UV-B Physiological Changes Under Conditions of Distress and Eustress in Sweet Basil
Abstract
:1. Introduction
2. Results
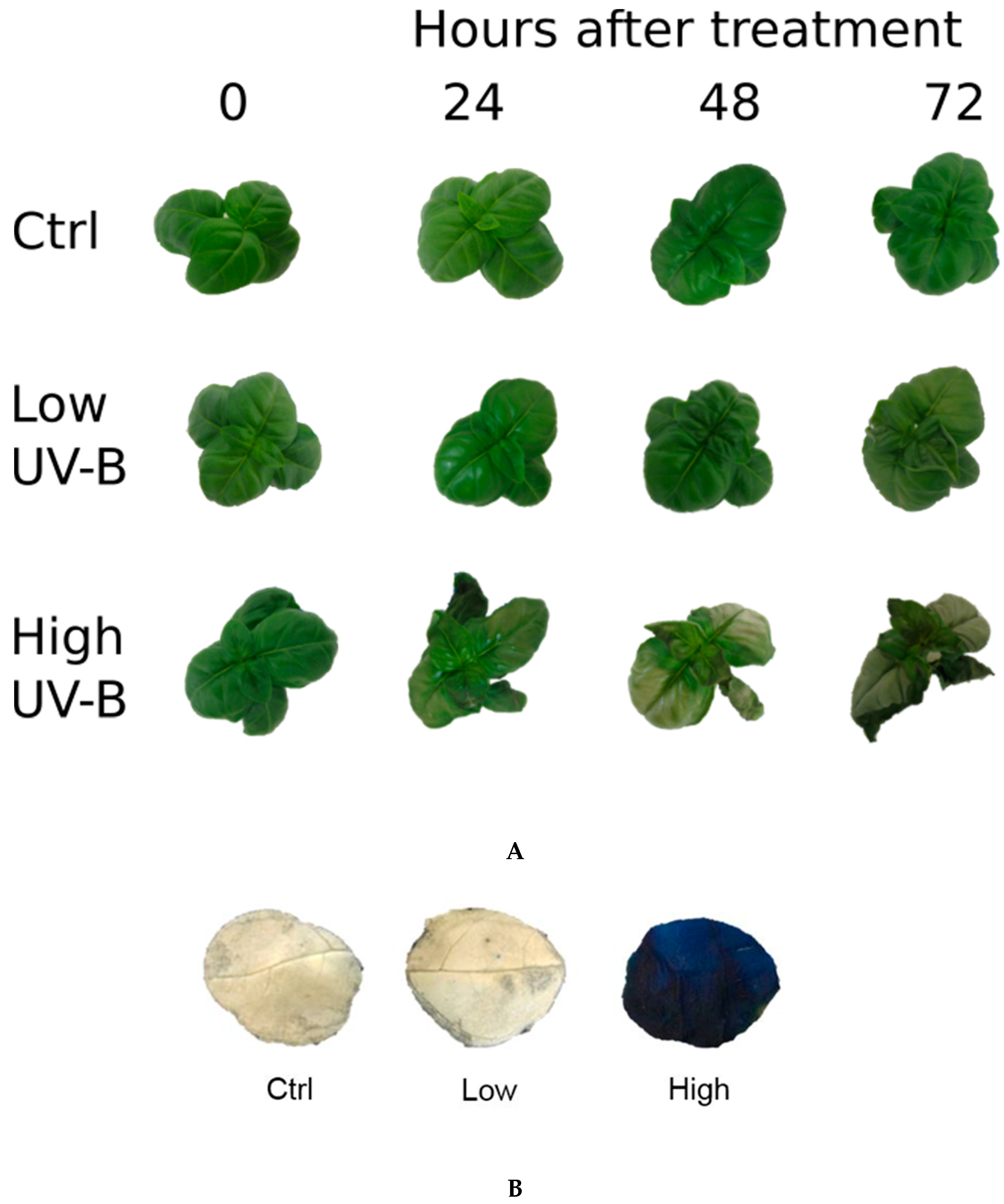
2.1. Visual Effects of UV-B Exposures
2.2. The Impact of UV-B Radiation on PSII Photochemistry
2.3. Overall Effects of UV Radiations on Photosynthetic Pigments
2.4. Overall Effects of UV Radiations on Phenolic Acid Accumulation
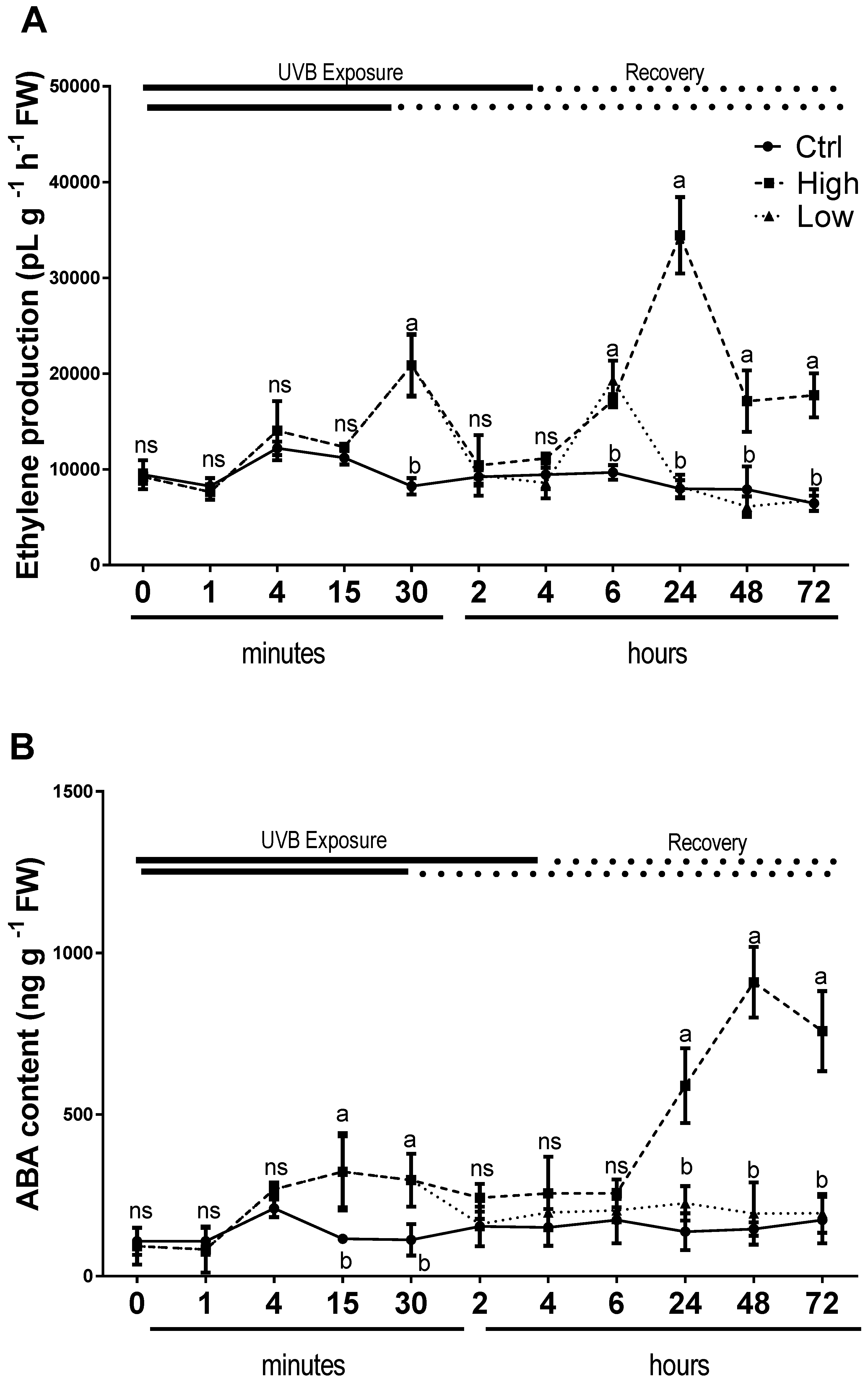
2.5. Endogenous Hormones Were Affected Differently by Low and High UV-B Light Conditions
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Condition
4.2. UV-B Treatments
4.3. Chlorophyll a Fluorescence Transient Analysis and Parameters
4.4. Photosynthetic Pigments Quantification by HPLC
4.5. Determination of Phenolic Acids by HPLC Analysis
4.6. Hormone Analysis
4.7. Evans Blue Staining
4.8. Experimental Design and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moan, J. Visible light and UV radiation. In Radiation at Home, Out- Doors and in the Workplace; Brune, D., Hellborg, R., Persson, B.R.R., Pääkkönen, R., Eds.; Scandinavian Science Publisher: Oslo, Norway, 2001; pp. 69–85. [Google Scholar]
- Bais, A.F.; Lucas, R.M.; Bornman, J.F.; Williamson, C.E.; Sulzberger, B.; Austin, A.T.; Wilson, S.R.; Andrady, A.L.; Bernhard, G.; McKenzie, R.L.; et al. Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP environmental effects assessment panel, update 2017. Photochem. Photobiol. Sci. 2018, 17, 127–179. [Google Scholar] [CrossRef] [PubMed]
- Frohnmeyer, H.; Staiger, D. Ultraviolet-B radiation-mediated responses in plants. Balancing damage and protection. Plant Physiol. 2003, 133, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Casati, P.; Walbot, V. Rapid transcriptome responses of maize (Zea mays) to UV-B in irradiated and shielded tissues. Genome Biol. 2004, 5, R16. [Google Scholar] [CrossRef] [PubMed]
- Blanding, C.R.; Simmons, S.J.; Casati, P.; Walbot, V.; Stapleton, A.E. Coordinated regulation of maize genes during increasing exposure to ultraviolet radiation: Identification of ultraviolet-responsive genes, functional processes and associated potential promoter motifs. Plant Biotechnol. J. 2007, 5, 677–695. [Google Scholar] [CrossRef]
- Brown, B.A.; Jenkins, G.I. UV-B signaling pathways with different fluence-rate response profiles are distinguished in mature Arabidopsis leaf tissue by requirement for UVR8, HY5, and HYH. Plant Physiol. 2008, 146, 576–588. [Google Scholar] [CrossRef]
- Jenkins, G.I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef]
- Pontin, M.A.; Piccoli, P.N.; Francisco, R.; Bottini, R.; Martinez-Zapater, J.M.; Lijavetzky, D. Transcriptome changes in grapevine (Vitis vinifera L.) cv. Malbec leaves induced by ultraviolet-B radiation. BMC Plant Biol. 2010, 10, 224. [Google Scholar] [CrossRef]
- Hideg, E.; Jansen, M.A.; Strid, A. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef]
- Mosadegh, H.; Trivellini, A.; Ferrante, A.; Lucchesini, M.; Vernieri, P.; Mensuali, A. Applications of UV-B lighting to enhance phenolic accumulation of sweet basil. Sci. Hortic. 2018, 229, 107–116. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Bornman, J.F.; Ballaré, C.L.; Flint, S.D.; Kulandaivelue, G. Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochem. Photobiol. Sci. 2003, 2, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Jansen, M.; Bornman, J.F. UV-B radiation: From generic stressor to specific regulator. Physiol. Plant. 2012, 145, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.A.K.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant. Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Surabhi, G.K.; Reddy, K.R.; Singh, S.K. Photosynthesis, fluorescence, shoot biomass and seed weight responses of three cowpea (Vigna unguiculata (L.) Walp.) cultivars with contrasting sensitivity to UV-B radiation. Environ. Exp. Bot. 2009, 66, 160–171. [Google Scholar] [CrossRef]
- Nawkar, G.M.; Maibam, P.; Park, J.H.; Sahi, V.P.; Lee, S.Y.; Kang, C.H. UV-Induced cell death in plants. Int. J. Mol. Sci. 2013, 14, 1608–1628. [Google Scholar] [CrossRef] [PubMed]
- Rusaczonek, A.; Czarnocka, W.; Kacprzak, S.; Witoń, D.; Ślesak, I.; Szechyńska-Hebda, M.; Karpiński, S. Role of phytochromes A and B in the regulation of cell death and acclimatory responses to UV stress in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 6679–6695. [Google Scholar] [CrossRef]
- Melis, A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage in vivo? Trends Plant. Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing Ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B 2014, 137, 55–66. [Google Scholar] [CrossRef]
- Zavafer, A.; Koinuma, W.; Chow, S.W.; Cheah, M.H.; Mino, H. Mechanism of photodamage of the oxygen evolving Mn cluster of photosystem II by excessive light energy. Sci. Rep. 2017, 7, 7604. [Google Scholar] [CrossRef]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crops Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Benca, J.P.; Duijnstee, I.A.P.; Looy, C.V. UV-B–induced forest sterility: Implications of ozone shield failure in Earth’s largest extinction. Sci. Adv. 2018, 4, e1700618. [Google Scholar] [CrossRef]
- Nigel, P. Plant responses to UV-B: Time to look beyond stratospheric ozone depletion? New Phytol. 2001, 150, 1–8. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Zivcak, M.; Brestic, M.; Kalaji, H.M. Govindjee Photosynthetic responses of sun- and shade-grown barley leaves to high light: Is the lower PSII connectivity in shade leaves associated with protection against excess of light? Photosynth. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, M.H.; Govindjee; Bosa, K.; Kos’cielniak, J.; Zuk-Gołaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: The sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Van Gorkom, H.; Schelvis, J. Kok’s oxygen clock: What makes it tick? The structure of P680 and consequences of its oxidizing power. Photosynth. Res. 1993, 38, 297–301. [Google Scholar] [CrossRef]
- Srivastava, A.; Guisséa, B.; Greppin, H.; Strasser, R.J. Regulation of antenna structure and electron transport in Photosystem II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim. Biophys. Acta 1997, 1320, 95–106. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant 2016, 38, 102. [Google Scholar] [CrossRef]
- Govindachary, S.; Bukhov, N.G.; Joly, D.; Carpentier, R. Photosystem II inhibition by moderate light under low temperature in intact leaves of chilling-sensitive and – tolerant plants. Physiol. Plant 2004, 121, 322–333. [Google Scholar]
- Kalaji, M.H.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as an early indicator of light stress in barley. J. Photochem. Photobiol. B 2012, 112, 1–6. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Lim, J.E.; Landry, L.G.; Last, R.L. Arabidopsis UVR8 regulates ultraviolet-B signal transduction and tolerance and contains sequence similarity to human regulator of chromatin condensation 1. Plant Physiol. 2001, 130, 234–243. [Google Scholar] [CrossRef]
- Jain, K.; Kataria, S.; Guruprasad, K.N. Changes in antioxidant defenses of cucumber cotyledons in response to UV-B and to the free radical generating compound AAPH. Plant Sci. 2003, 165, 551–557. [Google Scholar] [CrossRef]
- Hollosy, F. Effects of ultraviolet radiation on plant cells. Micron 2002, 33, 179–197. [Google Scholar] [CrossRef]
- Jordan, B.R.; James, P.E.; Strid, A.; Anthony, R.G. The effect of ultraviolet-B radiation on gene expression and pigment composition in etiolated and green pea leaf tissue UV-B induced changes are gene-specific and dependent upon the developmental stage. Plant Cell Environ. 1994, 17, 45–54. [Google Scholar] [CrossRef]
- Sztatelman, O.; Grzyb, J.; Gabryś, H.; Banaś, A.K. The effect of UV-B on Arabidopsis leaves depends on light conditions after treatment. BMC Plant Biol. 2015, 15, 281. [Google Scholar] [CrossRef] [PubMed]
- Huarancca Reyes, T.; Scartazza, A.; Castagna, A.; Cosio, E.G.; Ranieri, A.; Guglielminetti, L. Physiological effects of short acute UV-B treatments in Chenopodium quinoa Willd. Sci Rep. 2018, 8, 371. [Google Scholar] [CrossRef] [PubMed]
- Golaszewska, Z.K.; Upadhyaya, M.K.; Golaszewski, J. The effect of UV-B radiation on plant growth and development. Plant Soil Environ. 2003, 49, 135–140. [Google Scholar] [CrossRef]
- Zvezdanović, J.; Cvetić, T.; Veljović-Jovanović, S.; Marković, D. Chlorophyll bleaching by UV-irradiation in vitro and in situ: Absorption and fluorescence studies. Radiation Phys. Chem. 2009, 78, 25–32. [Google Scholar] [CrossRef]
- Jahns, P.; Holzwarth, A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Mathur, S.; Mehta, P.; Jajoo, A.; Bharti, S. Analysis of elevated temperature induced inhibition of Photosystem II using Chl a fluorescence induction kinetics. Plant Biol. 2011, 13, 1–6. [Google Scholar] [CrossRef]
- Fujii, R.; Yamano, N.; Hashimoto, H.; Misawa, N.; Ifuku, K. Photoprotection vs. Photoinhibition of Photosystem II in Transplastomic Lettuce (Lactuca sativa) Dominantly Accumulating Astaxanthin. Plant Cell Physiol. 2016, 57, 1518–1529. [Google Scholar] [CrossRef]
- Wingler, A.; Marès, M.; Pourtau, N. Spatial patterns and metabolic regulation of photosynthetic parameters during leaf senescence. New Phytol. 2004, 161, 781–789. [Google Scholar] [CrossRef]
- Sozer, O.; Komenda, J.; Ughy, B.; Domonkos, I.; Laczkó-Dobos, H.; Malec, P.; Gombos, Z.; Kis, M. Involvement of carotenoids in the synthesis and assembly of protein subunits of photosynthetic reaction centers of Synechocystis sp. PCC 6803. Plant Cell Physiol. 2010, 51, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Santabarbara, S.; Casazza, A.P.; Ali, K.; Economou, C.K.; Wannathong, T.; Zito, F.; Redding, K.E.; Rappaport, F.; Purton, S. The requirement for carotenoids in the assembly and function of the photosynthetic complexes in Chlamydomonas reinhardtii. Plant Physiol. 2013, 161, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Toth, T.N.; Chukhutsina, V.; Domonkos, I.; Knoppová, J.; Komenda, J.; Kis, M.; Lénárt, Z.; Garab, G.; Kovács, L.; Gombos, Z.; et al. Carotenoids are essential for the assembly of cyanobacterial photosynthetic complexes. Biochim. Biophys. Acta 2015, 1847, 1153–1165. [Google Scholar] [CrossRef] [Green Version]
- Dao, T.T.H.; Linthorst, H.J.M.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Kiferle, C.; Lucchesini, M.; Mensuali Sodi, A.; Maggini, R.; Raffaelli, A.; Pardossi, A. Rosmarinic acid content in basil plants grown in vitro and in hydroponics. Cent. Eur. J. Biol. 2011, 6, 946–957. [Google Scholar] [CrossRef]
- Dewanjee, S.; Gangopadhyay, M.; Das, U.; Sahu, R.; Samanta, A.; Banerjee, P. Signal transducer and oxidative stress mediated modulation of phenylpropanoid pathway to enhance rosmarinic acid biosynthesis in fungi elicited whole plant culture of Solenostemon scutellarioides. Enzyme Microb. Technol. 2014, 66, 1–9. [Google Scholar] [CrossRef]
- Vanhaelewyn, L.; Prinsen, E.; VanDerStraeten, D.; Vandenbussche, F. Hormone-controlled UV-B responses in plants. J. Exp. Bot. 2016, 67, 4469–4482. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Pellegrini, E.; Trivellini, A.; Campanella, A.; Francini, A.; Lorenzini, G.; Nali, C.; Vernieri, P. Signaling molecules and cell death in Melissa officinalis plants exposed to ozone. Plant Cell Rep. 2013, 32, 1965–1980. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Lucchesini, M.; Ferrante, A.; Carmassi, G.; Scatena, G.; Vernieri, P.; Mensuali-Sodi, A. Survive or die? A molecular insight into salt-dependant signaling network. Env. Exp. Bot. 2016, 132, 140–153. [Google Scholar] [CrossRef]
- Valluru, R.; Davies, W.J.; Reynolds, M.P.; Dodd, I.C. Foliar abscisic acid-to-ethylene accumulation and response regulate shoot growth sensitivity to mild drought in wheat. Front. Plant Sci. 2016, 7, 461. [Google Scholar] [CrossRef] [PubMed]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S.P. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, M.I.R. Ethylene Role in Plant Growth, Development and Senescence: Interaction with Other Phytohormones. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tossi, V.; Lamattina, L.; Cassia, R. An increase in the concentration of abscisic acid is critical for nitric oxide-mediated plant adaptive responses to UV-B irradiation. New Phytol. 2009, 181, 871–879. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Chen, M.W.; Ji, B.H.; Jiao, D.M.; Liang, J.S. Roles of xanthophylls and exogenous ABA in protection against NaCl- induced photodamage in rice (Oryza sativa L) and cabbage (Brassica campestris). J. Exp. Bot. 2011, 62, 4617–4625. [Google Scholar] [CrossRef]
- Barickman, T.C.; Kopsell, D.A.; Sams, C.E. Abscisic acid increases carotenoid and chlorophyll concentrations in leaves and fruit of two tomato genotypes. J. Am. Soc. Hort. Sci. 2014, 139, 261–266. [Google Scholar] [CrossRef]
- Mackerness, S.A.H.; Surplus, S.L.; Blake, P.C.; John, F.; Buchanan-Wollaston, V.; Jordan, B.R.; Thomas, B. Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: Role of signaling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Env. 1999, 22, 1413–1423. [Google Scholar] [CrossRef]
- Nara, A.; Takeuchi, Y. Ethylene evolution from tobacco leaves irradiated with UV-B. J. Plant Res. 2002, 115, 247–253. [Google Scholar] [CrossRef]
- McLeod, A.R.; Fry, S.C.; Loake, G.J.; Messenger, D.J.; Reay, D.S.; Smith, K.A.; Yun, B.W. Ultraviolet radiation drives methane emissions from terrestrial plant pectins. New Phytol. 2008, 180, 124–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.R.; Khan, N.A. Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 2014, 251, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Trivellini, A.; Malorgio, F.; Carmassi, G.; Vernieri, P.; Serra, G. Effect of seawater aerosol on leaves of six plant species potentially useful for ornamental purposes in coastal areas. Sci. Hortic. 2011, 128, 332–341. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Xu, J.; Su, T.; Liu, G.; Ren, D. Ethylene signaling is required for the acceleration of cell death induced by the activation of AtMEK5 in Arabidopsis. Cell Res. 2008, 18, 422–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameter | Calculation | Description |
|---|---|---|
| Extracted and technical fluorescence parameters | ||
| Fo | Fluorescence intensity at 50 µs | Fluorescence intensity when all reaction centers (RCs) are open |
| Fj | Fluorescence intensity at 2 ms at J-step | |
| Fk | Fluorescence intensity at 300 µs at K-step | |
| Fm | Maximal fluorescence intensity | Fluorescence intensity when all RCs are closed |
| Vj | Vj = (Fj − Fo)/(Fm − Fo) | Relative variable fluorescence at 2 ms. For unconnected PSII units, this equals the fraction of closed RCs expressed as a proportion of the total number of RCs |
| Vk | Vk = (Fk − Fo)/(Fm − Fo) | Relative variable fluorescence at 300 μs |
| Fv/Fo | (Fm − Fo)/Fo | Proportional to the activity of the water-splitting complex on the donor side of the PSII |
| Fk/Fj | To probe the extent of inactivation of the PSII donor side | |
| Mo | Mo = 4(F300 − Fo)/(Fm − Fo) | Slope of the normalized curve at the origin of the fluorescence rise. Net rate of closed reaction centers accumulation |
| Sm | Sm = Area/(Fm−Fo) | Standardized area above the fluorescence curve between Fo and Fm is proportional to the pool size of the electron acceptors QA on the reducing side of Photosystem II |
| Tfm | Time needed to reach Fm | |
| Efficiencies and quantum yields | ||
| ϕP0 | ϕP0 = 1 − (Fo/Fm) = Fv/Fm | Maximum quantum yield of primary PSII photochemistry. Probability that an absorbed photon will be trapped by the PSII RC with the resulting reduction of QA |
| ϕE0 | ϕE0 = [1 − (Fo/Fm)](1 − Vj) | Quantum yield for electron transport |
| ΨE0 | ΨE0 = 1-Vj | Efficiency of excitation energy to electron transport flux conversion. Probability that an exciton trapped by the PSII RC enters the electron transport chain |
| δR0 | δR0 = (1 − Vi) (1 − Vj) | Efficiency with which an electron from the intersystem electron carriers moves to reduce end electron acceptor side (RE) |
| δD0 | δD0 = 1 − ϕP0 | It expresses the probability that the energy of an adsorbed photon is dissipated as heat |
| ϕR0 | ϕR0 = δR0*ϕP0*ΨE0 | Quantum yield for the reduction of end acceptors of PSI per photon absorbed |
| Parameter | UV-B dose | Time after UV-B exposure | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | ||||||
| Fo | Ctrl | 567.8 ns | 569.5 b | 614.7 b | 626.0 b | ||||
| Low | 565.7 ns | 609.5 b | 668.0 b | 749.0 bc | |||||
| High | 678.3 ns | 816.5 a | ↑ | 953.3 a | ↑ | 907.0 ac | ↑ | ||
| Fm | Ctrl | 3167 a | 3011 a | 3238 a | 3198 a | ||||
| Low | 2641 a | 2814 a | 2341 b | ↓ | 1849 b | ↓ | |||
| High | 1929 b | ↓ | 1778 b | ↓ | 1194 c | ↓ | 862 c | ↓ | |
| Vj | Ctrl | 0.45 ns | 0.49 ns | 0.49 b | 0.52 b | ||||
| Low | 0.43 ns | 0.40 ns | 0.47 b | 0.48 b | |||||
| High | 0.45 ns | 0.50 ns | 0.67 a | ↑ | 1.11 a | ↑ | |||
| Vk | Ctrl | 0.30 ns | 0.31 ns | 0.31 ns | 0.33 b | ||||
| Low | 0.28 ns | 0.28 ns | 0.29 ns | 0.30 b | |||||
| High | 0.30 ns | 0.29 ns | 0.40 ns | 0.67 a | ↑ | ||||
| Fv/Fo | Ctrl | 5,58 a | 5,30 a | 5,27 a | 5.12 a | ||||
| Low | 4,68 a | 4,61 a | 3.54 b | ↓ | 2.50 b | ↓ | |||
| High | 2,9 b | ↓ | 2,41 b | ↓ | 1.30 c | ↓ | 1.00 c | ↓ | |
| Fk/Fj | Ctrl | 0.77 b | 0.76 b | 0.77 b | 0.76 c | ||||
| Low | 0.78 b | 0.82 b | 0.80 b | 0.84 b | ↑ | ||||
| High | 0.86 a | ↑ | 0.89 a | ↑ | 0.95 c | ↑ | 0.99 a | ↑ | |
| Mo | Ctrl | 1.19 ns | 1.27 ns | 1.27 b | 1.35 b | ||||
| Low | 1.10 ns | 1.10 ns | 1.10 b | 1.20 b | |||||
| High | 1.20 ns | 1.17 ns | 1.70 a | ↑ | 2.67 a | ↑ | |||
| Sm | Ctrl | 17.83 b | 19.76 ns | 20.25 ns | 20.25 a | ||||
| Low | 31.34 b | 33.50 ns | 32.14 ns | 28.76 a | |||||
| High | 71.74 a | ↑ | 41.33 ns | 13.33 ns | 0.33 b | ↓ | |||
| Tfm | Ctrl | 195.0 b | 205.0 b | 205.0 ns | 210.0 a | ||||
| Low | 260.0 b | 280.0 b | 390.0 ns | 285.0 a | |||||
| High | 750.0 a | ↑ | 510.0 a | ↑ | 224.8 ns | 1.5 b | ↓ | ||
| ϕP0 | Ctrl | 0.82 ns | 0.81 a | 0.81 a | 0.80 a | ||||
| Low | 0.78 ns | 0.78 a | 0.69 a | 0.60 b | ↓ | ||||
| High | 0.62 ns | 0.46 b | ↓ | 0.18 b | ↓ | 0.01 c | ↓ | ||
| ϕE0 | Ctrl | 0.45 ns | 0.41 a | 0.41 a | 0.41 a | ||||
| Low | 0.45 ns | 0.47 a | 0.37 a | 0.32 a | |||||
| High | 0.35 ns | 0.26 b | ↓ | 0.08 b | ↓ | 0.001 b | ↓ | ||
| ΨE0 | Ctrl | 0.55 ns | 0.51 ns | 0.51 a | 0.51 a | ||||
| Low | 0.57 ns | 0.60 ns | 0.52 a | 0.52 a | |||||
| High | 0.55 ns | 0.49 ns | 0.33 b | ↓ | 0.03 b | ↓ | |||
| δR0 | Ctrl | 0.48 b | 0.46 ns | 0.44 ns | 0.49 a | ||||
| Low | 0.59 b | 0.54 ns | 0.52 ns | 0.49 a | |||||
| High | 0.75 a | ↑ | 0.54 ns | 0.33 ns | 0.17 b | ↓ | |||
| ϕR0 | Ctrl | 0.14 ns | 0.15 ns | 0.15 a | 0.15 a | ||||
| Low | 0.20 ns | 0.20 ns | 0.20 a | 0.09 a | |||||
| High | 0.17 ns | 0.10 ns | 0.02 b | ↓ | 0.000 b | ↓ | |||
| δD0 | Ctrl | 0.18 ns | 0.19 b | 0.19 b | 0.19 c | ||||
| Low | 0.21 ns | 0.22 b | 0.31 b | 0.40 b | ↑ | ||||
| High | 0.37 ns | 0.53 a | ↑ | 0.82 a | ↑ | 0.99 a | ↑ | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosadegh, H.; Trivellini, A.; Lucchesini, M.; Ferrante, A.; Maggini, R.; Vernieri, P.; Mensuali Sodi, A. UV-B Physiological Changes Under Conditions of Distress and Eustress in Sweet Basil. Plants 2019, 8, 396. https://doi.org/10.3390/plants8100396
Mosadegh H, Trivellini A, Lucchesini M, Ferrante A, Maggini R, Vernieri P, Mensuali Sodi A. UV-B Physiological Changes Under Conditions of Distress and Eustress in Sweet Basil. Plants. 2019; 8(10):396. https://doi.org/10.3390/plants8100396
Chicago/Turabian StyleMosadegh, Haana, Alice Trivellini, Mariella Lucchesini, Antonio Ferrante, Rita Maggini, Paolo Vernieri, and Anna Mensuali Sodi. 2019. "UV-B Physiological Changes Under Conditions of Distress and Eustress in Sweet Basil" Plants 8, no. 10: 396. https://doi.org/10.3390/plants8100396
APA StyleMosadegh, H., Trivellini, A., Lucchesini, M., Ferrante, A., Maggini, R., Vernieri, P., & Mensuali Sodi, A. (2019). UV-B Physiological Changes Under Conditions of Distress and Eustress in Sweet Basil. Plants, 8(10), 396. https://doi.org/10.3390/plants8100396







