Two Wheat Cultivars with Contrasting Post-Embryonic Root Biomass Differ in Shoot Re-Growth after Defoliation: Implications for Breeding Grazing Resilient Forages
Abstract
:1. Introduction
2. Results
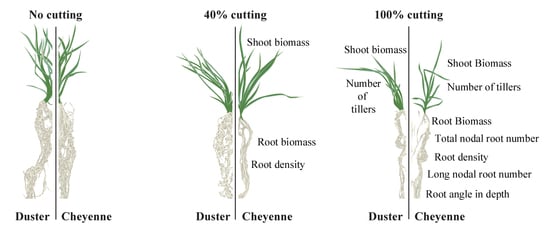
2.1. Two Wheat Cultivars with Contrasting Forage Productivity in the Field Presented Differences in Root Features and Shoot Biomass in Controlled Greenhouse Conditions
2.2. Maximum Rooting Depth and Seminal Root Production Were not Affected by Defoliation
2.3. Cheyenne and Duster Exhibited Differences in Root Biomass, Root Density, Total Nodal Root Number, and Number of Long Nodal Roots in Response to Defoliation
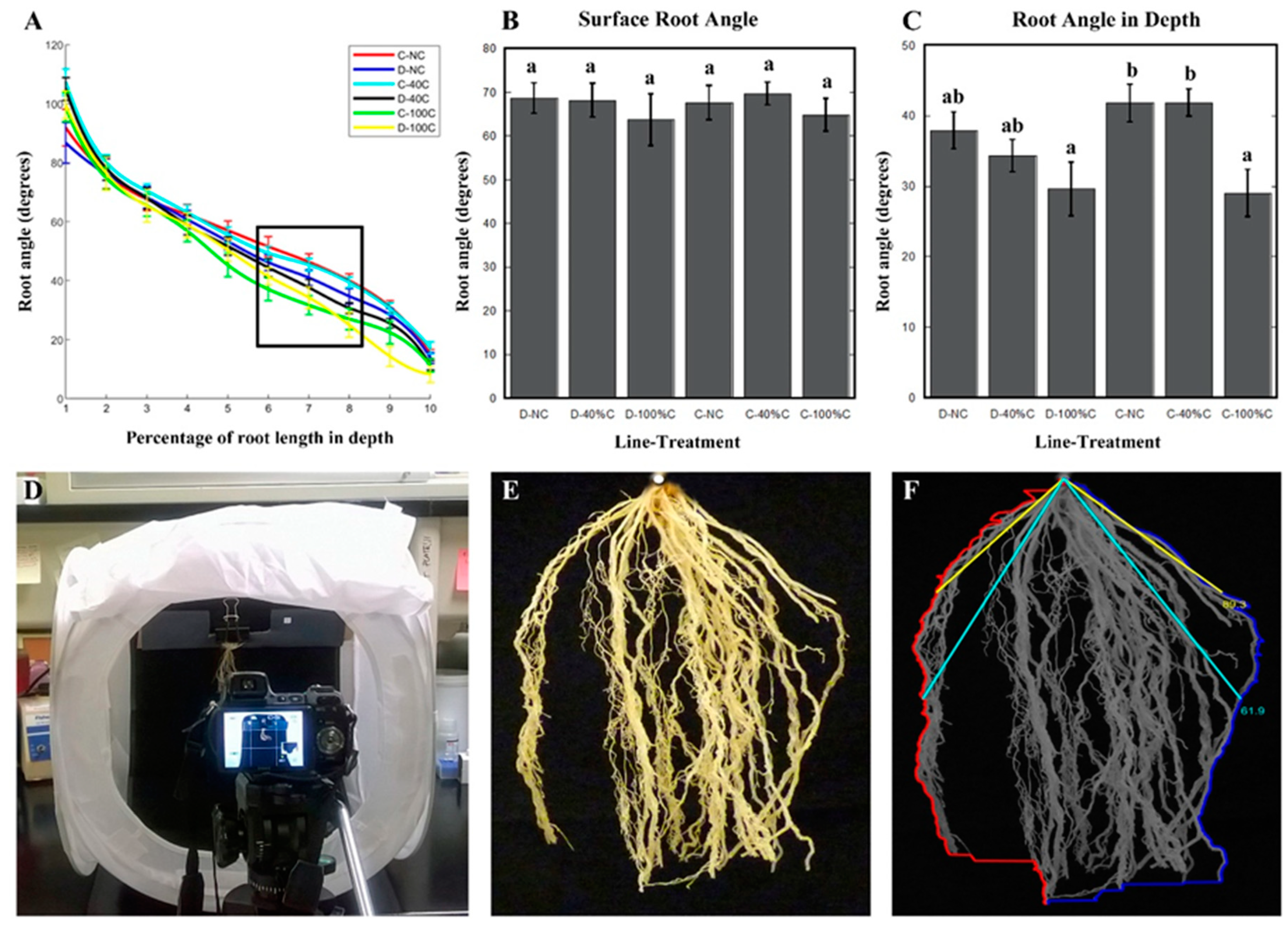
2.4. Root Angle at Depth Was Affected by Defoliation in Cheyenne, While Root Angle Close to Soil Surface Was not Influenced by Defoliation Treatment or Genotype
2.5. Cheyenne Showed Lower Re-Growth Vigor after Shoot Removal Compared to Duster
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Shoot and Root Phenotyping Methodology
4.3. Computing Software for Root Density and Root Angle Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kemp, D.R.; Culvenor, R.A. Improving the grazing and drought tolerance of temperate perennial grasses. N. Z. J. Agric. Res. 1994, 37, 365–378. [Google Scholar] [CrossRef]
- Herbel, C.H.; Pieper, R. Grazing management. In Semiarid Lands and Deserts: Soil Resources and Reclamation; Skujin, J., Ed.; Marcel Deccer Inc.: New York, NY, USA, 1991; pp. 361–385. [Google Scholar]
- Peterson, R.A. Factors Affecting Resistance to Heavy Grazing in Needle-and-Thread Grass. J. Range Manag. 1962, 15, 183–189. [Google Scholar] [CrossRef]
- Winter, S.R.; Thompson, E.K. Grazing Winter Wheat: I. Response of Semidwarf Cultivars to Grain and Grazed Production Systems. Agron. J. 1990, 82, 33–37. [Google Scholar] [CrossRef]
- Brougham, R.; Harris, W. Rapidity and extent of changes in genotypic structure induced by grazing in a ryegrass population. N. Z. J. Agric. Res. 1967, 10, 56–65. [Google Scholar] [CrossRef]
- Holliday, R. Fodder Production from Winter-Sown Cereals and Its Effect upon Grain Yield. F. Abs. 1956, 9, 129–135, 207–213. [Google Scholar]
- Redmon, L.A.; Horn, G.W.; Krenzer, E.G.; Bernardo, D.J. A review of livestock grazing and wheat grain yield: Boom or bust? Agron. J. 1995, 87, 137–147. [Google Scholar] [CrossRef]
- Virgona, J.; Gummer, F.; Angus, J. Effects of grazing on wheat growth, yield, development, water use, and nitrogen use. Aust. J. Agric. Res. 2006, 57, 1307–1319. [Google Scholar] [CrossRef]
- Jones, G.B.; Alpuerto, J.B.; Tracy, B.F.; Fukao, T. Physiological Effect of Cutting Height and High Temperature on Regrowth Vigor in Orchardgrass. Front. Plant Sci. 2017, 8, 805. [Google Scholar] [CrossRef]
- Pang, R.; Sun, Y.; Xu, X.; Song, M.; Ouyang, H. Effects of clipping and shading on 15NO3− and 15NH4+ recovery by plants in grazed and ungrazed temperate grasslands. Plant Soil 2018, 433, 339–352. [Google Scholar] [CrossRef]
- Benot, M.-L.; Morvan-Bertrand, A.; Mony, C.; Huet, J.; Sulmon, C.; Decau, M.-L.; Prud’homme, M.-P.; Bonis, A. Grazing intensity modulates carbohydrate storage pattern in five grass species from temperate grasslands. Acta Oecol. 2019, 95, 108–115. [Google Scholar] [CrossRef]
- Biswell, H.H.; Weaver, J.E. Effect of Frequent Clipping on the Development of Roots and Tops of Grasses in Prairie Sod. Ecology 1933, 14, 368–390. [Google Scholar] [CrossRef] [Green Version]
- Harrison, C.; Hodgson, C. Response of certain perennial grasses to cutting treatments. J. Am. Soc. Agron. 1939, 31, 418–430. [Google Scholar] [CrossRef]
- Fitts, O. A preliminary study of the root growth of fine grasses under turf conditions. Bull. Green Sect. U. S. Golf Assoc. 1925, 5, 58–62. [Google Scholar]
- Dittmer, H.J. Clipping Effects on Bermuda Grass Biomass. Ecology 1973, 54, 217–219. [Google Scholar] [CrossRef]
- Crider, F.J. Root growth stoppage following clipping. US Dep. Agric. Tech. Bull. 1955, 1102, 23. [Google Scholar]
- Parker, K.W.; Sampson, A.W. Influence of leafage removal on anatomical structure of roots of Stipa pulchra and Bromus hordeaceus. Plant Physiol. 1930, 5, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol. 2019, 223, 548–564. [Google Scholar] [CrossRef]
- Chochois, V.; Vogel, J.P.; Watt, M. Application of Brachypodium to the genetic improvement of wheat roots. J. Exp. Bot. 2012, 63, 3467–3474. [Google Scholar] [CrossRef] [Green Version]
- Watt, M.; Schneebeli, K.; Dong, P.; Wilson, I.W. The shoot and root growth of Brachypodium and its potential as a model for wheat and other cereal crops. Funct. Plant Biol. 2009, 36, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Manschadi, A.; Manske, G.; Vlek, P. Root architecture and resource acquisition: Wheat as a model plant. In Plant Roots: The Hidden Half, 4th ed.; CRC Press: London, UK, 2013; pp. 22.1–22.18. [Google Scholar]
- Kim, K.-S.; Anderson, J.D.; Newell, M.A.; Grogan, S.M.; Byrne, P.F.; Baenziger, P.S.; Butler, T.J. Genetic Diversity of Great Plains Hard Winter Wheat Germplasm for Forage. Crop Sci. 2016, 56, 2297–2305. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-S.; Anderson, J.D. Forage yield and nutritive value of winter wheat varieties in the southern Great Plains. Euphytica 2015, 202, 445–457. [Google Scholar] [CrossRef]
- Edwards, J.T.; Hunger, R.M.; Smith, E.L.; Horn, G.W.; Chen, M.-S.; Yan, L.; Bai, G.; Bowden, R.L.; Klatt, A.; Rayas-Duarte, P. ‘Duster’ wheat: A durable, dual-purpose cultivar adapted to the southern Great Plains of the USA. J. Plant Regist. 2012, 6, 37–48. [Google Scholar] [CrossRef]
- Clark, J.A. Registration of Improved Wheat Varieties, VI 1. Agron. J. 1931, 23, 1010–1012. [Google Scholar] [CrossRef]
- Higaki, T.; Kutsuna, N.; Sano, T.; Kondo, N.; Hasezawa, S. Quantification and cluster analysis of actin cytoskeletal structures in plant cells: Role of actin bundling in stomatal movement during diurnal cycles in Arabidopsis guard cells. Plant J. 2010, 61, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Dyachok, J.; Sparks, J.A.; Liao, F.; Wang, Y.S.; Blancaflor, E.B. Fluorescent protein-based reporters of the actin cytoskeleton in living plant cells: Fluorophore variant, actin binding domain, and promoter considerations. Cytoskeleton 2014, 71, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P. Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. 2013, 112, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097. [Google Scholar] [CrossRef]
- Das, A.; Schneider, H.; Burridge, J.; Ascanio, A.K.M.; Wojciechowski, T.; Topp, C.N.; Lynch, J.P.; Weitz, J.S.; Bucksch, A. Digital imaging of root traits (DIRT): A high-throughput computing and collaboration platform for field-based root phenomics. Plant Methods 2015, 11, 51. [Google Scholar] [CrossRef]
- Pound, M.P.; French, A.P.; Atkinson, J.A.; Wells, D.M.; Bennett, M.J.; Pridmore, T. RootNav: Navigating Images of Complex Root Architectures. Plant Physiol. 2013, 162, 1802–1814. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, P.; Kirkegaard, J.; Lilley, J.; Gregory, P.; Rebetzke, G. A tillering inhibition gene influences root–shoot carbon partitioning and pattern of water use to improve wheat productivity in rainfed environments. J. Exp. Bot. 2015, 67, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Christopher, J.; Christopher, M.; Jennings, R.; Jones, S.; Fletcher, S.; Borrell, A.; Manschadi, A.M.; Jordan, D.; Mace, E.; Hammer, G. QTL for root angle and number in a population developed from bread wheats (Triticum aestivum) with contrasting adaptation to water-limited environments. Theor. Appl. Genet. 2013, 126, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, J.; Yee, M.-C.; Goudinho Viana, W.; Rellán-Álvarez, R.; Feldman, M.; Priest, H.D.; Trontin, C.; Lee, T.; Jiang, H.; Baxter, I.; et al. Grasses suppress shoot-borne roots to conserve water during drought. Proc. Natl. Acad. Sci. USA 2016, 113, 8861–8866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futuyma, D.J.; Philippi, T.E. Genetic variation and covariation in responses to host plants by Alsophila pometaria (Lepidoptera: Geometridae). Evolution 1987, 41, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Weis, A.E.; Simms, E.L.; Hochberg, M.E. Will plant vigor and tolerance be genetically correlated? Effects of intrinsic growth rate and self-limitation on regrowth. Evol. Ecol. 2000, 14, 331–352. [Google Scholar] [CrossRef]
- Wasson, A.; Rebetzke, G.; Kirkegaard, J.; Christopher, J.; Richards, R.; Watt, M. Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. J. Exp. Bot. 2014, 65, 6231–6249. [Google Scholar] [CrossRef] [Green Version]
- Meister, R.; Rajani, M.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef]
- Rogers, E.D.; Benfey, P.N. Regulation of plant root system architecture: Implications for crop advancement. Curr. Opin. Biotechnol. 2015, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, J.; Krishnamurthy, L.; Upadhyaya, H.D.; Krishna, H.; Chandra, S.; Vadez, V.; Serraj, R. Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.). Euphytica 2005, 146, 213–222. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]




| TRAIT | UNITS |
|---|---|
| Number of tillers | n/a |
| Shoot biomass | Grams (g) |
| Root biomass | Grams (g) |
| Maximum rooting depth | Centimeters (cm) |
| Number of nodal roots | n/a |
| Number of seminal roots | n/a |
| Surface Root Angle | Degrees |
| Root Angle in depth | Degrees |
| Root Density | Pixels/millimeter square (px/mm2) |
| Trait | Cultivar | Treatment | Cultivar * Treatment |
|---|---|---|---|
| Number of tillers | *** | *** | ns |
| Shoot biomass | *** | *** | ** |
| Root biomass | *** | *** | *** |
| Maximum rooting depth | ns | ns | ns |
| Number of nodal roots | *** | *** | ns |
| Number of long nodal roots | * | *** | ns |
| Number of seminal roots | ns | ns | ns |
| Surface Root Angle | ns | ns | ns |
| Root Angle in depth | ns | ** | ns |
| Root Density | *** | *** | ns |
| ROOT TRAIT | Maximum Rooting Depth (cm) | Number of Seminal Roots |
|---|---|---|
| Average ± SEM | Average ± SEM | |
| Duster NO clipping | 100.1 ± 5.2 | 5.7 ± 0.3 |
| Duster 40% clipping | 106.7 ± 5.8 | 5.8 ± 0.3 |
| Duster 100% clipping | 105.4 ± 8.0 | 5.9 ± 0.3 |
| Cheyenne NO clipping | 98.2 ± 3.8 | 5.99 ± 0.2 |
| Cheyenne 40% clipping | 94.2 ± 4.0 | 5.4 ± 0.2 |
| Cheyenne 100% clipping | 96.8 ± 4.3 | 5.7 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paez-Garcia, A.; Liao, F.; Blancaflor, E.B. Two Wheat Cultivars with Contrasting Post-Embryonic Root Biomass Differ in Shoot Re-Growth after Defoliation: Implications for Breeding Grazing Resilient Forages. Plants 2019, 8, 470. https://doi.org/10.3390/plants8110470
Paez-Garcia A, Liao F, Blancaflor EB. Two Wheat Cultivars with Contrasting Post-Embryonic Root Biomass Differ in Shoot Re-Growth after Defoliation: Implications for Breeding Grazing Resilient Forages. Plants. 2019; 8(11):470. https://doi.org/10.3390/plants8110470
Chicago/Turabian StylePaez-Garcia, Ana, Fuqi Liao, and Elison B. Blancaflor. 2019. "Two Wheat Cultivars with Contrasting Post-Embryonic Root Biomass Differ in Shoot Re-Growth after Defoliation: Implications for Breeding Grazing Resilient Forages" Plants 8, no. 11: 470. https://doi.org/10.3390/plants8110470
APA StylePaez-Garcia, A., Liao, F., & Blancaflor, E. B. (2019). Two Wheat Cultivars with Contrasting Post-Embryonic Root Biomass Differ in Shoot Re-Growth after Defoliation: Implications for Breeding Grazing Resilient Forages. Plants, 8(11), 470. https://doi.org/10.3390/plants8110470