Impact of Combined Heat and Drought Stress on the Potential Growth Responses of the Desert Grass Artemisia sieberi alba: Relation to Biochemical and Molecular Adaptation
Abstract
:1. Introduction
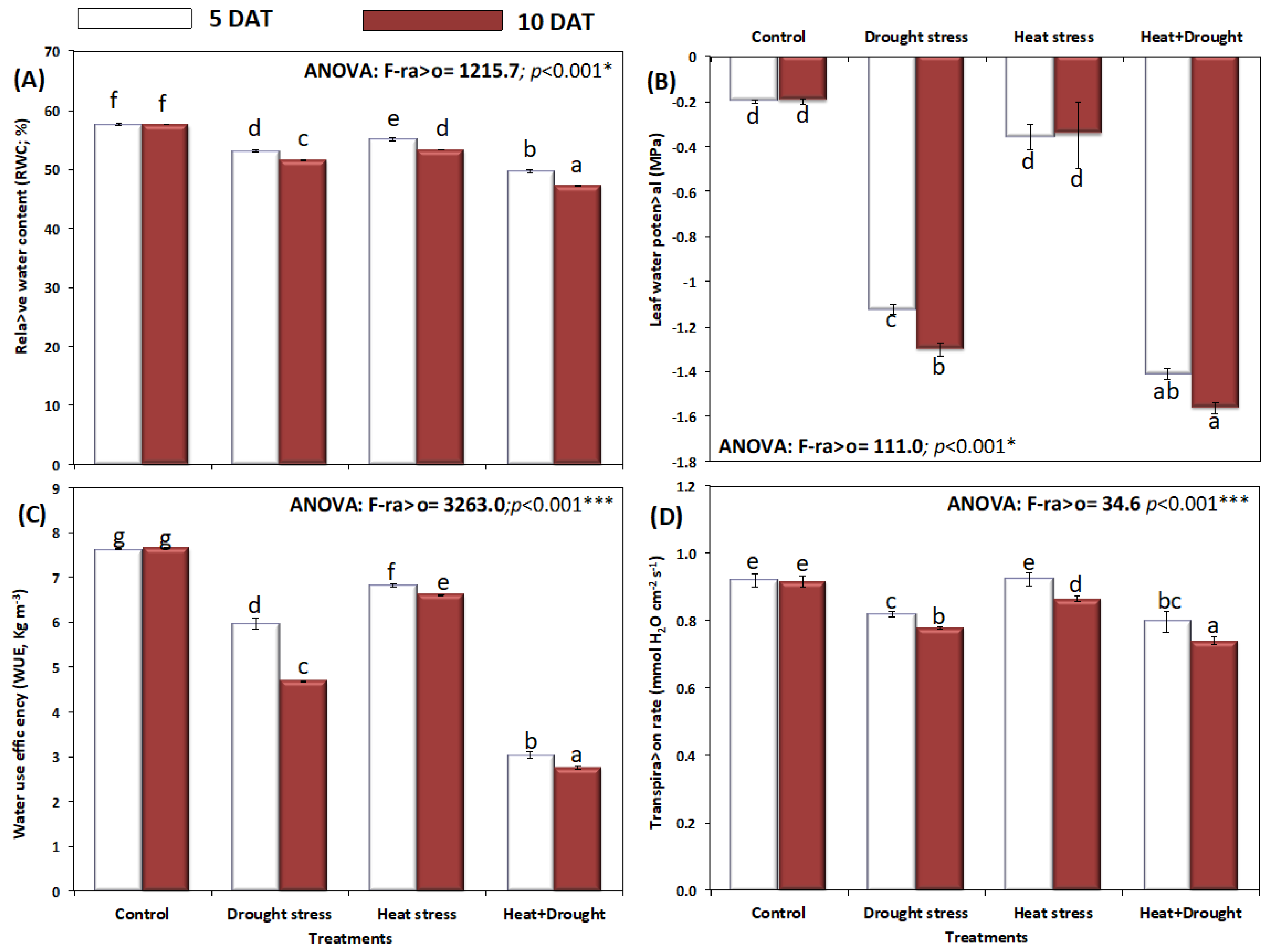
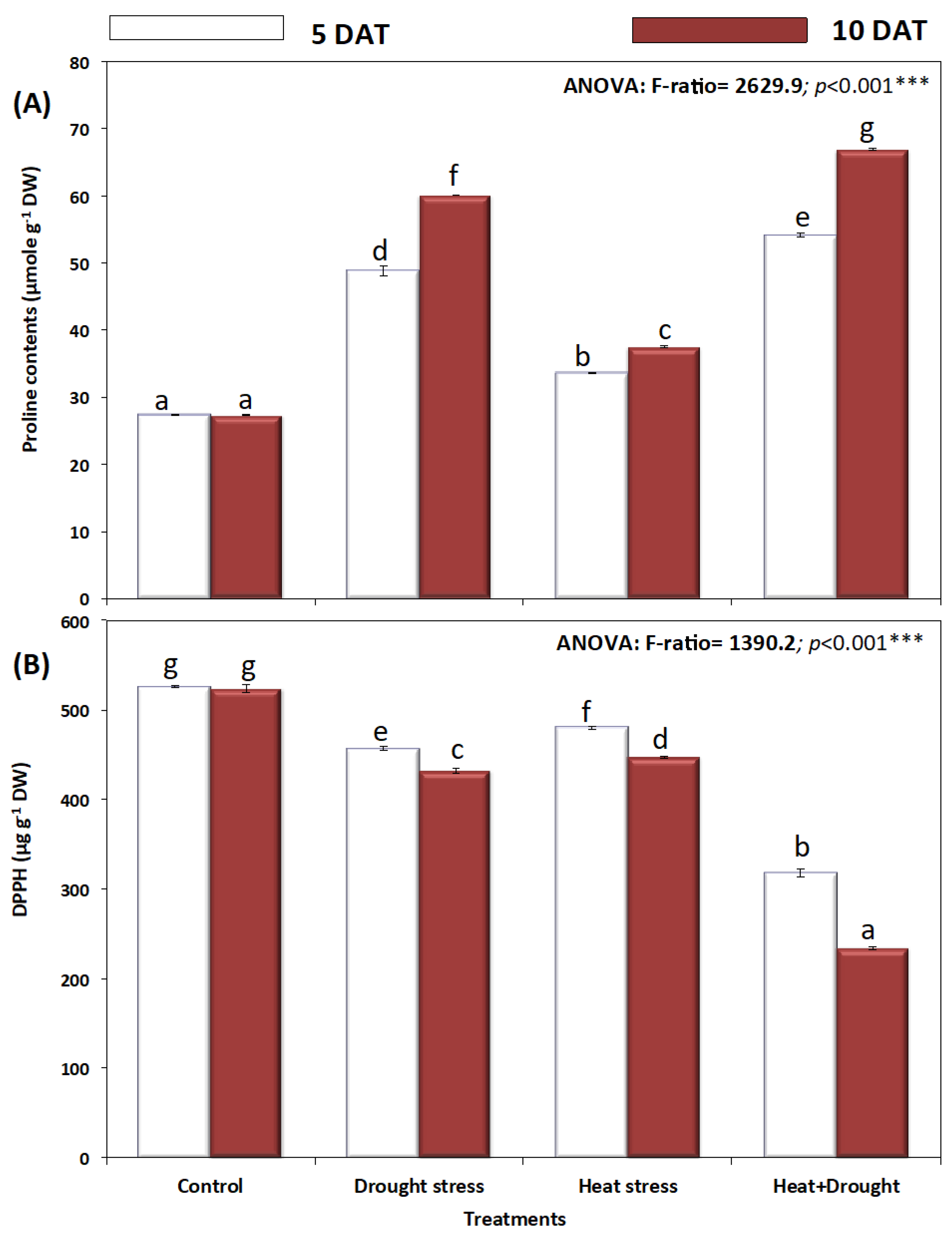
2. Results
3. Discussion
4. Materials and Methods
4.1. Pot Experiments and Stress Treatments
4.2. Growth Measurements
4.3. Physiological Traits
4.3.1. Relative Water Content and Leaf Water Potential (LWP)
4.3.2. Gas Exchange, Chlorophyll Fluorescence Parameters and Water Use Efficiency
4.3.3. Measurement of Chlorophyll
4.4. Oxidative Stress Parameters
4.4.1. Membrane Stability Index (MSI)
4.4.2. Lipid Peroxidation
4.4.3. Hydrogen Peroxide
4.4.4. Electrolyte Leakage
4.5. Determination of Proline Content
4.6. Total Antioxidant Activity
4.7. Determination of Mannitol, Sorbitol Inositol Content
4.8. Phytochemical Screening
4.9. RNA Isolation and Reverse Transcription of RNA (RT-PCR)
4.10. Gene Expression Levels
4.11. Statistical Analysis
5. Conclusions
Funding
Conflicts of Interest
References
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plantcell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Cantalapiedra, C.P.; García-Pereira, M.J.; Gracia, M.P.; Igartua, E.; Casas, A.M.; Contreras-Moreira, B. Large differences in gene expression responses to drought and heat stress between elite barley cultivar Scarlett and a spanish landrace. Front. Plant Sci. 2017, 8, 647. [Google Scholar] [CrossRef]
- Sekhon, H.S.; Singh, G.; Sharma, P.; Bains, T.S. Water use efficiency under stress environments. In Climate Change and Management of Cool Season Grain Legume Crops; Springer: Dordrecht, The Netherlands, 2010; pp. 207–227. [Google Scholar]
- Vadez, V.; Kholova, J.; Choudhary, S.; Zindy, P.; Terrier, M.; Krishnamurthy, L.; Kumar, P.R.; Turner, N.C. Responses to Increased Moisture Stress and Extremes: Whole Plant Response to Drought under Climate Change. In Crop Adaptation to Climate Change; Yadav, S.S., Redden, R.J., Hatfield, J.L., Lotze-Campen, H., Hall, A.E., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 186–197. ISBN 978-0-470-96092-9. [Google Scholar] [Green Version]
- Agarwal, G.; Garg, V.; Kudapa, H.; Doddamani, D.; Pazhamala, L.T.; Khan, A.W.; Thudi, M.; Lee, S.-H.; Varshney, R.K. Genome-wide dissection of AP2/ERF and HSP90 gene families in five legumes and expression profiles in chickpea and pigeonpea. Plant Biotechnol. J. 2016, 14, 1563–1577. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Effects of Drought, Heat and Their Interaction on the Growth, Yield and Photosynthetic Function of Lentil (Lens culinaris Medikus) Genotypes Varying in Heat and Drought Sensitivity. Front. Plant Sci. 2017, 8, 1776. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Extreme temperature responses, oxidative stress and antioxidant defense in plants. Abiotic Stress-Plant Responses Appl. Agric. 2013, 169–205. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant Response and Tolerance to Abiotic Oxidative Stress: Antioxidant Defense Is a Key Factor. In Crop Stress and its Management: Perspectives and Strategies; Venkateswarlu, B., Shanker, A.K., Shanker, C., Maheswari, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. ISBN 978-94-007-2219-4. [Google Scholar]
- Cao, P.B.; Azar, S.; SanClemente, H.; Mounet, F.; Dunand, C.; Marque, G.; Marque, C.; Teulières, C. Genome-wide analysis of the AP2/ERF family in Eucalyptus grandis: An intriguing over-representation of stress-responsive DREB1/CBF genes. PLoS ONE 2015, 10, e0121041. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.-P.; Scott, E.; Liu, J.-W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.-Y. Exogenous Melatonin Alleviates Cold Stress by Promoting Antioxidant Defense and Redox Homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.O.N.; Miller, G.A.D. ROS and redox signalling in the response of plants to abiotic stress. Plantcell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Tanakamaru, S.; Maitani, T.; Kimura, K. Recovery responses of photosynthesis, transpiration, and stomatal conductance in kidney bean following drought stress. Environ. Exp. Bot. 2005, 53, 205–214. [Google Scholar] [CrossRef]
- Prasad, P.V.; Pisipati, S.R.; Mutava, R.N.; Tuinstra, M.R. Sensitivity of grain sorghum to high temperature stress during reproductive development. Crop Sci. 2008, 48, 1911–1917. [Google Scholar] [CrossRef]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; Von Korff, M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef]
- Inamullah Isoda, A. Adaptive responses of soybean and cotton to water stress II. Changes in CO2 assimilation rate, chlorophyll fluorescence and photochemical reflectance index in relation to leaf temperature. Plant Prod. Sci. 2005, 8, 131–138. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci. 2001, 41, 436–442. [Google Scholar] [CrossRef]
- Radwan, U.A.A. Photosynthetic and leaf anatomical characteristics of the drought-resistant Balanites aegyptiaca (L.) Del. seedlings. Am. Eurasian J. Agric. Environ. Sci. 2007, 2, 680–688. [Google Scholar]
- Souza, R.P.; Machado, E.C.; Silva, J.A.B.; Lagôa, A.; Silveira, J.A.G. Photosynthetic gas exchange, chlorophyll fluorescence and some associated metabolic changes in cowpea (Vigna unguiculata) during water stress and recovery. Environ. Exp. Bot. 2004, 51, 45–56. [Google Scholar] [CrossRef]
- Edreva, A.M.; Velikova, V.B.; Tsonev, T.D. Phenylamides in plants. Russ. J. Plant Physiol. 2007, 54, 287–301. [Google Scholar] [CrossRef]
- Szabados, L.; Kovács, H.; Zilberstein, A.; Bouchereau, A. Plants in extreme environments: Importance of protective compounds in stress tolerance. In Advances in Botanical Research; Elsevier Science Direct: Amsterdam, The Netherlands, 2011; Volume 57, pp. 105–150. [Google Scholar]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.-K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Krasensky, J.; Jonak, C. Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J. Exp. Bot. 2012, 63, 1593–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Armijo, L.; Sha, W.; Cortes, D.; Shulaev, V.; Mittler, R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2008, 283, 34197–34203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, F.; Li, D.; Zhang, H.; Huang, R. Expression of ethylene response factor JERF1 in rice improves tolerance to drought. Planta 2010, 232, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.V.V.; Djanaguiraman, M.; Jagadish, S.V.K.; Ciampitti, I.A. Drought and High Temperature Stress and Traits Associated with Tolerance. Sorghum State Art Future Perspect. 2018. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Pisipati, S.R.; Momčilović, I.; Ristic, Z. Independent and Combined Effects of High Temperature and Drought Stress During Grain Filling on Plant Yield and Chloroplast EF-Tu Expression in Spring Wheat. J. Agron. Crop Sci. 2011, 197, 430–441. [Google Scholar] [CrossRef]
- Obata, T.; Witt, S.; Lisec, J.; Palacios-Rojas, N.; Florez-Sarasa, I.; Araus, J.L.; Cairns, J.E.; Yousfi, S.; Fernie, A.R. Metabolite profiles of maize leaves in drought, heat and combined stress field trials reveal the relationship between metabolism and grain yield. Plant Physiol. 2015, 169, 2665–2683. [Google Scholar] [CrossRef] [Green Version]
- Wani, S.H.; Dutta, T.; Neelapu, N.R.R.; Surekha, C. Transgenic approaches to enhance salt and drought tolerance in plants. Plant Gene 2017, 11, 219–231. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptations in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A.; Van Staden, J. Dissecting the roles of osmolyte accumulation during stress. Plantcell Environ. 1998, 21, 535–553. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Tomar, N.S.; Tittal, M.; Argal, S.; Agarwal, R.M. Plant growth under water/salt stress: ROS production; antioxidants and significance of added potassium under such conditions. Physiol. Mol. Biol. Plants 2017, 23, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Hassani, A.; Dellal, A.; Belkhodja, M.; Kaid–Harche, M. Effect of Salinity on Water and Some Osmolytes in Barley (Hordeum Vulgare). Eur. J. Sci. Res. 2008, 23, 61–69. [Google Scholar]
- Zivcak, M.; Brestic, M.; Sytar, O. Osmotic adjustment and plant adaptation to drought stress. In Drought Stress Tolerance in Plants; Springer: Cham, Switzerland, 2016; Volume 1, pp. 105–143. [Google Scholar]
- Moufid, A.; Eddouks, M. Artemisial herbal allbal: A Popular Plant with Potential Medicinal Properties. Pak. J. Biol. Sci. 2012, 15, 1152–1159. [Google Scholar] [PubMed]
- Nejat, N.; Mantri, N. Plant immune system: Crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Signal 2017, 2, O2. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.J.; Gowing, D.J.G.; Lark, R.M.; Leeds-Harrison, P.B.; Miller, A.J.; Wells, D.M.; Whalley, W.R.; Whitmore, A.P. Sensing the physical and nutritional status of the root environment in the field: A review of progress and opportunities. J. Agric. Sci. 2005, 143, 347–358. [Google Scholar] [CrossRef]
- Dodd, I.C. Root-To-Shoot Signalling: Assessing The Roles of ‘Up’ In the Up and Down World of Long-Distance Signalling in Planta. Plant Soil 2005, 274, 251–270. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Goodger, J.Q.D. Chemical root to shoot signaling under drought. Trends Plant Sci. 2008, 13, 281–287. [Google Scholar] [CrossRef]
- Wahid, A.; Perveen, M.; Gelani, S.; Basra, S.M.A. Pretreatment of seed with H2O2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. J. Plant Physiol. 2007, 164, 283–294. [Google Scholar] [CrossRef]
- El Soda, M.; Nadakuduti, S.S.; Pillen, K.; Uptmoor, R. Stability parameter and genotype mean estimates for drought stress effects on root and shoot growth of wild barley pre-introgression lines. Mol. Breed. 2010, 26, 583–593. [Google Scholar] [CrossRef]
- Dreesen, F.E.; De Boeck, H.J.; Janssens, I.A.; Nijs, I. Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ. Exp. Bot. 2012, 79, 21–30. [Google Scholar] [CrossRef]
- Zlatev, Z.; Lidon, F.C. An overview on drought induced changes in plant growth, water relations and photosynthesis. Emir. J. Food Agric. 2012, 24, 57–72. [Google Scholar]
- Awasthi, R.; Kaushal, N.; Vadez, V.; Turner, N.C.; Berger, J.; Siddique, K.H.; Nayyar, H. Individual and combined effects of transient drought and heat stress on carbon assimilation and seed filling in chickpea. Funct. Plant Biol. 2014, 41, 1148–1167. [Google Scholar] [CrossRef] [Green Version]
- Templer, S.E.; Ammon, A.; Pscheidt, D.; Ciobotea, O.; Schuy, C.; McCollum, C.; Sonnewald, U.; Hanemann, A.; Förster, J.; Ordon, F. Metabolite profiling of barley flag leaves under drought and combined heat and drought stress reveals metabolic QTLs for metabolites associated with antioxidant defense. J. Exp. Bot. 2017, 68, 1697–1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earnshaw, M.J.; Hendry, G. Stress indicators: Electrolyte leakage. In Methods in Comparative Plant Ecology; Chapman and Hall: London, UK, 1993; pp. 152–154. [Google Scholar]
- Conde, A.; Chaves, M.M.; Gerós, H. Membrane transport, sensing and signaling in plant adaptation to environmental stress. Plant Cell Physiol. 2011, 52, 1583–1602. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaushal, N.; Nayyar, H.; Gaur, P. Abscisic acid induces heat tolerance in chickpea (Cicer arietinum L.) seedlings by facilitated accumulation of osmoprotectants. Acta Physiol. Plant. 2012, 34, 1651–1658. [Google Scholar] [CrossRef]
- Liu, J.; Xie, X.; Du, J.; Sun, J.; Bai, X. Effects of simultaneous drought and heat stress on Kentucky bluegrass. Sci. Hortic. 2008, 115, 190–195. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Mittler, R. The combined effect of drought stress and heat shock on gene expression in tobacco. Plant Physiol. 2002, 130, 1143–1151. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Rivero, R.M.; Martínez, V.; Gómez-Cadenas, A.; Arbona, V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016, 16, 105. [Google Scholar] [CrossRef]
- Killi, D.; Bussotti, F.; Raschi, A.; Haworth, M. Adaptation to high temperature mitigates the impact of water deficit during combined heat and drought stress in C3 sunflower and C4 maize varieties with contrasting drought tolerance. Physiol. Plant. 2017, 159, 130–147. [Google Scholar] [CrossRef]
- Melis, A. Photosystem-II damage and repair cycle in chloroplasts: What modulates the rate of photodamage in vivo? Trends Plant Sci. 1999, 4, 130–135. [Google Scholar] [CrossRef]
- Robinson, J.M.; Bunce, J.A. Influence of drought-induced water stress on soybean and spinach leaf ascorbate-dehydroascorbate level and redox status. Int. J. Plant Sci. 2000, 161, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Barta, C.; Dunkle, A.M.; Wachter, R.M.; Salvucci, M.E. Structural changes associated with the acute thermal instability of Rubisco activase. Arch. Biochem. Biophys. 2010, 499, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Rabara, R.C.; Tripathi, P.; Reese, R.N.; Rushton, D.L.; Alexander, D.; Timko, M.P.; Shen, Q.J.; Rushton, P.J. Tobacco drought stress responses reveal new targets for Solanaceae crop improvement. BMC Genomics 2015, 16, 484–505. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zhang, J. Effects of water stress on photosystem II photochemistry and its thermostability in wheat plants. J. Exp. Bot. 1999, 50, 1199–1206. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Ristic, Z.; Bukovnik, U.; Prasad, P.V. Correlation between heat stability of thylakoid membranes and loss of chlorophyll in winter wheat under heat stress. Crop Sci. 2007, 47, 2067–2073. [Google Scholar] [CrossRef]
- Carmo-Silva, A.E.; Gore, M.A.; Andrade-Sanchez, P.; French, A.N.; Hunsaker, D.J.; Salvucci, M.E. Decreased CO2 availability and inactivation of Rubisco limit photosynthesis in cotton plants under heat and drought stress in the field. Environ. Exp. Bot. 2012, 83, 1–11. [Google Scholar] [CrossRef]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.-H. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.-O.; Rosenqvist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Ludlow, M.M.; Muchow, R.C. A Critical Evaluation of Traits for Improving Crop Yields in Water-Limited Environments In Advances in Agronomy; Brady, N.C., Ed.; Academic Press: Cambridge, MA, USA, 1990; Volume 43, pp. 107–153. [Google Scholar]
- Ahanger, M.A.; Agarwal, R.M. Salinity stress induced alterations in antioxidant metabolism and nitrogen assimilation in wheat (Triticum aestivum L) as influenced by potassium supplementation. Plant Physiol. Biochem. 2017, 115, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Sairam, R.K.; Shukla, D.S.; Saxena, D.C. Stress induced injury and antioxidant enzymes in relation to drought tolerance in wheat genotypes. Biol. Plant. 1997, 40, 357–364. [Google Scholar] [CrossRef]
- Sairam, R.K.; Srivastava, G.C. Water Stress Tolerance of Wheat (Triticum aestivum L.): Variations in Hydrogen Peroxide Accumulation and Antioxidant Activity in Tolerant and Susceptible Genotypes. J. Agron. Crop Sci. 2001, 186, 63–70. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Ahmad, P. Chapter 17—Role of Mineral Nutrients in Abiotic Stress Tolerance: Revisiting the Associated Signaling Mechanisms. In Plant Signaling Molecules; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Elsevier: Amsterdam, The Netherlands, 2019; pp. 269–285. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.; Sairam, R. High temperature stress tolerance in wheat genotypes: Role of antioxidant defence enzymes. Acta Agron. Hung. 2009, 57, 1–14. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef]
- Soliman, M.H.; Alayafi, A.A.; El Kelish, A.A.; Abu-Elsaoud, A.M. Acetylsalicylic acid enhance tolerance of Phaseolus vulgaris L. to chilling stress, improving photosynthesis, antioxidants and expression of cold stress responsive genes. Bot. Stud. 2018, 59, 6. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Urban, O.; Hlaváčová, M.; Klem, K.; Novotná, K.; Rapantová, B.; Smutná, P.; Horáková, V.; Hlavinka, P.; Škarpa, P.; Trnka, M. Combined effects of drought and high temperature on photosynthetic characteristics in four winter wheat genotypes. Field Crop. Res. 2018, 223, 137–149. [Google Scholar] [CrossRef]
- Sayed, S.A.; Gadallah, M.A.A.; Salama, F.M. Ecophysiological studies on three desert plants growing in Wadi Natash, Eastern Desert, Egypt. J. Biol. Earth Sci. 2013, 3, 135–143. [Google Scholar]
- Robson, T.M.; Klem, K.; Urban, O.; Jansen, M.A. Re-interpreting plant morphological responses to UV-B. radiation. Plantcell Environ. 2015, 38, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought tolerance: Role of organic osmolytes, growth regulators, and mineral nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Springer: New York, NY, USA, 2014; pp. 25–55. [Google Scholar]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars: Metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhani, N.; Heidari, R. Drought-induced Accumulation of Soluble Sugars and Proline in Two Maize Varieties. World Appl. Sci. J. 2008, 6, 448–453. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [Green Version]
- Bandurska, H.; Niedziela, J.; Chadzinikolau, T. Separate and combined responses to water deficit and UV-B radiation. Plant Sci. 2013, 213, 98–105. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Sangam, S.; Amrutha, R.N.; Laxmi, P.S.; Naidu, K.R. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 2005, 88, 15. [Google Scholar]
- Shekoofa, A.; Sinclair, T.R. Aquaporin Activity to Improve Crop Drought Tolerance. Cells 2018, 7, 123. [Google Scholar] [CrossRef]
- Wang, X.; Deng, Z.; Zhang, W.; Meng, Z.; Chang, X.; Lv, M. Effect of Waterlogging Duration at Different Growth Stages on the Growth, Yield and Quality of Cotton. PLoS ONE 2017, 12, e0169029. [Google Scholar] [CrossRef]
- Magwanga, R.O.; Lu, P.; Kirungu, J.N.; Lu, H.; Wang, X.; Cai, X.; Zhou, Z.; Zhang, Z.; Salih, H.; Wang, K.; et al. Characterization of the late embryogenesis abundant (LEA) proteins family and their role in drought stress tolerance in upland cotton. BMC Genet. 2018, 19, 6. [Google Scholar] [CrossRef]
- Shao, H.-B.; Chu, L.-Y.; Jaleel, C.A.; Manivannan, P.; Panneerselvam, R.; Shao, M.-A. Understanding water deficit stress-induced changes in the basic metabolism of higher plants–biotechnologically and sustainably improving agriculture and the ecoenvironment in arid regions of the globe. Crit. Rev. Biotechnol. 2009, 29, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Tunnacliffe, A.; Wise, M.J. The continuing conundrum of the LEA proteins. Naturwissenschaften 2007, 94, 791–812. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.M.; El-Bastawisy, Z.M.; El-Sayed, A.K.; Ebeed, H.T.; Nemat Alla, M.M. Roles of dehydrin genes in wheat tolerance to drought stress. J. Adv. Res. 2015, 6, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.S.; Chakradhar, T.; Reddy, R.A.; Nitnavare, R.B.; Mahanty, S.; Reddy, M.K. Role of Heat Shock Proteins in Improving Heat Stress Tolerance in Crop Plants. In Heat Shock Proteins and Plants; Asea, A.A.A., Kaur, P., Calderwood, S.K., Eds.; Heat Shock Proteins; Springer: Cham, Switzerland, 2016; pp. 283–307. ISBN 978-3-319-46340-7. [Google Scholar]
- Yuan-Yuan, S.U.N.; Yong-Jian, S.U.N.; Ming-Tian, W.; Xu-Yi, L.I.; Xiang, G.U.O.; Rong, H.U.; Jun, M.A. Effects of seed priming on germination and seedling growth under water stress in rice. Acta Agron. Sin. 2010, 36, 1931–1940. [Google Scholar]
- Liu, C.; Liu, Y.; Guo, K.; Fan, D.; Li, G.; Zheng, Y.; Yu, L.; Yang, R. Effect of drought on pigments, osmotic adjustment and antioxidant enzymes in six woody plant species in karst habitats of southwestern China. Environ. Exp. Bot. 2011, 71, 174–183. [Google Scholar] [CrossRef]
- Yang, W.; Sun, Y.; Chen, S.; Jiang, J.; Chen, F.; Fang, W.; Liu, Z. The effect of exogenously applied nitric oxide on photosynthesis and antioxidant activity in heat stressed chrysanthemum. Biol. Plant. 2011, 55, 737. [Google Scholar] [CrossRef]
- LICHTENTHALER, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents; Biochemical Society Transactions; Portland Press: London, UK, 1983. [Google Scholar]
- Deshmukh, P.S.; Sairam, R.K.; Shukla, D.S. Measurement of ion leakage as a screening technique for drought resistance in wheat genotypes-Short Communication. Indian J. Plant Physiol. 1991, 34, 89–91. [Google Scholar]
- Yin, D.; Chen, S.; Chen, F.; Guan, Z.; Fang, W. Morphological and physiological responses of two chrysanthemum cultivars differing in their tolerance to waterlogging. Environ. Exp. Bot. 2009, 67, 87–93. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Togawa, T.; Dunn, W.A.; Emmons, A.C.; Nagao, J.; Willis, J.H. Developmental expression patterns of cuticular protein genes with the R&R Consensus from Anopheles gambiae. Insect Biochem. Mol. Biol. 2008, 38, 508–519. [Google Scholar] [PubMed] [Green Version]









| Treatments | Control | Drought (D) | Heat (H) | H+D | Kruskal-Wallis Test |
|---|---|---|---|---|---|
| Flavonoids | - | + | + | ++ a | <0.05* |
| Tannins | - | - | + a | + a | <0.05* |
| Phenols | + | + | + | ++ a | <0.05* |
| Saponins | - | + a | - | + a | <0.05* |
| Glycoside | + | + | + | ++ a | <0.05* |
| Alkaloids | + | + | ++ a | ++ a | <0.05* |
| Steroids | + | ++ a | ++ a | ++ a | <0.05* |
| Terpenoids | + | + | ++ a | ++ a | <0.05* |
| Soluble Sugars | - | - | - | - | n.s. |
| Sterols | - | - | - | - | n.s. |
| Gene | Primer | Primer Sequence | Τm (°C) |
|---|---|---|---|
| β-Actin | For Rev | 5′-GGTTCACTTGAAGGGTGGTG-3′ 5′-TGAGGTGTACCTGTCCTCGTT-3′ | 60 |
| Aquaporin1 (SsPIP1-1) | For Rev | 5′-GTTCCTATCCTTGCCCCACT-3′ 5′-AGGCGTGATCCCTGTTGTAG-3′ | 60 |
| HSP 70 | For Rev | 5′-CAGATGAGGCCGTGGCTTAT-3′ 5′-GGGAGTCACATCCAACAGCAA-3′ | 60 |
| Osmotin-34 | For Rev | 5′-GAACGGAGGGTGTCACAAAATC-3′ 5′-CGTAGTGGGTCCACAAGTTCCT-3′ | 60 |
| LEA-1 | For Rev | 5′-CAGCGAAGTTTGGATGGAATG-3′ 5′-ACCTGTCGCCAATCAGAAGAT-3′ | 60 |
| DHN1 | For Rev | 5′-GAGGAGGAGGGAGATGACGAAGAC-3′ 5′-GAGGAGGAGGGAGATGACGAAGAC-3′ | 60 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhaithloul, H.A.S. Impact of Combined Heat and Drought Stress on the Potential Growth Responses of the Desert Grass Artemisia sieberi alba: Relation to Biochemical and Molecular Adaptation. Plants 2019, 8, 416. https://doi.org/10.3390/plants8100416
Alhaithloul HAS. Impact of Combined Heat and Drought Stress on the Potential Growth Responses of the Desert Grass Artemisia sieberi alba: Relation to Biochemical and Molecular Adaptation. Plants. 2019; 8(10):416. https://doi.org/10.3390/plants8100416
Chicago/Turabian StyleAlhaithloul, Haifa Abdulaziz S. 2019. "Impact of Combined Heat and Drought Stress on the Potential Growth Responses of the Desert Grass Artemisia sieberi alba: Relation to Biochemical and Molecular Adaptation" Plants 8, no. 10: 416. https://doi.org/10.3390/plants8100416
APA StyleAlhaithloul, H. A. S. (2019). Impact of Combined Heat and Drought Stress on the Potential Growth Responses of the Desert Grass Artemisia sieberi alba: Relation to Biochemical and Molecular Adaptation. Plants, 8(10), 416. https://doi.org/10.3390/plants8100416




