Effect of Elevated CO2 Concentration on the Disease Severity of Compatible and Incompatible Interactions of Brassica napus–Leptosphaeria maculans Pathosystem
Abstract
:1. Introduction
2. Results
2.1. Inferred Phenotyping
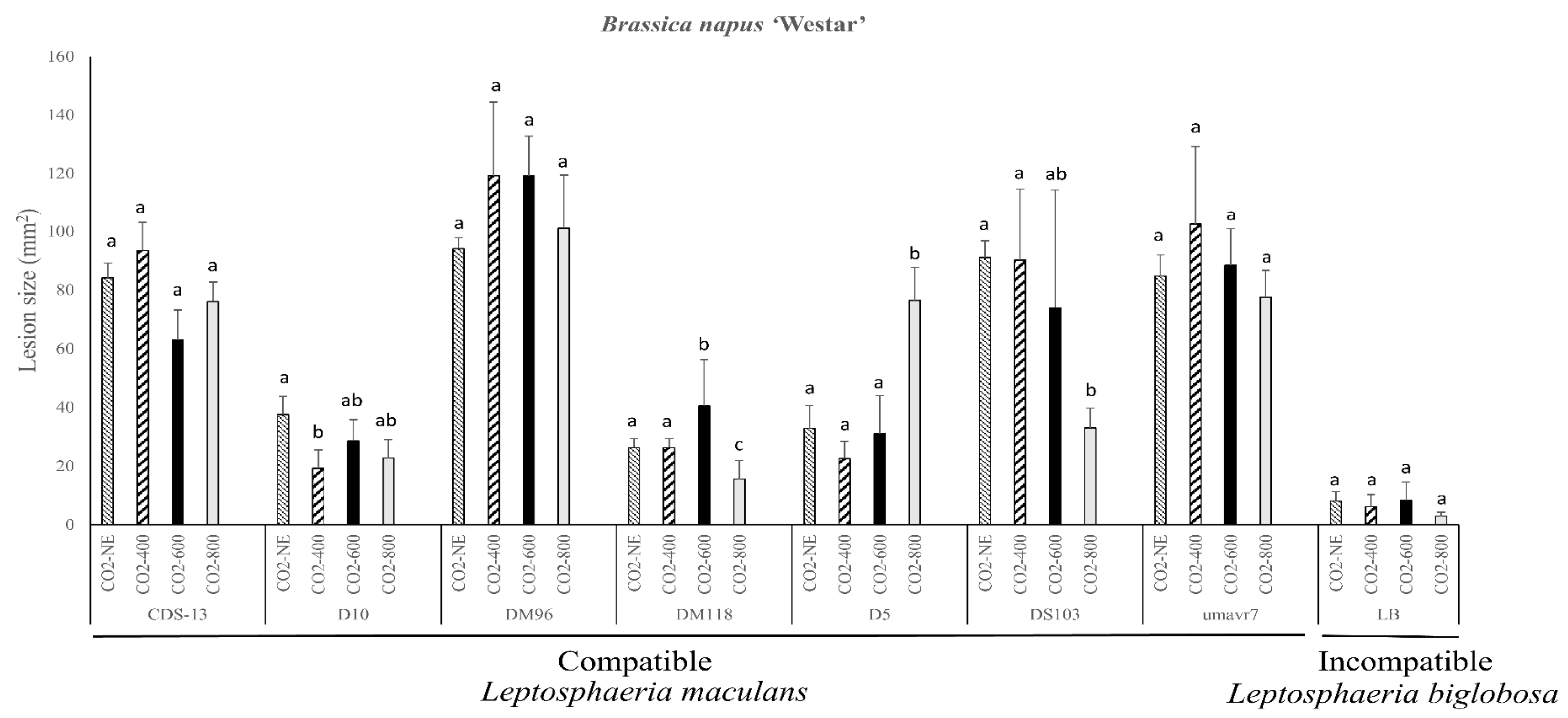
2.2. Pathogenicity Evaluation of the Susceptible B. napus Variety Westar
2.3. Pathogenicity Evaluation of Compatible and Incompatible Interactions between Leptosphaeria spp. and B. napus
2.4. Pathogenicity Evaluation of Compatible and Incompatible Interactions on Different Brassica napus Varieties and Lines Caused by Leptosphaeria maculans
3. Discussion
4. Materials and Methods
4.1. Brassica napus Varieties and Lines and Leptosphaeria maculans Isolates
4.2. Plant Cotyledon Inoculation and CO2 Treatment
4.3. Disease Rating and Lesion Size Measurement
4.4. Statistical Methodology
Author Contributions
Funding
Conflicts of Interest
References
- Kerr, R.A. Global warming is changing the world. Science 2007, 316, 188–190. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007; 104p. [Google Scholar]
- Meehl, G.A.; Washington, W.M.; Collins, W.D.; Arblaster, J.M.; Hu, A.X.; Buja, L.E.; Strand, W.G.; Teng, H.Y. How much more global warming and sea level rise? Science 2005, 307, 1769–1772. [Google Scholar] [CrossRef] [PubMed]
- Tubiello, F.N.; Donatelli, M.; Rosenzweig, C.; Stockle, C.O. Effects of climate change and elevated CO2 on cropping systems: Model predictions at two Italian locations. Eur. J. Agron. 2000, 13, 179–189. [Google Scholar] [CrossRef]
- Soares, A.S.; Driscoll, S.P.; Olmos, E.; Harbinson, J.; Arrabaca, M.C.; Foyer, C.H. Adaxial/abaxial specification in the regulation of photosynthesis and stomatal opening with respect to light orientation and growth with CO2 enrichment in the C4 species Paspalum dilatatum. New Phytol. 2008, 177, 186–198. [Google Scholar] [PubMed]
- Drake, B.G.; Gonzalez Meler, M.A.; Long, S.P. More efficient plants: A consequence of rising atmospheric CO2? Ann. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 609–639. [Google Scholar] [CrossRef]
- Saxe, H.; Ellsworth, D.S.; Heath, J. Tree and forest functioning in an enriched CO2 atmosphere. New Phytol. 1998, 139, 395–436. [Google Scholar] [CrossRef]
- Faralli, M.; Grove, I.G.; Hare, M.C.; Kettlewell, P.S.; Fiorani, F. Rising CO2 from historical concentrations enhances the physiological performance of Brassica napus seedlings under optimal water supply but not under reduced water availability. Plant Cell Environ. 2017, 40, 317–325. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Ahammed, G.J.; Li, Z.; Wei, J.; Shen, C.; Yan, P.; Zhang, L.; Han, W. Stimulation in primary and secondary metabolism by elevated carbon dioxide alters green tea quality in Camellia sinensis L. Sci. Rep. 2017, 7, 7937. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef]
- Amthor, J.S. Terrestrial higher-plant response to increasing atmospheric CO2 in relation to the global carbon cycle. Glob. Chang. Biol. 1995, 1, 243–274. [Google Scholar] [CrossRef]
- Scholthof, K.B.G. The disease triangle: Pathogens, the environment society. Nat. Rev. Microbiol. 2007, 5, 152–156. [Google Scholar] [CrossRef] [PubMed]
- McElrone, A.J.; Reid, C.D.; Hoye, K.A.; Hart, E.; Jackson, R.B. Elevated CO2 reduces disease incidence and severity of a red maple fungal pathogen vis changes in host physiology and leaf chemistry. Glob. Chang. Biol. 2005, 11, 1828–1836. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Sun, Z.; Shao, S.; Hu, L.; Ye, M.; Zhou, Y.; Xia, X.; Yu, J.; Shi, K. Antagonism between phytohormone signaling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J. Exp. Bot. 2015, 66, 1951–1963. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Chitarra, W.; Pugliese, M.; Gilardi, G.; Gullino, L.M.; Garibaldi, A. Effect of elevated atmospheric CO2 and temperature on disease severity of Fusarium oxysporum f. sp. lactucae on lettuce plants. Appl. Soil Ecol. 2013, 72, 1–6. [Google Scholar] [CrossRef]
- McElrone, A.J.; Hamilton, J.G.; Krafnick, A.J.; Aldea, M.; Knepp, R.G.; DeLucia, E.H. Combined effects of elevated CO2 and natural climatic variation on leaf spot diseases of redbud and sweetgum trees. Environ. Pollut. 2010, 158, 108–114. [Google Scholar] [CrossRef]
- Mitchell, C.E.; Reich, P.B.; Tilman, D.; Groth, J.V. Effects of elevated CO2, nitrogen deposition, and decreased species diversity on foliar fungal plant disease. Glob. Chang. Biol. 2003, 9, 438–451. [Google Scholar] [CrossRef]
- Chitarra, W.; Siciliano, I.; Ferrocino, I.; Gullino, M.L.; Garibaldi, A. Effect of elevated atmospheric CO2 and temperature on disease severity of rocket plants caused by Fusarium wilt under phytotron conditions. PLoS ONE 2015, 10, e0140769. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Brun, H.; Barbetti, M.J.; Rimmer, S.R. World-wide importance of Phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 3–15. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Zhang, X.; Selin, C.; Zou, Z.; Liban, S.H.; McLaren, D.L.; Kubinec, A.; Parks, P.S.; Rashid, M.H.; Padmathilake, K.R.; et al. A six-year investigation of the dynamics of Avirulence allele profiles, blackleg incidence, and mating type alleles of Leptosphaeria maculans population associated with canola crops in Manitoba, Canada. Plant Dis. 2016, 102, 4. [Google Scholar] [CrossRef]
- Kutcher, H.R.; Balesdent, M.H.; Rimmer, S.R.; Rouxel, T.; Chèvre, A.M.; Delourme, R.; Brun, H. Frequency of avirulence genes in Leptosphaeria maculans in western Canada. Can. J. Plant Pathol. 2010, 32, 77–85. [Google Scholar] [CrossRef]
- Ansan-Melayah, D.; Balesden, M.H.; Delourme, R.; Pilet, M.L.; Renard, M.; Tanguy, X.; Renard, M.; Rouxel, T. Genes for race specific resistance against blackleg disease in Brassica napus L. Plant Breed. 1998, 117, 373–378. [Google Scholar] [CrossRef]
- Balesdent, M.H.; Attard, A.; Kuhn, M.L.; Rouxel, T. New avirulence genes in the phytopathogenic fungus Leptosphaeria maculans. Phytopathology 2002, 92, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Gout, L.; Fudal, I.; Kuhn, M.L.; Blaise, F.; Eckert, M.; Cattolico, L.; Balesdent, M.H.; Rouxel, T. Lost in the middle of nowhere: The AvrLm1 avirulence gene of the Dothideomycete Leptosphaeria maculans. Mol. Microbiol. 2006, 60, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.; McLanders, J.; Campbell, E.; Edwards, D.; Batley, J. Genomic advances will herald new insights into the Brassica: Leptosphaeria maculans pathosystem. Plant Biol. 2012, 14, S1–S10. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Lowe, R.G.T.; Elliott, C.E.; Dubois, D.J.; Howlett, B.J. An avirulence gene, AvrLmJ1, from the blackleg fungus, Leptosphaeria maculans, confers avirulence to Brassica juncea varieties. Mol. Plant Pathol. 2014, 15, 523–530. [Google Scholar] [CrossRef]
- Ghanbarnia, K.; Fudal, I.; Larkan, N.J.; Links, M.G.; Balesdent, M.H.; Profotova, B.; Fernando, W.G.D.; Borhan, M.H. Rapid identification of the Leptosphaeria maculans avirulence gene AvrLm2 using an intraspecific comparative genomics approach. Mol. Plant Pathol. 2015, 16, 699–709. [Google Scholar] [CrossRef]
- Plissonneau, C.; Daverdin, G.; Ollivier, B.; Blaise, F.; Degrave, A.; Fudal, I.; Rouxel, T.; Balesdent, M.H. A game of hide and seek between avirulence genes AvrLm4-7 and AvrLm3 in Leptosphaeria maculans. New Phytol. 2016, 209, 1613–1624. [Google Scholar] [CrossRef]
- Rimmer, S.R. Resistance genes to Leptosphaeria maculans in Brassica napus. Can. J. Plant Pathol. 2006, 28, S288–S297. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Marcroft, S.J.; Barbetti, M.J.; Hua, L.; Salisbury, P.A.; Gout, L.; Rouxel, T.; Howlett, B.J.; Balesdent, M.H. Dual control of avirulence in Leptosphaeria maculans towards a Brassica napus cultivar with “sylvestris-derived” resistance suggests involvement of two resistance genes. Plant Pathol. 2009, 58, 305–313. [Google Scholar] [CrossRef]
- Eber, F.; Lourgant, K.; Brun, H. Analysis of Brassica nigra chromosomes allows identification of a new effective Leptosphaeria maculans resistance gene introgressed in Brassica napus. In Proceedings of the 13th International Rapeseed Congress, Prague, Czech Republic, 5–9 June 2011; pp. 5–9. [Google Scholar]
- Long, Y.; Wang, Z.; Sun, Z.; Fernando, W.G.D.; McVetty, B.E.P.; Li, G. Identification of two blackleg resistance genes and fine mapping of one of these two genes in a Brassica napus canola variety ‘Surpass 400’. Theor. Appl. Genet. 2011, 122, 1223–1231. [Google Scholar] [CrossRef]
- Balesdent, M.H.; Fudal, I.; Ollivier, B.; Bally, P.; Grandaubert, J.; Eber, F.; Chèvre, A.M.; Leflon, M.; Rouxel, T. The dispensable chromosome of Leptosphaeria maculans shelters an effector gene conferring avirulence towards Brassica rapa. New Phytol. 2013, 198, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.; Peng, G. Quantitative resistance against an isolate of Leptosphaeria maculans (blackleg) in selected Canadian canola cultivars remains effective under increased temperatures. Plant Pathol. 2018, 67, 1329–1338. [Google Scholar] [CrossRef]
- Kimball, B.A.; Kobayashi, K.; Bindi, M. Responses of agricultural crops to free-air CO2 enrichment. Adv. Agron. 2002, 77, 293–368. [Google Scholar]
- West, J.S.; Townsend, J.A.; Stevens, M.; Fitt, B.D.L. Comparative biology of different plant pathogens to estimate effects of climate change on crop diseases in Europe. Eur. J. Plant Pathol. 2012, 133, 315–331. [Google Scholar] [CrossRef] [Green Version]
- Pongam, P.; Osborn, T.C.; Williams, P.H. Genetic analysis and identification of amplified fragment length polymorphism markers linked to the alm1 avirulence gene of Leptosphaeria maculans. Phytopathology 1998, 88, 1068–1072. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, G.; Kutcher, H.R.; Balesdent, M.H.; Delourme, R.; Fernando, W.G.D. Breakdown of Rlm3 resistance in the Brassica napus–Leptosphaeria maculans pathosystem in western Canada. Eur. J. Plant Pathol. 2016, 145, 659–674. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, G.; Parks, P.; Hu, B.; Li, Q.; Jiang, L.; Niu, Y.; Huang, S.; Fernando, W.G.D. Identifying seedling and adult plant resistance of Chinese Brassica napus germplasm to Leptosphaeria maculans. Plant Pathol. 2017, 66, 752–762. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Pangga, I.B.; Chakraborty, S.; Yates, D. Canopy size and induced resistance in Stylosanthes scabra determine anthracnose severity at high CO2. Phytopathology 2004, 94, 221–227. [Google Scholar] [CrossRef]
- Eastburn, D.; Degennaro, M.; DeLucia, E.H.; Dermody, O.; McElrone, A.J. Elevated atmospheric CO2 and ozone alter soybean diseases at SoyFACE. Glob. Chang. Biol. 2010, 16, 320–330. [Google Scholar] [CrossRef]
- Thompson, G.B.; Brown, J.K.M.; Woodward, F.I. The effects of host carbon dioxide, nitrogen and water supply on the infection of wheat by powdery mildew and aphids. Plant Cell Environ. 1993, 16, 687–694. [Google Scholar] [CrossRef]
- Thompson, G.B.; Drake, B.G. Insects and fungi on a C3 sedge and a C4 grass exposed to elevated atmospheric CO2 concentrations in open-top chambers in the field. Plant Cell Environ. 1994, 17, 1161–1167. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ishiguro, K.; Nakajima, T.; Kim, H.Y.; Okada, M.; Kobayashi, K. Effects of elevated atmospheric CO2 concentration on the infection of rice blast and sheath blight. Phytopathology 2006, 96, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Tiedemann, A.V.; Teng, P.S. Climate change: Potential impact on plant diseases. Environ. Pollut. 2000, 108, 317–326. [Google Scholar] [CrossRef]
- Coakley, S.M.; Scherm, H.; Chakraborty, S. Climate change and plant disease management. Ann. Rev. Phytopathol. 1999, 37, 399–426. [Google Scholar] [CrossRef]
- Hibberd, J.M.; Whitbread, R.; Farrar, J.F. Effect of elevated concentrations of CO2 on infection of barley by Erysiphe graminis. Physiol. Mol. Plant Pathol. 1996, 48, 37–53. [Google Scholar] [CrossRef]
- Williams, P.H.; Delwiche, P.A. Screening for resistance to blackleg of crucifers in the seedling stage. In Proceedings of the A Eucarpia-Conference on the Breeding of Cruciferous Crops, Wageningen, The Netherlands, 1–3 October 1979; pp. 164–170. [Google Scholar]


| Disease Rating | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| B. napus Varieties and Lines | CO2 | CDS-13 d | D5 d | D10 d | DM96 d | DM118 d | DS103 d | umavr7 d | LB e |
| Westar/No R gene a | NE b | 7.25 (S) c | 7.33 (S) | 7.50 (S) | 8.00 (S) | 7.00 (S) | 9.00 (S) | 8.33 (S) | 3.50 (R) |
| 400 | 8.33 (S) | 7.50 (S) | 9.00 (S) | 7.25 (S) | 8.33 (S) | 7.55 (S) | 9.00 (S) | 2.67 (R) | |
| 600 | 8.00 (S) | 8.00 (S) | 8.33 (S) | 7.00 (S) | 9.00 (S) | 8.00 (S) | 7.50 (S) | 3.33 (R) | |
| 800 | 7.50 (S) | 8.33 (S) | 6.25 (S) | 6.83 (S) | 6.15 (S) | 7.00 (S) | 6.83 (S) | 2.33 (R) | |
| 1065/LepR1 a | NE | 8.00 (S) | 4.25 (R) | 7.87 (S) | 3.33 (R) | 5.85 (IR) | 7.50 (S) | 8.33 (S) | 4.87 (IR) |
| 400 | 8.33 (S) | 6.00 (S) | 7.50 (S) | 3.00 (R) | 5.25 (IR) | 6.83 (S) | 5.25 (IR) | 4.50 (R) | |
| 600 | 7.50 (S) | 4.60 (R) | 7.50 (S) | 2.87 (R) | 6.00 (S) | 7.15 (S) | 6.67 (S) | 3.87 (R) | |
| 800 | 7.50 (S) | 5.00 (IR) | 6.67 (S) | 2.87 (R) | 5.00 (IR) | 5.25 (IR) | 8.00 (S) | 3.00 (R) | |
| 1135/LepR2 a | NE | 8.33 (S) | 6.67 (S) | 7.00 (S) | 8.13 (S) | 7.00 (S) | 8.33 (S) | 7.50 (S) | 5.25 (IR) |
| 400 | 7.50 (S) | 6.50 (S) | 6.50 (S) | 7.50 (S) | 6.83 (S) | 9.00 (S) | 8.00 (S) | 4.50 (R) | |
| 600 | 6.67 (S) | 7.00 (S) | 6.67 (S) | 7.87 (S) | 8.33 (S) | 8.00 (S) | 6.87 (S) | 5.00 (IR) | |
| 800 | 7.00 (S) | 6.50 (S) | 5.65 (IR) | 6.87 (S) | 7.00 (S) | 6.25 (S) | 7.33 (S) | 3.87 (R) | |
| 02-22-2-1/Rlm3 a | NE | 8.33 (S) | 7.50 (S) | 6.67 (S) | 4.00 (R) | 2.67 (R) | 8.33 (S) | 8.33 (S) | 5.00 (IR) |
| 400 | 8.00 (S) | 6.00 (S) | 7.33 (S) | 2.33 (R) | 1.67 (R) | 7.86 (S) | 7.50 (S) | 4.50 (R) | |
| 600 | 8.00 (S) | 6.67 (S) | 9.00 (S) | 3.67 (R) | 1.33 (R) | 7.50 (S) | 5.75 (IR) | 3.33 (R) | |
| 800 | 6.83 (S) | 6.00 (S) | 5.33 (IR) | 3.00 (R) | 1.33 (R) | 6.83 (S) | 5.00 (IR) | 3.00 (R) | |
| 01-23-2-1/Rlm7 a | NE | 1.33 (R) | 2.13 (R) | 6.50 (S) | 7.50 (S) | 7.25 (S) | 1.50 (R) | 6.25 (S) | 2.67 (R) |
| 400 | 2.67 (R) | 2.50 (R) | 6.67 (S) | 7.13 (S) | 8.00 (S) | 2.33 (R) | 7.00 (S) | 3.00 (R) | |
| 600 | 2.67 (R) | 3.00 (R) | 7.00 (S) | 7.00 (S) | 6.87 (S) | 3.00 (R) | 9.00 (S) | 3.00 (R) | |
| 800 | 2.33 (R) | 1.67 (R) | 6.33 (S) | 6.87 (S) | 6.25 (S) | 2.33 (R) | 8.15 (S) | 3.00 (R) | |
| Jet Neuf/Rlm4 a | NE | 7.50 (S) | 1.67 (R) | 8.15 (S) | 8.15 (S) | 8.25 (S) | 8.33 (S) | 8.33 (S) | 4.00 (R) |
| 400 | 6.87 (S) | 1.33 (R) | 8.00 (S) | 7.50 (S) | 6.87 (S) | 8.00 (S) | 6.67 (S) | 3.50 (R) | |
| 600 | 6.00 (S) | 2.50 (R) | 7.50 (S) | 7.50 (S) | 7.50 (S) | 7.86 (S) | 7.15 (S) | 3.00 (R) | |
| 800 | 6.50 (S) | 2.00 (R) | 6.00 (S) | 7.00 (S) | 7.86 (S) | 6.83 (S) | 7.50 (S) | 2.33 (R) | |
| Goé Land/Rlm9 a | NE | 7.50 (S) | 8.15 (S) | 2.50 (R) | 8.33 (S) | 8.33 (S) | 7.86 (S) | 8.15 (S) | 5.00 (IR) |
| 400 | 7.33 (S) | 7.50 (S) | 3.50 (R) | 6.87 (S) | 7.00 (S) | 7.00 (S) | 7.33 (S) | 4.13 (R) | |
| 600 | 8.33 (S) | 7.00 (S) | 2.13 (R) | 6.50 (S) | 8.33 (S) | 6.83 (S) | 7.00 (S) | 3.50 (R) | |
| 800 | 7.00 (S) | 6.67 (S) | 1.87 (R) | 6.00 (S) | 6.67 (S) | 6.33 (S) | 6.83 (S) | 3.00 (R) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Z.; Liu, F.; Chen, C.; Fernando, W.G.D. Effect of Elevated CO2 Concentration on the Disease Severity of Compatible and Incompatible Interactions of Brassica napus–Leptosphaeria maculans Pathosystem. Plants 2019, 8, 484. https://doi.org/10.3390/plants8110484
Zou Z, Liu F, Chen C, Fernando WGD. Effect of Elevated CO2 Concentration on the Disease Severity of Compatible and Incompatible Interactions of Brassica napus–Leptosphaeria maculans Pathosystem. Plants. 2019; 8(11):484. https://doi.org/10.3390/plants8110484
Chicago/Turabian StyleZou, Zhongwei, Fei Liu, Changqin Chen, and W. G. Dilantha Fernando. 2019. "Effect of Elevated CO2 Concentration on the Disease Severity of Compatible and Incompatible Interactions of Brassica napus–Leptosphaeria maculans Pathosystem" Plants 8, no. 11: 484. https://doi.org/10.3390/plants8110484
APA StyleZou, Z., Liu, F., Chen, C., & Fernando, W. G. D. (2019). Effect of Elevated CO2 Concentration on the Disease Severity of Compatible and Incompatible Interactions of Brassica napus–Leptosphaeria maculans Pathosystem. Plants, 8(11), 484. https://doi.org/10.3390/plants8110484






