31 ± 1.76 | 7.21 ± 0.55 | 284.1 ± 28.2 |
| S70% | 4.27 ± 0.12 | 2.63 ± 0.07 | 235.24 ± 4.3 | 52.18 ± 3.06 | 9.19 ± 2.6 | 9.42 ± 0.49 * | 231.5 ± 51.8 |
| S20% | 4.1 ± 0.25 | 2.55 ± 0.18 | 207.17 ± 16.89 | 18.2 ± 2.53 * | 2.5 ± 0.41 * | 6.26 ± 0.39 | 333.8 ± 46.6 |
| S5% | 3.99 ± 0.43 | 2.54 ± 0.3 | 209.4 ± 28.53 | 10.56 ± 2.25 * | 0.91 ± 0.28 * | 6.6 ± 0.64 | 161.9 ± 9.6 * |
| SEED Lot | Germination Capacity (%) a | T50 (hours) b | T’50 (hours) b | Speed of Germination (hour−1) c | CRG d | Normal Seedling Establishment Rate (%) e |
|---|---|---|---|---|---|---|
| S400% | 100 ± 0.0 | 28.7 ± 1.0 | 28.7 ± 1.0 | 3.37 ± 0.1 | 2.03 ± 0.03 | 86.7 ± 1.8 ° |
| S70% | 100 ± 0.0 | 26.6 ± 0.9 | 26.6 ± 0.9 | 3.62 ± 0.11 | 2.1 ± 0.03 | 74.7 ± 2.3 *° |
| S20% | 98 ± 1.2 | 24.6 ± 1.1 | 24.3 ± 1.2 | 3.91 ± 0.26 | 2.19 ± 0.05 | 66 ± 8.1 *° |
| S5% | 75.3 ± 4.7 * | 35.8 ± 1.2 * | 31.6 ± 1.1 | 2.27 ± 0.13 * | 1.96 ± 0.01 | 69 ± 2.8 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Hooghe, P.; Picot, D.; Brunel-Muguet, S.; Kopriva, S.; Avice, J.-C.; Trouverie, J. Germinative and Post-Germinative Behaviours of Brassica napus Seeds Are Impacted by the Severity of S Limitation Applied to the Parent Plants. Plants 2019, 8, 12. https://doi.org/10.3390/plants8010012
D’Hooghe P, Picot D, Brunel-Muguet S, Kopriva S, Avice J-C, Trouverie J. Germinative and Post-Germinative Behaviours of Brassica napus Seeds Are Impacted by the Severity of S Limitation Applied to the Parent Plants. Plants. 2019; 8(1):12. https://doi.org/10.3390/plants8010012
Chicago/Turabian StyleD’Hooghe, Philippe, Dimitri Picot, Sophie Brunel-Muguet, Stanislav Kopriva, Jean-Christophe Avice, and Jacques Trouverie. 2019. "Germinative and Post-Germinative Behaviours of Brassica napus Seeds Are Impacted by the Severity of S Limitation Applied to the Parent Plants" Plants 8, no. 1: 12. https://doi.org/10.3390/plants8010012
APA StyleD’Hooghe, P., Picot, D., Brunel-Muguet, S., Kopriva, S., Avice, J.-C., & Trouverie, J. (2019). Germinative and Post-Germinative Behaviours of Brassica napus Seeds Are Impacted by the Severity of S Limitation Applied to the Parent Plants. Plants, 8(1), 12. https://doi.org/10.3390/plants8010012







