Faster Removal of 2-Phosphoglycolate through Photorespiration Improves Abiotic Stress Tolerance of Arabidopsis
Abstract
1. Introduction
2. Results
2.1. PGLP Overexpressors Sustain CO2 Assimilation and Electron Transport at Combined High Light and Temperature
2.2. Faster 2-PG Degradation is Beneficial for Photosynthetic CO2 Assimilation under Water-Limiting Conditions
2.3. High PGLP Activity is Beneficial for Photosynthesis at Increased Growth Temperatures
2.4. Improved 2-PG Degradation Translates to Higher Transitory Starch Stocks under Temperature Stress
2.5. Minor Changes in the Amino and Organic Acid Contents during Temperature Stress
2.6. Expression of Photorespiratory Proteins Increases after High Temperature Exposure in Wild Type but not in PGLP Overexpressor Lines
3. Discussion
4. Material and Methods
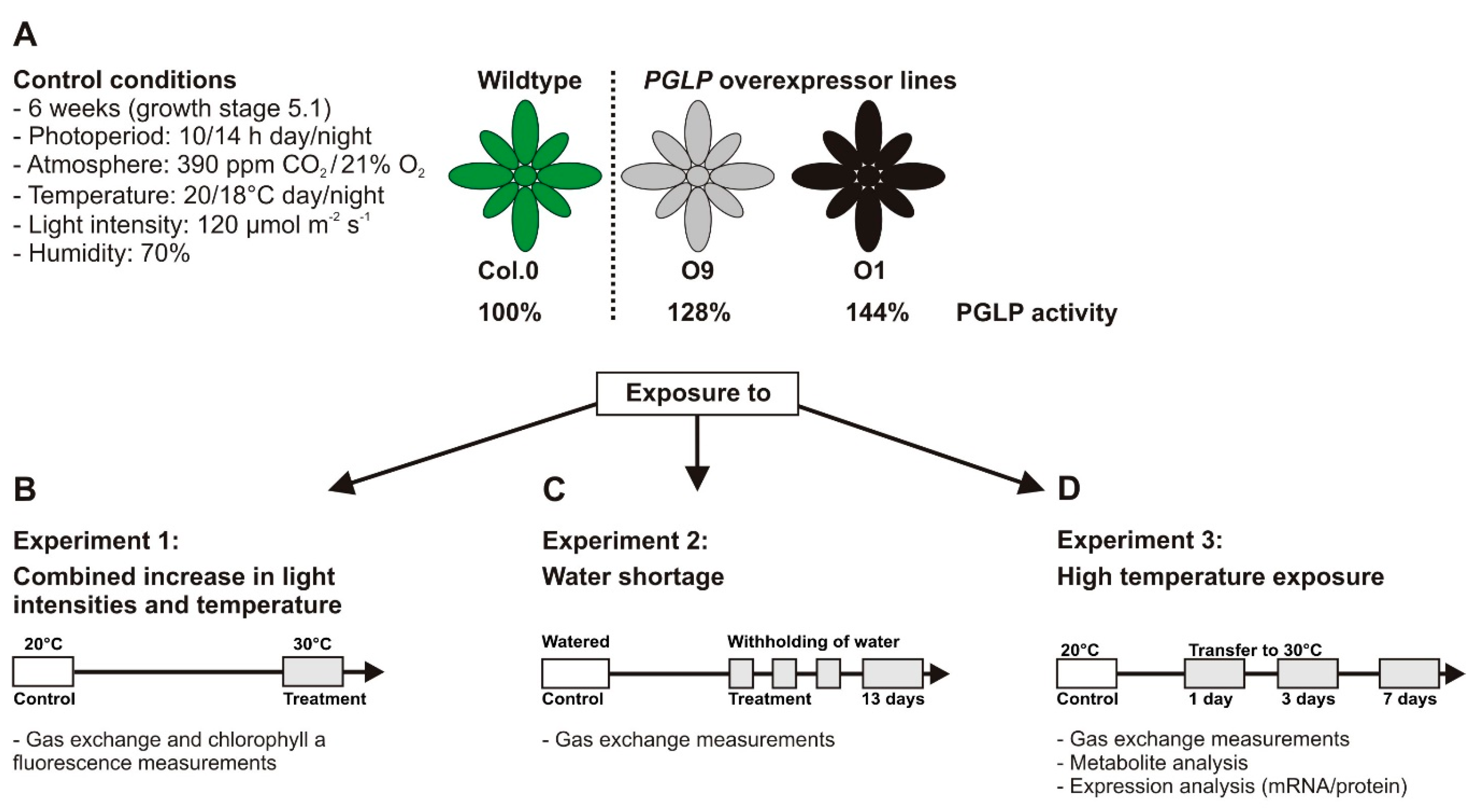
4.1. Plant Material and Standard Growth Conditions
4.2. Stress Conditions
4.3. qRT-PCR Analysis and Immunological Studies
4.4. Determination of Starch and Metabolite Analysis
4.5. Gas Exchange and Chlorophyll a Fluorescence Measurements
4.6. Statistical Analysis
4.7. Accession Numbers
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eisenhut, M.; Ruth, W.; Haimovich, M.; Bauwe, H.; Kaplan, A.; Hagemann, M. The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc. Natl. Acad. Sci. USA 2008, 105, 17199–17204. [Google Scholar] [CrossRef] [PubMed]
- Zelitch, I.; Schultes, N.P.; Peterson, R.B.; Brown, P.; Brutnell, T.P. High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiol. 2009, 149, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bauwe, H.; Hagemann, M.; Kern, R.; Timm, S. Photorespiration has a dual origin and manifold links to central metabolism. Curr. Opin. Plant Biol. 2012, 15, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Timm, S.; Mielewczik, M.; Florian, A.; Frankenbach, S.; Dreissen, A.; Hocken, N.; Fernie, A.R.; Walter, A.; Bauwe, H. High-to-low CO2 acclimation reveals plasticity of the photorespiratory pathway and indicates regulatory links to cellular metabolism of Arabidopsis. PLoS ONE 2012, 7, e42809. [Google Scholar] [CrossRef]
- Rademacher, N.; Kern, R.; Fujiwara, T.; Mettler-Altmann, T.; Miyagishima, S.Y.; Hagemann, M.; Eisenhut, M.; Weber, A.P. Photorespiratory glycolate oxidase is essential for the survival of the red alga Cyanidioschyzon merolae under ambient CO2 conditions. J. Exp. Bot. 2016, 67, 3165–3175. [Google Scholar] [CrossRef]
- Levey, M.; Timm, S.; Mettler-Altmann, T.; Borghi, G.L.; Koczor, M.; Arrivault, S.; Weber, A.P.M.; Bauwe, H.; Gowik, U.; Westhoff, P. Efficient 2-phosphoglycolate degradation is required to maintain carbon assimilation and allocation in the C4 plant Flaveria bidentis. J. Exp. Bot. 2019, 70, 575–587. [Google Scholar] [CrossRef]
- Bowes, G.; Ogren, W.L.; Hageman, R.H. Phosphoglycolate production catazyded by ribulose diphosphate carboxylase. Biochem. Biophys. Res. Commun. 1971, 45, 716–722. [Google Scholar] [CrossRef]
- Anderson, L.E. Chloroplast and cytoplasmic enzymes. 2. Pea leaf triose phosphate isomerases. Biochim. Biophys. Acta 1971, 235, 237–244. [Google Scholar] [CrossRef]
- Kelly, G.J.; Latzko, E. Inhibition of spinach-leaf phosphofructokinase by 2-phosphoglycollate. FEBS Lett. 1976, 68, 55–58. [Google Scholar] [CrossRef]
- Flügel, F.; Timm, S.; Arrivault, S.; Florian, A.; Stitt, M.; Fernie, A.R.; Bauwe, H. The photorespiratory metabolite 2-phosphoglycolate regulates photosynthesis and starch accumulation in Arabidopsis. Plant Cell 2017, 29, 2537–2551. [Google Scholar] [CrossRef]
- Li, J.; Weraduwage, S.M.; Peiser, A.L.; Tietz, S.; Weise, S.E.; Strand, D.D.; Froehlich, J.E.; Kramer, D.M.; Hu, J.; Sharkey, T.D. A Cytosolic Bypass and G6P Shunt in Plants Lacking Peroxisomal Hydroxypyruvate Reductase. Plant Physiol. 2019, 180, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, T.D. Estimating the rate of photorespiration in leaves. Physiol. Plant 1988, 73, 147–152. [Google Scholar] [CrossRef]
- Walker, B.J.; VanLoocke, A.; Bernacchi, C.J.; Ort, D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016, 67, 107–129. [Google Scholar] [CrossRef] [PubMed]
- Slattery, A.R.; Ort, D.R. Carbon assimilation in crops at high temperature. Plant Cell Environ. 2019, 42, 2750–2758. [Google Scholar] [CrossRef]
- Peterhänsel, C.; Maurino, V.G. Photorespiration redesigned. Plant Physiol. 2011, 155, 49–55. [Google Scholar] [CrossRef]
- Xin, C.P.; Tholen, D.; Devloo, V.; Zhu, X.G. The benefits of photorespiratory bypasses: How can they work? Plant Physiol. 2015, 167, 574–585. [Google Scholar] [CrossRef]
- South, P.F.; Cavanagh, A.P.; Lopez-Calcagno, P.E.; Raines, C.A.; Ort, D.R. Optimizing photorespiration for improved crop productivity. J. Integr. Plant Biol. 2018, 60, 1217–1230. [Google Scholar] [CrossRef]
- Timm, S.; Florian, A.; Arrivault, S.; Stitt, M.; Fernie, A.R.; Bauwe, H. Glycine decarboxylase controls photosynthesis and plant growth. FEBS Lett. 2012, 586, 3692–3697. [Google Scholar] [CrossRef]
- Timm, S.; Wittmiß, M.; Gamlien, S.; Ewald, R.; Florian, A.; Frank, M.; Wirtz, M.; Hell, R.; Fernie, A.R.; Bauwe, H. Mitochondrial dihydrolipoyl dehydrogenase activity shapes photosynthesis and photorespiration of Arabidopsis thaliana. Plant Cell 2015, 27, 1968–1984. [Google Scholar] [CrossRef]
- Simkin, A.; Lopez-Calcagno, P.; Davey, P.; Headland, L.; Lawson, T.; Timm, S.; Bauwe, H.; Raines, C. Simultaneous stimulation of the SBPase, FBP aldolase and the photorespiratory GDC-H protein increases CO2 assimilation, vegetative biomass and seed yield in Arabidopsis. Plant Biotechnol. J. 2017, 15, 805–816. [Google Scholar] [CrossRef]
- López-Calcagno, P.E.; Fisk, S.; Brown, K.L.; Bull, S.E.; South, P.F.; Raines, C.A. Overexpressing the H-protein of the glycine cleavage system increases biomass yield in glasshouse and field-grown transgenic tobacco plants. Plant Biotechnol. J. 2019, 17, 141–151. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Wang, K.; Ryu, C.M.; Kaundal, A.; Mysore, K.S. Glycolate oxidasemodulates reactive oxygen species-mediated signaltransduction during nonhost resistance inNicotianabenthamianaandArabidopsis. Plant Cell 2012, 24, 336–352. [Google Scholar] [CrossRef] [PubMed]
- Sørhagen, K.; Laxa, M.; Peterhänsel, C.; Reumann, S. The emerging role of photorespiration and non-photorespiratory peroxisomal metabolism in pathogen defense. Plant Biol. 2013, 15, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, A.; Takeba, G. Photorespiration protects C3 plants from photooxidation. Nature 1996, 384, 557–560. [Google Scholar] [CrossRef]
- Wingler, A.; Lea, P.J.; Quick, W.P.; Leegood, R.C. Photorespiration: Metabolic pathways and their role in stress protection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1517–1529. [Google Scholar] [CrossRef]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging concept for the role of photorespiration as an important part of abiotic stress. Plant Biol. 2013, 15, 713–722. [Google Scholar] [CrossRef]
- Saji, S.; Bathula, S.; Kubo, A.; Tamaoki, M.; Aono, M.; Sano, T.; Tobe, K.; Timm, S.; Bauwe, H.; Nakajima, N.; et al. Ozone-Sensitive Arabidopsis Mutants with Deficiencies in Photorespiratory Enzymes. Plant Cell Physiol. 2017, 58, 914–924. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.J.; Wang, J.H.; Hu, H. Photorespiration is the major alternative electron sink under high light in alpine evergreen sclerophyllous Rhododendron species. Plant Sci. 2019. [Google Scholar] [CrossRef]
- Sunil, B.; Saini, D.; Bapatla, R.B.; Aswani, V.; Raghavendra, A.S. Photorespiration is complemented by cyclic electron flow and alternative oxidase pathway to optimize photosynthesis and protect against abiotic stress. Photosynth. Res. 2019, 139, 67–79. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Mikkelsen, T.N.; Ambus, P.; Bauwe, H.; Lea, P.J.; Gardeström, P. Photorespiration Contributes to Stomatal Regulation and Carbon Isotope Fractionation: A Study with Barley, Potato and Arabidopsis Plants Deficient in Glycine Decarboxylase. Photosynth. Res. 2004, 81, 139–152. [Google Scholar] [CrossRef]
- Eisenhut, M.; Bräutigam, A.; Timm, S.; Florian, A.; Tohge, T.; Fernie, A.R.; Bauwe, H.; Weber, A. Photorespiration is crucial to the dynamic response of photosynthetic metabolism to altered CO2 availability. Mol. Plant 2017, 10, 437–461. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mauve, C.; Lamothe-Sibold, M.; Guérard, F.; Glab, N.; Hodges, M.; Jossier, M. Photorespiratory serine hydroxymethyltransferase 1 activity impacts abiotic stress tolerance and stomatal closure. New Phytol. 2019, 42, 2567–2583. [Google Scholar] [CrossRef] [PubMed]
- Boyes, D.C.; Zayed, A.M.; Ascenzi, R.; McCaskill, A.J.; Hoffman, N.E.; Davis, K.R.; Gorlach, J. Growth stage-based phenotypic analysis of Arabidopsis: A model for high throughput functional genomics in plants. Plant Cell 2001, 13, 1499–1510. [Google Scholar] [CrossRef] [PubMed]
- Florian, A.; Nikoloski, Z.; Sulpice, R.; Timm, S.; Araújo, W.L.; Tohge, T.; Bauwe, H.; Fernie, A. Analysis of short-term metabolic alterations in Arabidopsis following changes in the prevailing environmental conditions. Mol. Plant 2014, 7, 893–911. [Google Scholar] [CrossRef]
- Li, J.; Hu, J. Using Co-Expression Analysis and Stress-Based Screens to Uncover Arabidopsis Peroxisomal Proteins Involved in Drought Response. PLoS ONE 2015, 10, e0137762. [Google Scholar] [CrossRef]
- Buckley, T.N. How stoma respond to water status? New Phytol. 2019, 224, 21–36. [Google Scholar] [CrossRef]
- Timm, S.; Florian, A.; Wittmiß, M.; Jahnke, K.; Hagemann, M.; Fernie, A.R.; Bauwe, H. Serine acts as a metabolic signal for the transcriptional control of photorespiration-related genes in Arabidopsis. Plant Physiol. 2013, 162, 379–389. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Wang, X.P.; Sun, H.; Han, S.J.; Li, W.F.; Cui, N.; Lin, G.M.; Zhang, J.Y.; Cheng, W.; Cao, D.D.; et al. Coordinating carbon and nitrogen metabolic signaling through the cyanobacterial global repressor NdhR. Proc. Natl. Acad. Sci. USA 2018, 115, 403–408. [Google Scholar] [CrossRef]
- Cross, J.M.; von Korff, M.; Altmann, T.; Bartzetko, L.; Sulpice, R.; Gibon, Y.; Palacios, N.; Stitt, M. Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol. 2006, 142, 1574–1588. [Google Scholar] [CrossRef]
- Sievers, N.; Muders, K.; Henneberg, M.; Klähn, S.; Effmert, M.; Junghans, H.; Hagemann, M. Establishing glucosylglycerol synthesis in potato (Solanum tuberosum L. cv. albatros) by expression of the ggpPS gene from Azotobacter vinelandii. J. Plant Sci. Mol. Breed. 2013, 2, 1. [Google Scholar] [CrossRef]
- Reinholdt, O.; Schwab, S.; Zhang, Y.; Reichheld, J.P.; Fernie, A.R.; Hagemann, M.; Timm, S. Redox-regulation of photorespiration through mitochondrial thioredoxin o1. Plant Physiol. 2019, 181, 442–457. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timm, S.; Woitschach, F.; Heise, C.; Hagemann, M.; Bauwe, H. Faster Removal of 2-Phosphoglycolate through Photorespiration Improves Abiotic Stress Tolerance of Arabidopsis. Plants 2019, 8, 563. https://doi.org/10.3390/plants8120563
Timm S, Woitschach F, Heise C, Hagemann M, Bauwe H. Faster Removal of 2-Phosphoglycolate through Photorespiration Improves Abiotic Stress Tolerance of Arabidopsis. Plants. 2019; 8(12):563. https://doi.org/10.3390/plants8120563
Chicago/Turabian StyleTimm, Stefan, Franziska Woitschach, Carolin Heise, Martin Hagemann, and Hermann Bauwe. 2019. "Faster Removal of 2-Phosphoglycolate through Photorespiration Improves Abiotic Stress Tolerance of Arabidopsis" Plants 8, no. 12: 563. https://doi.org/10.3390/plants8120563
APA StyleTimm, S., Woitschach, F., Heise, C., Hagemann, M., & Bauwe, H. (2019). Faster Removal of 2-Phosphoglycolate through Photorespiration Improves Abiotic Stress Tolerance of Arabidopsis. Plants, 8(12), 563. https://doi.org/10.3390/plants8120563





