Exogenous Dopamine Application Promotes Alkali Tolerance of Apple Seedlings
Abstract
:1. Introduction
2. Results
2.1. Screening of Suitable Dopamine Concentrations
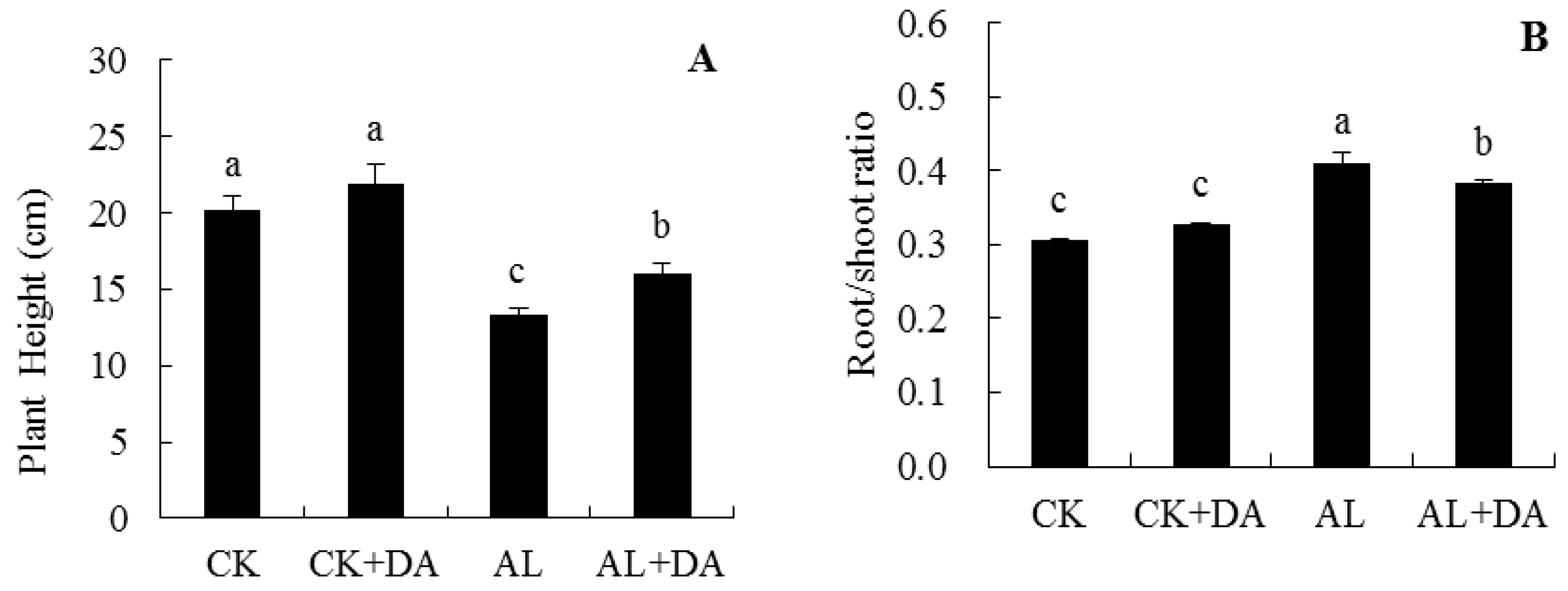
2.2. Effects of Dopamine Application on Plant Growth
2.3. Effects of Dopamine Application on Root System Architecture
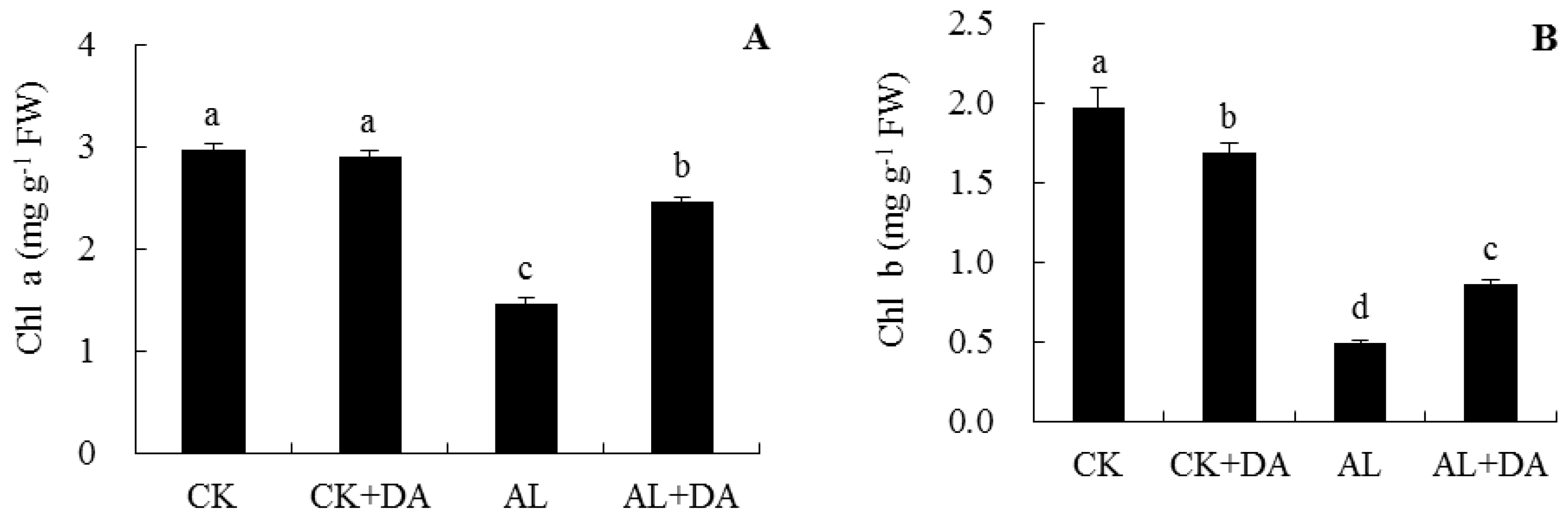
2.4. Effects of Dopamine Application on Chlorophyll Content and Fv/Fm
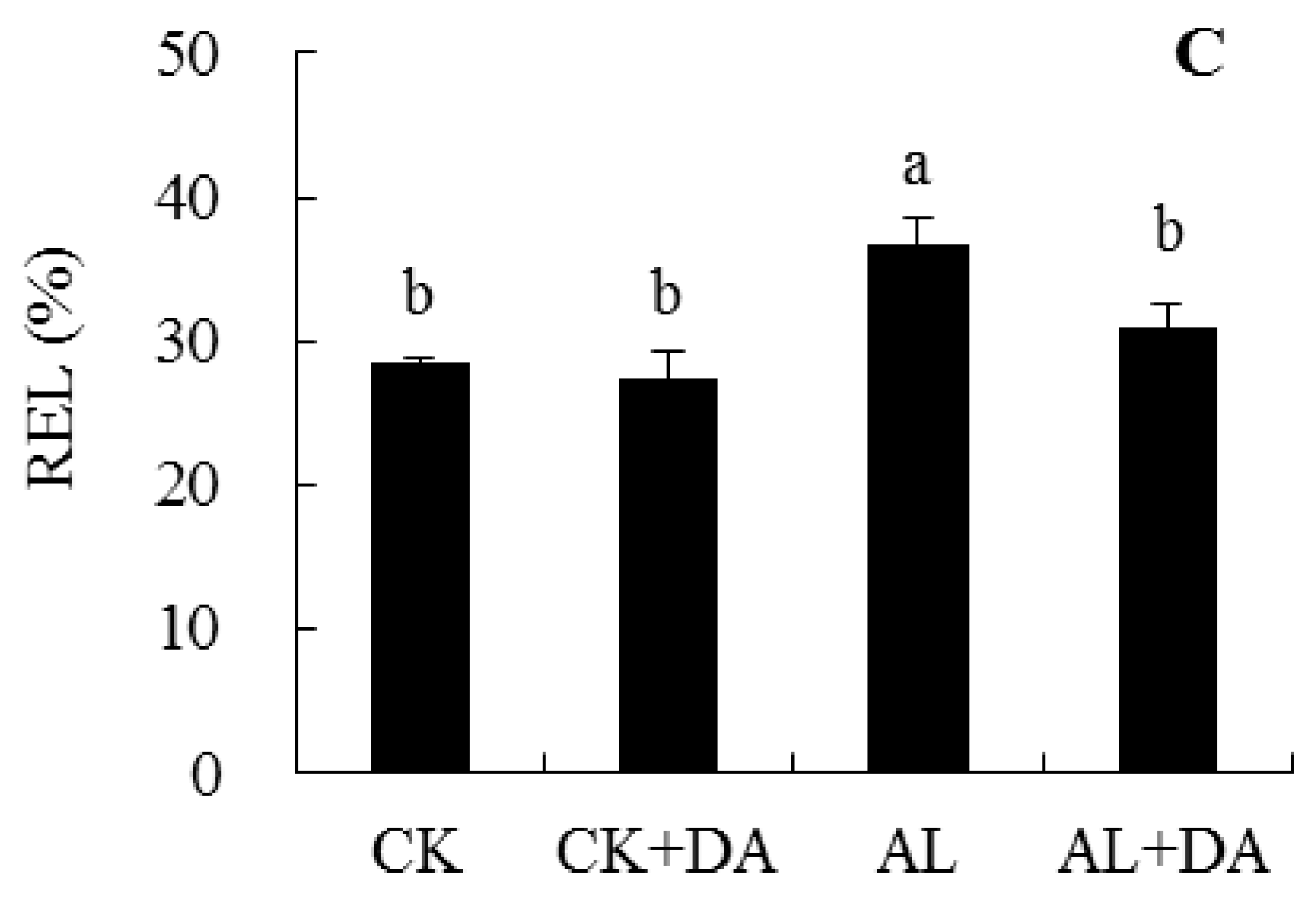
2.5. Effects of Dopamine Application on the Accumulation of Reactive Oxygen Species
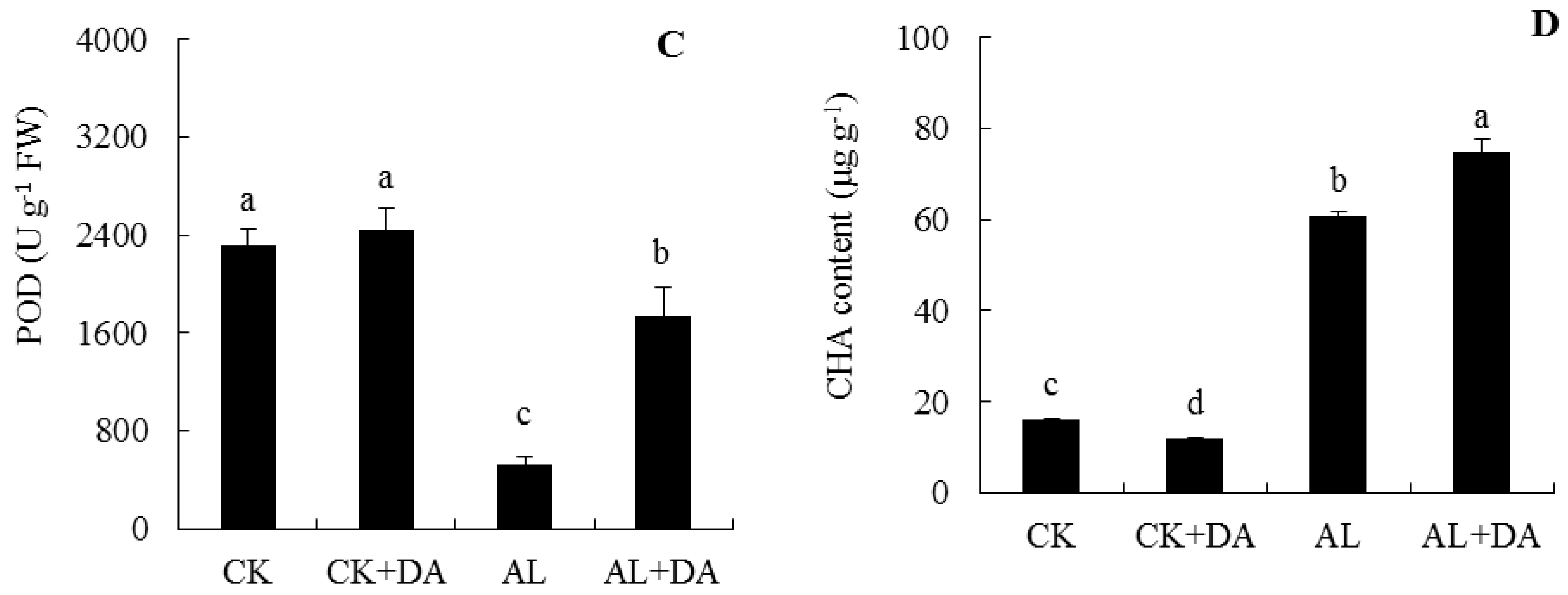
2.6. Effects of Dopamine Application on Antioxidant Capacity
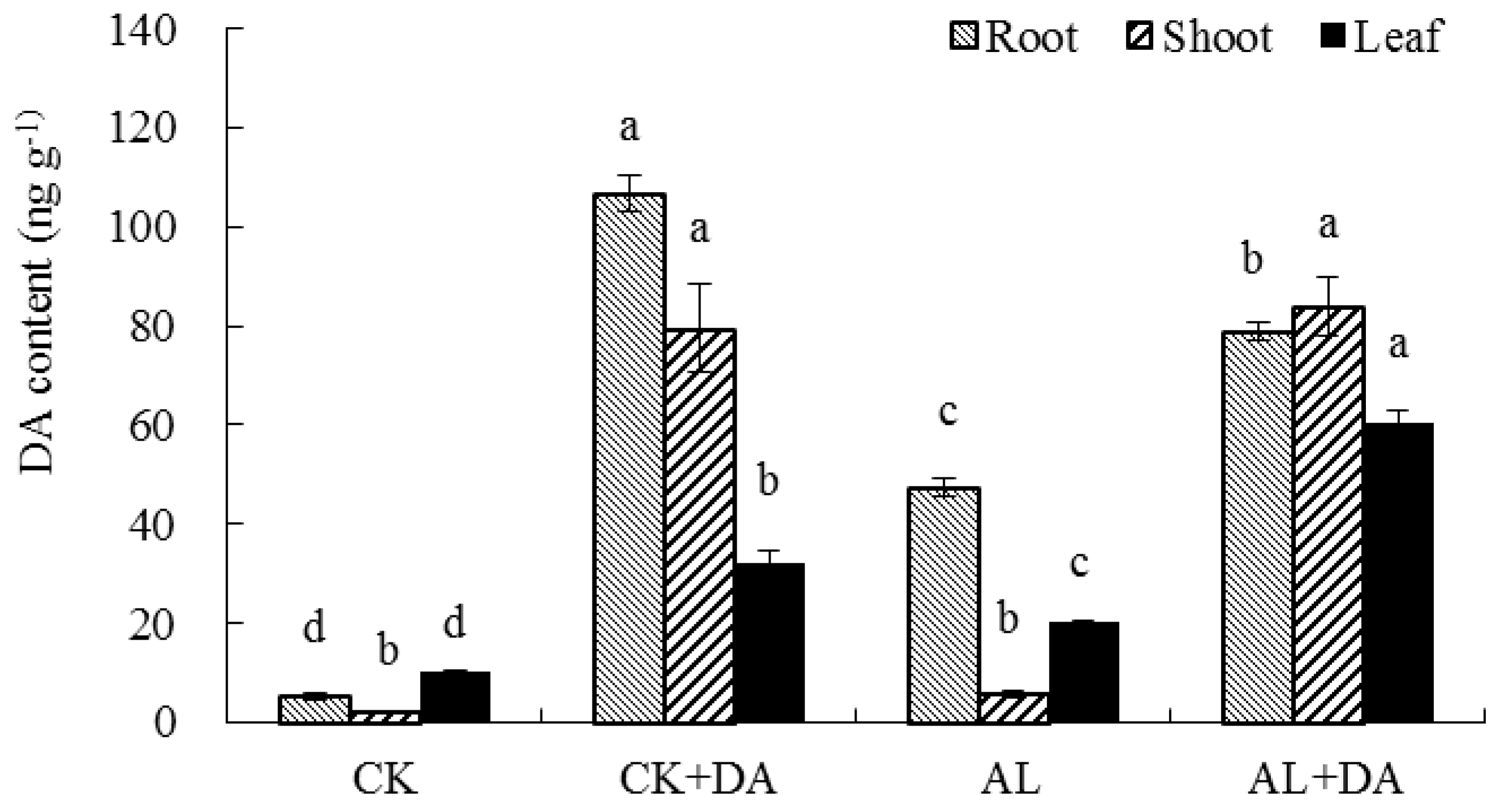
2.7. Endogenous Dopamine Content
3. Discussion
3.1. Growth
3.2. Pn, Chlorophyll Content, and Fv/Fm
3.3. Antioxidant System
3.4. Dopamine Content
4. Materials and Methods
4.1. Plant Materials
4.2. Screening of Suitable Concentrations of Exogenous Dopamine Application
4.3. Mitigation Effect of Exogenous Dopamine Application
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guo, R.; Shi, L.; Yan, C.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H.R. Ionomic and metabolic responses to neutral salt or alkaline salt stresses in maize (Zea mays L.) seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, D.C.; Sheng, Y.M. Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ. Exp. Bot. 2005, 54, 8–21. [Google Scholar] [CrossRef]
- Yang, C.W.; Xu, H.H.; Wang, L.L.; Liu, J.; Shi, D.C.; Wang, D.L. Comparative effects of salt-stress and alkali-stress on the growth, photosynthesis, solute accumulation, and ion balance of barley plants. Photosynthetica 2009, 47, 79–86. [Google Scholar] [CrossRef]
- Zhang, K.X.; Wen, T.; Dong, J.; Ma, F.W.; Bai, T.H.; Wang, K.; Li, C.Y. Comprehensive evaluation of tolerance to alkali stress by 17 genotypes of apple rootstocks. J. Integr. Agric. 2016, 15, 1499–1509. [Google Scholar] [CrossRef] [Green Version]
- Gong, X.Q.; Shi, S.T.; Dou, F.F.; Song, Y.; Ma, F.W. Exogenous melatonin alleviates alkaline stress in Malus hupehensis Rehd. by regulating the biosynthesis of polyamines. Molecules 2017, 22, 1542. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Dong, L.J.; Wang, L.; Ma, F.W.; Zou, Y.J.; Li, C.Y. Changes in root architecture and endogenous hormone levels in two Malus rootstocks under alkali stress. Sci. Hortic. 2017, 235, 198–204. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Das, P.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. Oxidative environment and redox homeostasis in plants: Dissecting out significant contribution of major cellular organelles. Front. Environ. Sci. 2015, 2, 70–81. [Google Scholar] [CrossRef] [Green Version]
- Hung, P.V. Phenolic Compounds of Cereals and Their Antioxidant Capacity. Crit. Rev. Food Sci. Nutr. 2016, 56, 25–35. [Google Scholar] [CrossRef]
- Liang, N.J.; Kitts, D.D. Role of Chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Cheng, D.; Zeng, X.Q.; Cao, J.K.; Jiang, W.B. Evidences for chlorogenic acid—A major endogenous polyphenol involved in regulation of ripening and senescence of apple fruit. PLoS ONE 2016, 11, e0146940. [Google Scholar] [CrossRef]
- Liang, B.W.; Gao, T.T.; Zhao, Q.; Ma, C.Q.; Chen, Q.; Wei, Z.W.; Li, C.Y.; Li, C.; Ma, F.W. Effects of exogenous dopamine on the uptake, transport, and resorption of apple ionome under moderate drought. Front. Plant Sci. 2018, 9, 755. [Google Scholar] [CrossRef]
- Li, C.; Sun, X.K.; Chang, C.; Jia, D.F.; Wei, Z.W.; Li, C.Y.; Ma, F.W. Dopamine alleviates salt-induced stress in Malus Hupehensis. Physiol. Plant. 2015, 153, 584–602. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.F. Superoxide as an obligatory, catalytic intermediate in photosynthetic reduction of oxygen by adrenaline and dopamine. Antioxid. Redox Signal. 2003, 5, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szopa, J.; Wilczyncñki, G.; Fiehn, O.; Wenczel, A.; Willmitzer, L. Identification and quantification of catecholamines in potato plants (Solanum tuberosum) by GC-MS. Phytochemistry 2001, 58, 315–320. [Google Scholar] [CrossRef]
- Skirycz, A.; Widrych, A.; Szopa, J. Expression of human dopamine receptor in potato (Solanum tuberosum) results in altered tuber carbon metabolism. BMC Plant Biol. 2005, 5, 1471–2229. [Google Scholar] [CrossRef] [Green Version]
- Roshchina, V.V. Biomediators in chloroplasts of higher plants. 3. Effect of dopamine on photochemical activity. Photosynthetica 1990, 24, 117–121. [Google Scholar]
- Elstner, E.F.; Konze, J.R.; Selman, B.R.; Stoffer, C. Ethylene formation in sugar beet leaves: Evidence forthe involvement of 3-hydroxytyramine and phenoloxidase after wounding. Plant Physiol. 1976, 58, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Kamisaka, S. Catecholamine stimulation of the gibberellin action that induces lettuce hypocotyls elongation. Plant Cell Physiol. 1979, 20, 1199–1207. [Google Scholar] [CrossRef]
- Protacio, C.M.; Dai, Y.R.; Lewis, E.F.; Flores, H.E. Growth-stimulation by catecholamines in plant-tissue organ-cultures. Plant Physiol. 1992, 98, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.W.; Li, C.Y.; Ma, C.Q.; Wei, Z.W.; Wang, Q.; Huang, D.; Chen, Q.; Li, C.; Ma, F.W. Dopamine alleviates nutrient deficiency-induced stress in Malus hupehensis. Plant Physiol. Biochem. 2017, 119, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Shi, F.C.; Fukuda, K.J.; Yang, Y.L. Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2010, 56, 725–733. [Google Scholar] [CrossRef]
- Liu, X.; Liang, W.; Li, Y.X.; Li, M.J.; Ma, B.Q.; Liu, C.H.; Ma, F.W.; Li, C.Y. Transcriptome analysis reveals the effects of alkali stress on root system architecture and endogenous hormones in apple rootstocks. J. Integr. Agric. 2019, 18, 2264–2271. [Google Scholar] [CrossRef]
- Kulma, A.; Szopa, J. Catecholamines are active compounds in plants. Plant Sci. 2007, 172, 433–440. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Y.; Zhou, K.; Li, Y.; Chen, X.F.; Liu, B.B.; Li, C.Y.; Gong, X.Q.; Ma, F.W. Exogenous myo-inositol alleviates salinity-induced stress in Malus hupehensis Rehd. Plant Physiol. Biochem. 2018, 133, 116–126. [Google Scholar] [CrossRef]
- Xiang, L.; Hu, L.; Xu, W.N.; Zhen, A.; Zhang, L.; Hu, X.H. Exogenous γ-aminobutyric acid improves the structure and function of photosystem II in muskmelon seedlings exposed to salinity-alkalinity stress. PLoS ONE 2016, 11, e0164847. [Google Scholar] [CrossRef]
- Li, Y.X.; Liu, B.Y.; Peng, Y.X.; Liu, C.L.; Zhang, X.Z.; Zhang, Z.Z.; Liang, W.; Ma, F.W.; Li, C.Y. Exogenous GABA alleviates alkaline stress in Malus hupehensis by regulating the accumulation of organic acids. Sci. Hortic. 2019. [Google Scholar] [CrossRef]
- Ohama, N.; Sato, H.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Transcriptional regulatory network of plant heat stress response. Trends Plant Sci. 2017, 22, 53–65. [Google Scholar] [CrossRef]
- Xie, Y.P.; Chen, P.X.; Yan, Y.; Bao, C.N.; Li, X.W.; Wang, L.P.; Shen, X.X.; Li, H.Y.; Liu, X.F.; Niu, C.D.; et al. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol. 2018, 218, 201–218. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Tejada, S.; Setzer, W.N.; Gortzi, O.; Sureda, A.; Braidy, N.; Daglia, M.; Manayi, A.; Nabavi, S.M. Chlorogenic acid and mental diseases: From chemistry to medicine. Curr. Neuropharmacol. 2017, 15, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef] [PubMed]
- Medda, R.; Padiglia, A.; Floris, G. Plant copper-amine oxidases. Phytochemistry 1995, 39, 1–9. [Google Scholar] [CrossRef]
- Bruhn, J.G.; Lundström, J. Alkaloids of Carnegiea gigantean. Arizonine, a new tetrahydroisoquinoline alkaloid. Lloydia 1976, 39, 197–203. [Google Scholar] [PubMed]
- Swiedrych, A.; Lorenc-Kukula, K.; Skirycz, A.; Szopa, J. The catecholamine biosynthesis route in potato is affected by stress. Plant Physiol. Biochem. 2004, 42, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Endress, R.; Jäger, A.; Kreis, W. Catecholamine biosynthesis dependent on the dark in betacyanin-forming portulaca callus. J. Plant Physiol. 1984, 115, 291–295. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stat. 1950, 347, 357–359. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Gao, J.F. Plants Physiology Experimentation Guidance; The World Press: Xi’an, China, 2000; pp. 101–103. (In Chinese) [Google Scholar]
- Kim, H.R.; Kim, T.H.; Hong, S.H.; Kim, H.G. Direct detection of tetrahydrobiopterin (BH4) and dopamine in rat brain using liquid chromatography coupled electrospray tandem mass spectrometry. Biochem. Biophys. Res. Commun. 2012, 419, 632–637. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, P.M.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in Honeycrisp apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]






| CK | CK+DA | AL | AL+DA | |
|---|---|---|---|---|
| Root Length (cm) | 540.24 ± 77.15ab | 580.60 ± 33.75a | 363.19 ± 14.03d | 456.95 ± 23.93bc |
| Root Diameter (mm) | 0.57 ± 0.02b | 0.60 ± 0.05ab | 0.59 ± 0.04ab | 0.70 ± 0.05a |
| Root Volume (cm3) | 1.38 ± 0.07b | 2.38 ± 0.42a | 1.33 ± 0.14b | 2.42 ± 0.40a |
| Tips | 1031.75 ± 25.82a | 1011.00 ± 100.33a | 569.00 ± 50.01b | 817.25 ± 191.62ab |
| Forks | 2418.00 ± 244.71b | 3272.33 ± 211.76a | 1914.00 ± 99.27c | 2406.67 ± 38.39bc |
| Root Surface Area (cm2) | 65.64 ± 5.48bc | 89.91 ± 4.94a | 61.76 ± 3.11c | 76.85 ± 5.99b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, X.; Li, Y.; Zhang, X.; Liu, C.; Liang, W.; Li, C.; Ma, F.; Li, C. Exogenous Dopamine Application Promotes Alkali Tolerance of Apple Seedlings. Plants 2019, 8, 580. https://doi.org/10.3390/plants8120580
Jiao X, Li Y, Zhang X, Liu C, Liang W, Li C, Ma F, Li C. Exogenous Dopamine Application Promotes Alkali Tolerance of Apple Seedlings. Plants. 2019; 8(12):580. https://doi.org/10.3390/plants8120580
Chicago/Turabian StyleJiao, Xueyi, Yuxing Li, Xiuzhi Zhang, Chenlu Liu, Wei Liang, Chao Li, Fengwang Ma, and Cuiying Li. 2019. "Exogenous Dopamine Application Promotes Alkali Tolerance of Apple Seedlings" Plants 8, no. 12: 580. https://doi.org/10.3390/plants8120580
APA StyleJiao, X., Li, Y., Zhang, X., Liu, C., Liang, W., Li, C., Ma, F., & Li, C. (2019). Exogenous Dopamine Application Promotes Alkali Tolerance of Apple Seedlings. Plants, 8(12), 580. https://doi.org/10.3390/plants8120580





