Genetic Engineering for Global Food Security: Photosynthesis and Biofortification
Abstract
:1. Introduction
2. Photosynthesis
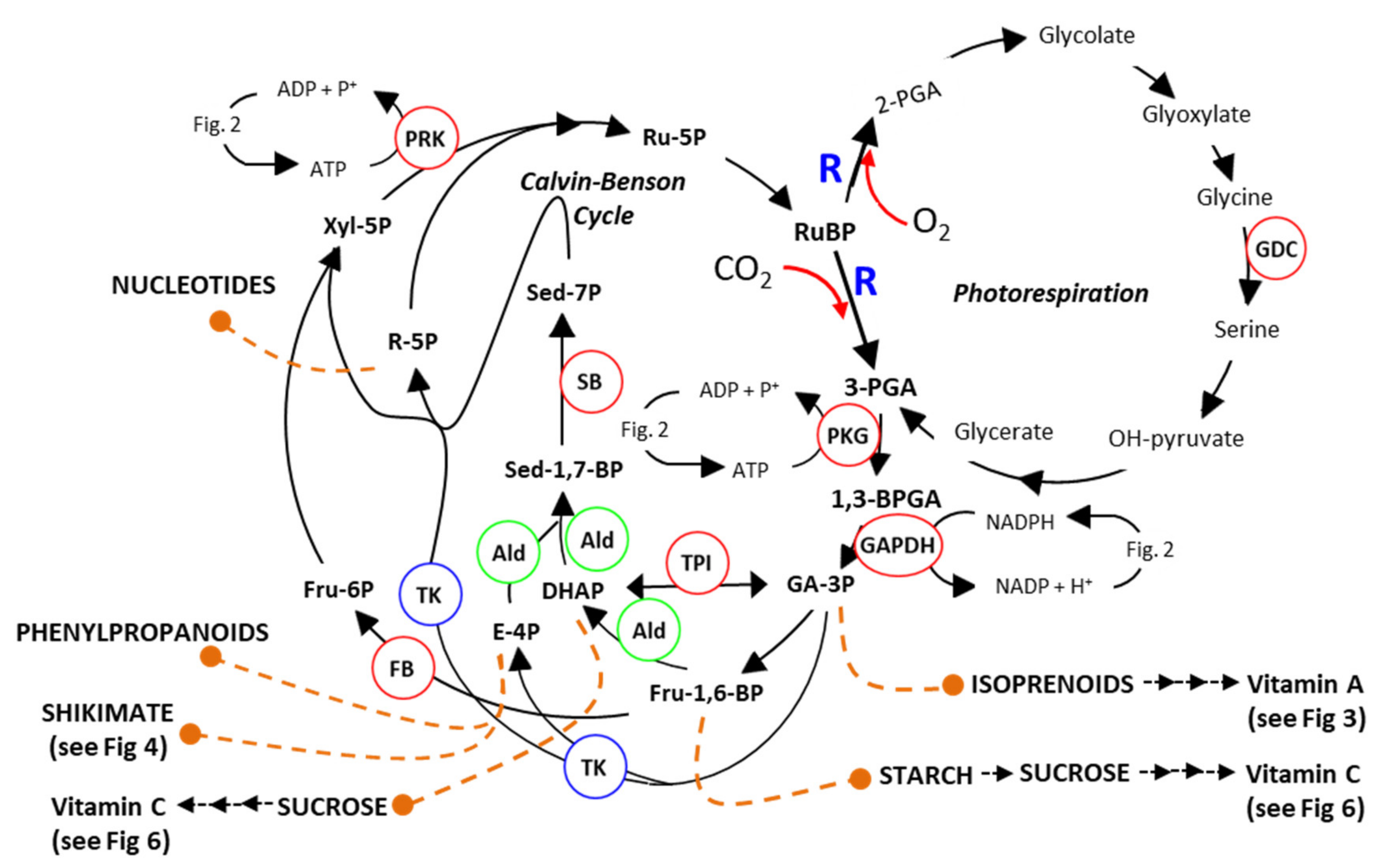
2.1. Manipulation of Calvin–Benson Cycle
2.2. Can Increasing Photorespiration Increase Yield?
2.3. Enhancing Photosynthetic Electron Transport Increases Yield
2.4. Multi-Targeted Approaches to Improve Photosynthetic Efficiency Can Have a Synergistic Effect
2.5. Increasing Photosynthetic Carbon Availability Can Positively Affect Vitamin Content (Biofortification)
3. Biofortification
3.1. Increasing Pro-Vitamin A Content in Planta
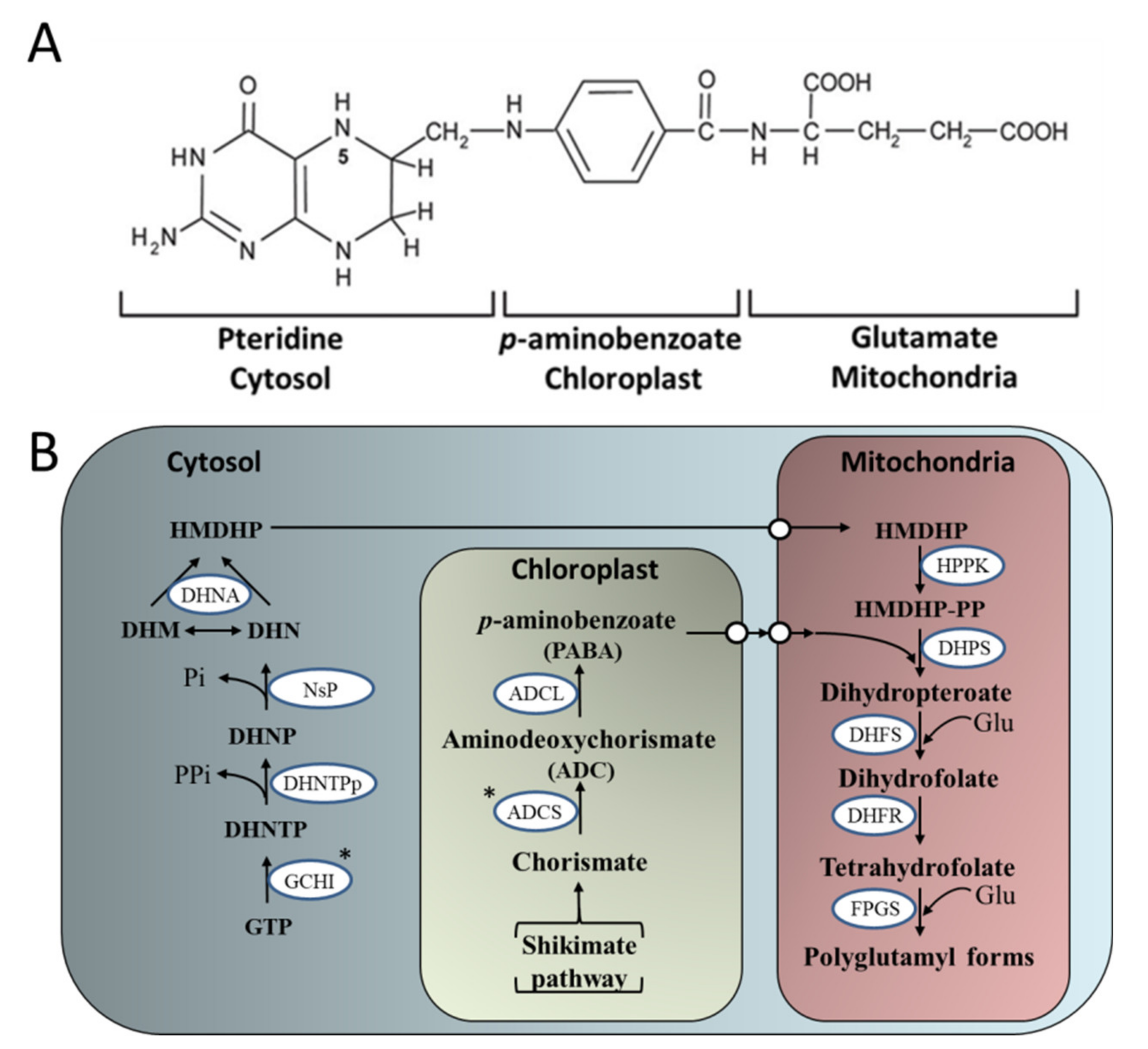
3.2. Genetic Manipulation of Folate (Vitamin B9) Accumulation
3.3. Cobalamin (Vitamin B12)
3.4. L-ascorbic Acid (Vitamin C)
3.5. Tocopherols (Vitamin E)
3.6. A Multi-Targeted Approach to Multi-Vitamin Crops
4. Future Prospects and Conclusions
Funding
Conflicts of Interest
References
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [Green Version]
- Lawson, T.; Kramer, D.M.; Raines, C.A. Improving yield by exploiting mechanisms underlying natural variation of photosynthesis. Curr. Opin. Biotechnol. 2012, 23, 215–220. [Google Scholar] [CrossRef]
- Driever, S.M.; Lawson, T.; Andralojc, P.J.; Raines, C.A.; Parry, M.A. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J. Exp. Bot. 2014, 65, 4959–4973. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO). State of Food Insecurity in the World 2015; FAO: Rome, Italy, 2015. [Google Scholar]
- United Nations Desa Population Division. World Population Prospects 2019: Data Booklet. Available online: https://population.un.org/wpp/Publications/Files/WPP2019_DataBooklet.pdf (accessed on 19 November 2019).
- Fischer, R.A.T.; Edmeades, G.O. Breeding and cereal yield progress. Crop. Sci. 2010, 50, S85–S98. [Google Scholar] [CrossRef] [Green Version]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–9536. [Google Scholar] [CrossRef] [Green Version]
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [Green Version]
- RSOL. Royal Society of London, Reaping the Benefits: Science and the Sustainable Intensification of Global Agriculture; Royal Society: London, UK, 2009. [Google Scholar]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [Green Version]
- World Bank. World Development Report 2008: Agriculture for Development; World Bank: Washington, DC, USA, 2008. [Google Scholar]
- Dirzo, R.; Raven, P.H. Global state of biodiversity and loss. Annu. Rev. Environ. Resour. 2003, 28, 137–167. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [Green Version]
- Godfray, H.C.J. The challenge of feeding 9–10 billion people equitably and sustainably. J. Agric. Sci. 2014, 152, S2–S8. [Google Scholar] [CrossRef]
- GOFS. The Government Office for Science Foresight. The Future of Food and Farming. Final Project Report; The Government Office for Science: London, UK, 2011. [Google Scholar]
- Vitousek, P.M.; Aber, J.D.; Howarth, R.W.; Likens, G.E.; Matson, P.A.; Schindler, D.W.; Schlesinger, W.H.; Tilman, D. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef] [Green Version]
- Burney, J.A.; Davis, S.J.; Lobell, D.B. Greenhouse gas mitigation by agricultural intensification. Proc. Natl. Acad. Sci. USA 2010, 107, 12052–12057. [Google Scholar] [CrossRef] [Green Version]
- Bouis, H.E.; Eozenou, P.; Rahman, A. Food prices, household income, and resource allocation: Socioeconomic perspectives on their effects on dietary quality and nutritional status. Food Nutr. Bull. 2011, 32, S14–S23. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.P. Photosynthesis. Essays Biochem. 2016, 60, 255–273. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Andralojc, P.J.; Scales, J.C.; Salvucci, M.E.; Carmo-Silva, A.E.; Alonso, H.; Whitney, S.M. Rubisco activity and regulation as targets for crop improvement. J. Exp. Bot. 2013, 64, 717–730. [Google Scholar] [CrossRef]
- Wu, A.; Hammer, G.L.; Doherty, A.; von Caemmerer, S.; Farquhar, G.D. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef]
- Simkin, A.J.; Lopez-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [Green Version]
- Orr, D.J.; Pereira, A.M.; da Fonseca Pereira, P.; Pereira-Lima, I.A.; Zsogon, A.; Araujo, W.L. Engineering photosynthesis: Progress and perspectives. F1000 Res. 2017, 6, 1891. [Google Scholar] [CrossRef]
- Bassham, J.A.; Calvin, M. The path of carbon in photosynthesis. In Die CO2-Assimilation / the Assimilation of Carbon Dioxide: In 2 Teilen / 2 Parts; Pirson, A., Ed.; Springer: Berlin/Heidelberg, Germany, 1960; pp. 884–922. [Google Scholar]
- Calvin, M.; Benson, A.A. The path of carbon in photosynthesis. Science 1948, 107, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Lawson, T.; Bryant, B.; Lefebvre, S.; Lloyd, J.C.; Raines, C.A. Decreased sbpase activity alters growth and development in transgenic tobacco plants. Plant Cell Environ. 2006, 29, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Harrison, E.P.; Olcer, H.; Lloyd, J.C.; Long, S.P.; Raines, C.A. Small decreases in sbpase cause a linear decline in the apparent rubp regeneration rate, but do not affect rubisco carboxylation capacity. J. Exp. Bot. 2001, 52, 1779–1784. [Google Scholar] [CrossRef] [Green Version]
- Harrison, E.P.; Willingham, N.M.; Lloyd, J.C.; Raines, C.A. Reduced sedoheptulose-1,7-bisphosphatase levels in transgenic tobacco lead to decreased photosynthetic capacity and altered carbohydrate accumulation. Planta 1998, 204, 27–36. [Google Scholar] [CrossRef]
- Haake, V.; Geiger, M.; Walch-Liu, P.; Engels, C.; Zrenner, R.; Stitt, M. Changes in aldolase activity in wild-type potato plants are important for acclimation to growth irradiance and carbon dioxide concentration, because plastid aldolase exerts control over the ambient rate of photosynthesis across a range of growth conditions. Plant J. 1999, 17, 479–489. [Google Scholar] [CrossRef]
- Haake, V.; Zrenner, R.; Sonnewald, U.; Stitt, M. A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. Plant J. 1998, 14, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Kossmann, J.; Muller-Rober, B.; Dyer, T.A.; Raines, C.A.; Sonnewald, U.; Willmitzer, L. Cloning and expression analysis of the plastidic fructose-1,6-bisphosphatase coding sequence from potato: Circumstantial evidence for the import of hexoses into chloroplasts. Planta 1992, 188, 7–12. [Google Scholar] [CrossRef]
- Rojas-Gonzalez, J.A.; Soto-Suarez, M.; Garcia-Diaz, A.; Romero-Puertas, M.C.; Sandalio, L.M.; Merida, A.; Thormahlen, I.; Geigenberger, P.; Serrato, A.J.; Sahrawy, M. Disruption of both chloroplastic and cytosolic fbpase genes results in a dwarf phenotype and important starch and metabolite changes in arabidopsis thaliana. J. Exp. Bot. 2015, 66, 2673–2689. [Google Scholar] [CrossRef] [Green Version]
- Sahrawy, M.; Avila, C.; Chueca, A.; Canovas, F.M.; Lopez-Gorge, J. Increased sucrose level and altered nitrogen metabolism in arabidopsis thaliana transgenic plants expressing antisense chloroplastic fructose-1,6-bisphosphatase. J. Exp. Bot. 2004, 55, 2495–2503. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.J.; Driscoll, S.P.; Andralojc, P.J.; Knight, J.S.; Gray, J.C.; Lawlor, D.W. Decrease of phosphoribulokinase activity by antisense rna in transgenic tobacco: Definition of the light environment under which phosphoribulokinase is not in large excess. Planta 2000, 211, 112–119. [Google Scholar] [CrossRef]
- Raines, C.A. The calvin cycle revisited. Photosynth. Res. 2003, 75, 1–10. [Google Scholar] [CrossRef]
- Stitt, M.; Schulze, D. Does rubisco control the rate of photosynthesis and plant-growth - an exercise in molecular ecophysiology. Plant Cell Environ. 1994, 17, 465–487. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (face)? A meta-analytic review of the responses of photosynthesis, canopy. N. Phytol. 2005, 165, 351–371. [Google Scholar] [CrossRef]
- Raines, C.A. Transgenic approaches to manipulate the environmental responses of the c3 carbon fixation cycle. Plant Cell Environ. 2006, 29, 331–339. [Google Scholar] [CrossRef]
- Lefebvre, S.; Lawson, T.; Fryer, M.; Zakhleniuk, O.V.; Lloyd, J.C.; Raines, C.A. Increased sedoheptulose-1,7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol. 2005, 138, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Simkin, A.J.; McAusland, L.; Headland, L.R.; Lawson, T.; Raines, C.A. Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J. Exp. Bot. 2015, 66, 4075–4090. [Google Scholar] [CrossRef]
- Simkin, A.J.; Lopez-Calcagno, P.E.; Davey, P.A.; Headland, L.R.; Lawson, T.; Timm, S.; Bauwe, H.; Raines, C.A. Simultaneous stimulation of sedoheptulose 1,7-bisphosphatase, fructose 1,6-bisphophate aldolase and the photorespiratory glycine decarboxylase h-protein increases CO2 assimilation, vegetative biomass and seed yield in arabidopsis. Plant Biotechnol. J. 2017, 15, 805–816. [Google Scholar] [CrossRef] [Green Version]
- Ding, F.; Wang, M.; Zhang, S.; Ai, X. Changes in sbpase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci. Rep. 2016, 6, 32741. [Google Scholar] [CrossRef]
- Rosenthal, D.M.; Locke, A.M.; Khozaei, M.; Raines, C.A.; Long, S.P.; Ort, D.R. Over-expressing the c3 photosynthesis cycle enzyme sedoheptulose-1-7 bisphosphatase improves photosynthetic carbon gain and yield under fully open air CO2 fumigation (face). BMC Plant Biol. 2011, 11, 123. [Google Scholar] [CrossRef] [Green Version]
- Driever, S.M.; Simkin, A.J.; Alotaibi, S.; Fisk, S.J.; Madgwick, P.J.; Sparks, C.A.; Jones, H.D.; Lawson, T.; Parry, M.A.J.; Raines, C.A. Increased sbpase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philos. Trans. R. Soc. B 2017, 372, 1730. [Google Scholar] [CrossRef] [Green Version]
- Uematsu, K.; Suzuki, N.; Iwamae, T.; Inui, M.; Yukawa, H. Increased fructose 1,6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. J. Exp. Bot. 2012, 63, 3001–3009. [Google Scholar] [CrossRef]
- Khozaei, M.; Fisk, S.; Lawson, T.; Gibon, Y.; Sulpice, R.; Stitt, M.; Lefebvre, S.C.; Raines, C.A. Overexpression of plastid transketolase in tobacco results in a thiamine auxotrophic phenotype. Plant Cell 2015, 27, 432–447. [Google Scholar] [CrossRef] [Green Version]
- Ludwig, L.J.; Canvin, D.T. The rate of photorespiration during photosynthesis and the relationship of the substrate of light respiration to the products of photosynthesis in sunflower leaves. Plant Physiol. 1971, 48, 712–719. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.J.; VanLoocke, A.; Bernacchi, C.J.; Ort, D.R. The costs of photorespiration to food production now and in the future. Annu. Rev. Plant Biol. 2016, 67, 107. [Google Scholar] [CrossRef] [Green Version]
- Walker, B.J.; South, P.F.; Ort, D.R. Physiological evidence for plasticity in glycolate/glycerate transport during photorespiration. Photosynth. Res. 2016, 129, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Tolbert, N.E. The c2 oxidative photosynthetic carbon cycle. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 1–25. [Google Scholar] [CrossRef]
- Bowes, G.; Ogren, W.L.; Hageman, R.H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem. Biophys. Res. Commun. 1971, 45, 716. [Google Scholar] [CrossRef]
- Anderson, L.E. Chloroplast and cytoplasmic enzymes. 2. Pea leaf triose phosphate isomerases. Biochim. Biophys. Acta 1971, 235, 237. [Google Scholar] [CrossRef]
- Sharkey, T.D. Estimating the rate of photorespiration in leaves. Physiol. Plant. 1988, 73, 147–152. [Google Scholar] [CrossRef]
- Busch, F.A. Current methods for estimating the rate of photorespiration in leaves. Plant Biol. 2013, 15, 648–655. [Google Scholar] [CrossRef]
- Heineke, D.; Bykova, N.; Gardestrom, P.; Bauwe, H. Metabolic response of potato plants to an antisense reduction of the p-protein of glycine decarboxylase. Planta 2001, 212, 880–887. [Google Scholar] [CrossRef]
- Lin, H.C.; Karki, S.; Coe, R.A.; Bagha, S.; Khoshravesh, R.; Balahadia, C.P.; Sagun, J.V.; Tapia, R.; Israel, W.K.; Montecillo, F.; et al. Targeted knockdown of gdch in rice leads to a photorespiratory-deficient phenotype useful as a building block for c4 rice. Plant Cell Physiol. 2016, 57, 919–932. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.Y.; Yu, Q.; Wang, Z.Q.; Pan, Y.F.; Lv, W.T.; Zhu, L.L.; Chen, R.Z.; He, G.C. Knockdown of gdch gene reveals reactive oxygen species-induced leaf senescence in rice. Plant Cell Environ. 2013, 36, 1476–1489. [Google Scholar] [CrossRef]
- Bykova, N.V.; Keerberg, O.; Parnik, T.; Bauwe, H.; Gardestrom, P. Interaction between photorespiration and respiration in transgenic potato plants with antisense reduction in glycine decarboxylase. Planta 2005, 222, 130–140. [Google Scholar] [CrossRef]
- Timm, S.; Florian, A.; Arrivault, S.; Stitt, M.; Fernie, A.R.; Bauwe, H. Glycine decarboxylase controls photosynthesis and plant growth. FEBS Lett. 2012, 586, 3692–3697. [Google Scholar] [CrossRef] [Green Version]
- Timm, S.; Florian, A.; Fernie, A.R.; Bauwe, H. The regulatory interplay between photorespiration and photosynthesis. J. Exp. Bot. 2016, 67, 2923–2929. [Google Scholar] [CrossRef] [Green Version]
- Timm, S.; Wittmiss, M.; Gamlien, S.; Ewald, R.; Florian, A.; Frank, M.; Wirtz, M.; Hell, R.; Fernie, A.R.; Bauwea, H. Mitochondrial dihydrolipoyl dehydrogenase activity shapes photosynthesis and photorespiration of arabidopsis thaliana. Plant Cell 2015, 27, 1968–1984. [Google Scholar] [CrossRef] [Green Version]
- López-Calcagno, P.E.; Fisk, S.J.; Brown, K.; Bull, S.E.; South, P.F.; Raines, C.A. Overexpressing the h-protein of the glycine cleavage system increases biomass yield in glasshouse and field-grown transgenic tobacco plants. Plant Biotechnol. J. 2018, 17, 141–151. [Google Scholar] [CrossRef]
- Kelly, G.J.; Latzko, E. Inhibition of spinach leaf phosphofructokinase by 2-phosphoglycollate. FEBS Lett. 1976, 68, 55–58. [Google Scholar] [CrossRef] [Green Version]
- Eisenhut, M.; Bauwe, H.; Hagemann, M. Glycine accumulation is toxic for the cyanobacterium synechocystis sp strain pcc 6803, but can be compensated by supplementation with magnesium ions. Fems. Microbiol. Lett. 2007, 277, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.S.; Li, Y.; Yang, Q.S.; Zhang, Z.S.; Chen, Y.; Zhang, S.; Peng, X.X. Suppression of glycolate oxidase causes glyoxylate accumulation that inhibits photosynthesis through deactivating rubisco in rice. Physiol. Plant. 2014, 150, 463–476. [Google Scholar] [CrossRef]
- Cook, C.M.; Mulligan, R.M.; Tolbert, N.E. Inhibition and stimulation of ribulose-1,5-bisphosphate carboxylase-oxygenase by glyoxylate. Arch. Biochem. Biophys. 1985, 240, 392–401. [Google Scholar] [CrossRef]
- Campbell, W.J.; Ogren, W.L. Glyoxylate inhibition of ribulosebisphosphate carboxylase/oxygenase activation in intact, lysed, and reconstituted chloroplasts. Photosynth. Res. 1990, 23, 257–268. [Google Scholar] [CrossRef]
- Chastain, C.J.; Ogren, W.L. Glyoxylate inhibition of ribulosebisphosphate carboxylase/oxygenase activation state In Vivo. Plant Cell Physiol. 1989, 30, 937–944. [Google Scholar]
- Flugel, F.; Timm, S.; Arrivault, S.; Florian, A.; Stitt, M.; Fernie, A.R.; Bauwe, H. The photorespiratory metabolite 2-phosphoglycolate regulates photosynthesis and starch accumulation in arabidopsis. Plant Cell 2017, 29, 2537–2551. [Google Scholar] [CrossRef] [Green Version]
- Hausler, R.E.; Bailey, K.J.; Lea, P.J.; Leegood, R.C. Control of photosynthesis in barley mutants with reduced activities of glutamine synthetase and glutamate synthase.3. Aspects of glyoxylate metabolism and effects of glyoxylate on the activation state of ribulose-1, 5-bisphosphate carboxylase-oxygenase. Planta 1996, 200, 388–396. [Google Scholar] [CrossRef]
- Roach, T.; Krieger-Liszkay, A. Regulation of photosynthetic electron transport and photoinhibition. Curr. Protein Pept. Sci. 2014, 15, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Chida, H.; Nakazawa, A.; Akazaki, H.; Hirano, T.; Suruga, K.; Ogawa, M.; Satoh, T.; Kadokura, K.; Yamada, S.; Hakamata, W.; et al. Expression of the algal cytochrome c6 gene in arabidopsis enhances photosynthesis and growth. Plant Cell Physiol. 2007, 48, 948–957. [Google Scholar] [CrossRef]
- Yadav, S.K.; Khatri, K.; Rathore, M.S.; Jha, B. Introgression of ufcyt c6, a thylakoid lumen protein from a green seaweed ulva fasciata delile enhanced photosynthesis and growth in tobacco. Mol. Biol. Rep. 2018, 45, 1745–1758. [Google Scholar] [CrossRef]
- Merchant, S.; Bogorad, L. The cu(ii)-repressible plastidic cytochrome c. Cloning and sequence of a complementary DNA for the pre-apoprotein. J. Biol. Chem. 1987, 262, 9062–9067. [Google Scholar]
- Simkin, A.J.; McAusland, L.; Lawson, T.; Raines, C.A. Over-expression of the rieskefes protein increases electron transport rates and biomass yield. Plant Physiol. 2017, 175, 134–145. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, M.; Lopez-Calcagno, P.E.; Raines, C.A.; Furbank, R.T.; von Caemmerer, S. Overexpression of the rieske fes protein of the cytochrome b6f complex increases c4 photosynthesis in setaria viridis. Commun. Biol. 2019, 2, 314. [Google Scholar] [CrossRef] [Green Version]
- Price, G.D.; Yu, J.W.; Voncaemmerer, S.; Evans, J.R.; Chow, W.S.; Anderson, J.M.; Hurry, V.; Badger, M.R. Chloroplast cytochrome b6f and atp synthase complexes in tobacco—transformation with antisense RNA against nuclear-encoded transcripts for the rieske fes and atp-delta polypeptides. Aust. J. Plant Physiol. 1995, 22, 285–297. [Google Scholar]
- Price, G.D.; von Caemmerer, S.; Evans, J.R.; Siebke, K.; Anderson, J.M.; Badger, M.R. Photosynthesis is strongly reduced by antisense suppression of chloroplastic cytochrome b6f complex in transgenic tobacco. Aust. J. Plant Physiol. 1998, 25, 445–452. [Google Scholar]
- Anderson, J.M.; Price, G.D.; Chow, W.S.; Hope, A.B.; Badger, M.R. Reduced levels of cytochrome bf complex in transgenic tobacco leads to marked photochemical reduction of the plastoquinone pool, without significant change in acclimation to irradiance. Photosynth. Res. 1997, 53, 215–227. [Google Scholar] [CrossRef]
- Ruuska, S.A.; Andrews, T.J.; Badger, M.R.; Price, G.D.; von Caemmerer, S. The role of chloroplast electron transport and metabolites in modulating rubisco activity in tobacco. Insights from transgenic plants with reduced amounts of cytochrome b/f complex or glyceraldehyde 3-phosphate dehydrogenase. Plant Physiol. 2000, 122, 491–504. [Google Scholar] [CrossRef] [Green Version]
- Yamori, W.; Takahashi, S.; Makino, A.; Price, G.D.; Badger, M.R.; von Caemmerer, S. The roles of atp synthase and the cytochrome b6/f complexes in limiting chloroplast electron transport and determining photosynthetic capacity. Plant Physiol. 2011, 155, 956–962. [Google Scholar] [CrossRef] [Green Version]
- Yamori, W.; Sakata, N.; Suzuki, Y.; Shikanai, T.; Makino, A. Cyclic electron flow around photosystem i via chloroplast nad(p)h dehydrogenase (ndh) complex performs a significant physiological role during photosynthesis and plant growth at low temperature in rice. Plant J. 2011, 68, 966–976. [Google Scholar] [CrossRef]
- Hurry, V.; Anderson, J.M.; Badger, M.R.; Price, G.D. Reduced levels of cytochrome b(6)lf in transgenic tobacco increases the excitation pressure on photosystem ii without increasing sensitivity to photoinhibition in vivo. Photosynth. Res. 1996, 50, 159–169. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kato, H.; Shinzaki, Y.; Horiguchi, S.; Shikanai, T.; Hase, T.; Endo, T.; Nishioka, M.; Makino, A.; Tomizawa, K.-i.; et al. Ferredoxin limits cyclic electron flow around psi (cef-psi) in higher plants—stimulation of cef-psi enhances non-photochemical quenching of chl fluorescence in transplastomic tobacco. Plant Cell Physiol. 2006, 47, 1355–1371. [Google Scholar] [CrossRef]
- Rodriguez, R.E.; Lodeyro, A.; Poli, H.O.; Zurbriggen, M.; Peisker, M.; Palatnik, J.F.; Tognetti, V.B.; Tschiersch, H.; Hajirezaei, M.-R.; Valle, E.M.; et al. Transgenic tobacco plants overexpressing chloroplastic ferredoxin-nadp(h) reductase display normal rates of photosynthesis and increased tolerance to oxidative stress. Plant Physiol. 2007, 143, 639–649. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Huang, H.-E.; Cheng, C.-F.; Ho, M.-H.; Ger, M.-J. Constitutive expression of a plant ferredoxin-like protein (pflp) enhances capacity of photosynthetic carbon assimilation in rice (oryza sativa). Transgenic Res. 2017, 26, 279–289. [Google Scholar] [CrossRef]
- Gong, H.Y.; Li, Y.; Fang, G.; Hu, D.H.; Jin, W.B.; Wang, Z.H.; Li, Y.S. Transgenic rice expressing ictb and fbp/sbpase derived from cyanobacteria exhibits enhanced photosynthesis and mesophyll conductance to CO2. PLoS ONE 2015, 10, e0140928. [Google Scholar] [CrossRef]
- Simkin, A.J.; Faralli, M.; Ramamoorthy, S.; Lawson, T. Photosynthesis in non-foliar tissues: Implications for yield. Plant J. 2019. [Google Scholar] [CrossRef]
- Hibberd, J.M.; Quick, W.P. Characteristics of c4 photosynthesis in stems and petioles of c3 flowering plants. Nature 2002, 415, 451–454. [Google Scholar] [CrossRef]
- Schwender, J.; Goffman, F.; Ohlrogge, J.B.; Shachar-Hill, Y. Rubisco without the calvin cycle improves the carbon efficiency of developing green seeds. Nature 2004, 432, 779–782. [Google Scholar] [CrossRef]
- Hiratsuka, S.; Suzuki, M.; Nishimura, H.; Nada, K. Fruit photosynthesis in satsuma mandarin. Plant Sci. 2015, 241, 65–69. [Google Scholar] [CrossRef]
- Sui, X.; Shan, N.; Hu, L.; Zhang, C.; Yu, C.; Ren, H.; Turgeon, R.; Zhang, Z. The complex character of photosynthesis in cucumber fruit. J. Exp. Bot. 2017, 68, 1625–1637. [Google Scholar] [CrossRef] [Green Version]
- Hetherington, S.E.; Smillie, R.M.; Davies, W.J. Photosynthetic activities of vegetative and fruiting tissues of tomato. J. Exp. Bot. 1998, 49, 1173–1181. [Google Scholar] [CrossRef]
- Carrara, S.; Pardossi, A.; Soldatini, G.F.; Tognoni, F.; Guidi, L. Photosynthetic activity of ripening tomato fruit. Photosynthetica 2001, 39, 75–78. [Google Scholar] [CrossRef]
- Maydup, M.L.; Antonietta, M.; Guiamet, J.J.; Graciano, C.; Lopez, J.R.; Tambussi, E.A. The contribution of ear photosynthesis to grain filling in bread wheat. Field Crop. Res. 2010, 119, 48–58. [Google Scholar] [CrossRef]
- Pengelly, J.J.L.; Kwasny, S.; Bala, S.; Evans, J.R.; Voznesenskaya, E.V.; Koteyeva, N.K.; Edwards, G.E.; Furbank, R.T.; von Caemmerer, S. Functional analysis of corn husk photosynthesis. Plant Physiol. 2011, 156, 503–513. [Google Scholar] [CrossRef] [Green Version]
- Idso, S.B.; Kimball, B.A.; Shaw, P.E.; Widmer, W.; Vanderslice, J.T.; Higgs, D.J.; Montanari, A.; Clark, W.D. The effect of elevated atmospheric CO2 on the vitamin c concentration of (sour) orange juice. Agric. Ecosyst. Environ. 2002, 90, 1–7. [Google Scholar] [CrossRef]
- Wang, S.Y.; Bunce, J.A.; Maas, J.L. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. J. Agric. Food Chem. 2003, 51, 4315–4320. [Google Scholar] [CrossRef]
- Mamatha, H.; Srinivasa Rao, N.K.; Laxman, R.H.; Shivashankara, K.S.; Bhatt, R.M.; Pavithra, K.C. Impact of elevated CO2 on growth, physiology, yield, and quality of tomato (lycopersicon esculentum mill) cv. Arka ashish. Photosynthetica 2014, 52, 519–528. [Google Scholar] [CrossRef]
- Wu, X.-J.; Sun, S.; Xing, G.-M.; Wang, G.-L.; Wang, F.; Xu, Z.-S.; Tian, Y.-S.; Hou, X.-L.; Xiong, A.-S. Elevated carbon dioxide altered morphological and anatomical characteristics, ascorbic acid accumulation, and related gene expression during taproot development in carrots. Front. Plant Sci. 2017, 7, 2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes-Nesi, A.; Carrari, F.; Lytovchenko, A.; Smith, A.M.O.; Ehlers Loureiro, M.; Ratcliffe, R.G.; Sweetlove, L.J.; Fernie, A.R. Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol. 2005, 137, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from harvestplus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Nisar, N.; Li, L.; Lu, S.; Khin, N.C.; Pogson, B.J. Carotenoid metabolism in plants. Mol. Plant 2015, 8, 68–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josse, E.M.; Simkin, A.J.; Gaffe, J.; Laboure, A.M.; Kuntz, M.; Carol, P. A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol. 2000, 123, 1427–1436. [Google Scholar] [CrossRef] [Green Version]
- De Moura, F.F.; Miloff, A.; Boy, E. Retention of provitamin a carotenoids in staple crops targeted for biofortification in africa: Cassava, maize and sweet potato. Crit. Rev. Food Sci. Nutr. 2015, 55, 1246–1269. [Google Scholar] [CrossRef] [Green Version]
- Telfer, A. What is beta-carotene doing in the photosystem ii reaction centre? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2002, 357, 1431–1439. [Google Scholar] [CrossRef] [Green Version]
- Von Lintig, J.; Dreher, A.; Kiefer, C.; Wernet, M.F.; Vogt, K. Analysis of the blind drosophila mutant ninab identifies the gene encoding the key enzyme for vitamin a formation invivo. Proc. Natl. Acad. Sci. USA 2001, 98, 1130–1135. [Google Scholar] [CrossRef]
- Wyss, A.; Wirtz, G.; Woggon, W.; Brugger, R.; Wyss, M.; Friedlein, A.; Bachmann, H.; Hunziker, W. Cloning and expression of beta,beta-carotene 15,15’-dioxygenase. Biochem. Biophys. Res. Commun. 2000, 271, 334–336. [Google Scholar] [CrossRef]
- Lindqvist, A.; Andersson, S. Biochemical properties of purified recombinant human beta-carotene 15, 15’-monooxygenase. J. Biol. Chem. 2002, 277, 23942–23948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.K.; Shang, X.M.; Ko, E.Y.; Choi, J.H.; Keum, Y.-S. Stability of carotenoids and tocopherols in ready-to-eat baby-leaf lettuce and salad rocket during low-temperature storage. Int. J. Food Sci. Nutr. 2016, 67, 489–495. [Google Scholar] [CrossRef]
- Ruiz, D.; Egea, J.; Tomas-Barberan, F.A.; Gil, M.I. Carotenoids from new apricot (prunus armeniaca l.) varieties and their relationship with flesh and skin color. J. Agric. Food Chem. 2005, 53, 6368–6374. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.F.; Yousef, G.G.; Guzman, I.; Chebrolu, K.K.; Werner, D.J.; Parker, M.; Gasic, K.; Perkins-Veazie, P. Variation of carotenoids and polyphenolics in peach and implications on breeding for modified phytochemical profiles. J. Am. Soc. Hortic. Sci. 2014, 139, 676–686. [Google Scholar] [CrossRef] [Green Version]
- Baranska, M.; Baranski, R.; Schulz, H.; Nothnagel, T. Tissue-specific accumulation of carotenoids in carrot roots. Planta 2006, 224, 1028–1037. [Google Scholar] [CrossRef]
- Perrin, F.; Hartmann, L.; Dubois-Laurent, C.; Welsch, R.; Huet, S.; Hamama, L.; Briard, M.; Peltier, D.; Gagne, S.; Geoffriau, E. Carotenoid gene expression explains the difference of carotenoid accumulation in carrot root tissues. Planta 2017, 245, 737–747. [Google Scholar] [CrossRef] [Green Version]
- Rando, R.R. The chemistry of vitamin a and vision. Angew. Chem. Int. 1990, 29, 461–480. [Google Scholar] [CrossRef]
- West, C.E.; Rombout, J.H.; van der Zijpp, A.J.; Sijtsma, S.R. Vitamin a and immune function. Proc. Nutr. Soc. 1991, 50, 251–262. [Google Scholar] [CrossRef] [Green Version]
- Tanumihardjo, S.A. Vitamin a: Biomarkers of nutrition for development. Am. J. Clin. Nutr. 2011, 94, 658S–665S. [Google Scholar] [CrossRef] [Green Version]
- WHO. World Health Organisation: Micronutrient Deficiencies. Available online: http://www.who.int/nutrition/topics/vad/en/ (accessed on 19 November 2019).
- WHO. World Health Organization: Global Prevalence of Vitamin a Deficiency in Populations at Risk 1995–2005. Available online: https://apps.who.int/iris/bitstream/handle/10665/44110/9789241598019_eng.pdf (accessed on 19 November 2019).
- Hodge, J. Hidden hunger: Approaches to tackling micronutrient deficiencies. In Nourishing Millions: Stories of Change in Nutrition; Gillespie, S., Hodge, J., Yosef, S., Pandya-Lorch, R., Eds.; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2016; pp. 35–46. [Google Scholar]
- Simkin, A.J.; Moreau, H.; Kuntz, M.; Pagny, G.; Lin, C.; Tanksley, S.; McCarthy, J. An investigation of carotenoid biosynthesis in coffea canephora and coffea arabica. J. Plant Physiol. 2008, 165, 1087–1106. [Google Scholar] [CrossRef]
- Simkin, A.; McCarthy, J.; Petiard, V.; Lin, C.; Tanksley, S. Polynucleotides Encoding Carotenoid and Apocartenoid Biosynthetic Pathway Enzymes in Coffee. French Patent WO2007028115a2, 8 March 2007. [Google Scholar]
- Römer, S.; Fraser, P.D.; Kiano, J.W.; Shipton, C.A.; Misawa, N.; Schuch, W.; Bramley, P.M. Elevation of the provitamin a content of transgenic tomato plants. Nat. Biotechnol. 2000, 18, 666–669. [Google Scholar] [CrossRef]
- Fraser, P.D.; Romer, S.; Shipton, C.A.; Mills, P.B.; Kiano, J.W.; Misawa, N.; Drake, R.G.; Schuch, W.; Bramley, P.M. Evaluation of transgenic tomato plants expressing an additional phytoene synthase in a fruit-specific manner. Proc. Natl. Acad. Sci. USA 2002, 99, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Simkin, A.J.; Gaffe, J.; Alcaraz, J.P.; Carde, J.P.; Bramley, P.M.; Fraser, P.D.; Kuntz, M. Fibrillin influence on plastid ultrastructure and pigment content in tomato fruit. Phytochemistry 2007, 68, 1545–1556. [Google Scholar] [CrossRef]
- Aluru, M.; Xu, Y.; Guo, R.; Wang, Z.; Li, S.; White, W.; Wang, K.; Rodermel, S. Generation of transgenic maize with enhanced provitamin a content. J. Exp. Bot. 2008, 59, 3551–3562. [Google Scholar] [CrossRef] [Green Version]
- Strobbe, S.; De Lepeleire, J.; Van Der Straeten, D. From in planta function to vitamin-rich food crops: The ace of biofortification. Frontiers Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Cong, L.; Wang, C.; Chen, L.; Liu, H.; Yang, G.; He, G. Expression of phytoene synthase1 and carotene desaturase crti genes result in an increase in the total carotenoids content in transgenic elite wheat (triticum aestivum l.). J. Agric. Food Chem. 2009, 57, 8652–8660. [Google Scholar] [CrossRef]
- Shewmaker, C.K.; Sheehy, J.A.; Daley, M.; Colburn, S.; Ke, D.Y. Seed-specific overexpression of phytoene synthase: Increase in carotenoids and other metabolic effects. Plant J. Cell Mol. Biol. 1999, 20, 401–412X. [Google Scholar] [CrossRef]
- Diretto, G.; Al-Babili, S.; Tavazza, R.; Papacchioli, V.; Beyer, P.; Giuliano, G. Metabolic engineering of potato carotenoid content through tuber-specific overexpression of a bacterial mini-pathway. PLoS ONE 2007, 2, e350. [Google Scholar] [CrossRef] [Green Version]
- Beyene, G.; Solomon, F.R.; Chauhan, R.D.; Gaitan-Solis, E.; Narayanan, N.; Gehan, J.; Siritunga, D.; Stevens, R.L.; Jifon, J.; Van Eck, J.; et al. Provitamin a biofortification of cassava enhances shelf life but reduces dry matter content of storage roots due to altered carbon partitioning into starch. Plant Biotechnol. J. 2018, 16, 1186–1200. [Google Scholar] [CrossRef] [Green Version]
- Ducreux, L.J.M.; Morris, W.L.; Hedley, P.E.; Shepherd, T.; Davies, H.V.; Millam, S.; Taylor, M.A. Metabolic engineering of high carotenoid potato tubers containing enhanced levels of β-carotene and lutein. J. Exp. Bot. 2004, 56, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, M.; Misawa, N. Enrichment of carotenoids in flaxseed by introducing a bacterial phytoene synthase gene. Methods Mol. Biol. 2010, 643, 201–211. [Google Scholar]
- Fujisawa, M.; Watanabe, M.; Choi, S.K.; Teramoto, M.; Ohyama, K.; Misawa, N. Enrichment of carotenoids in flaxseed (linum usitatissimum) by metabolic engineering with introduction of bacterial phytoene synthase gene crtb. J. Biosci. Bioeng. 2008, 105, 636–641. [Google Scholar] [CrossRef]
- Che, P.; Zhao, Z.-Y.; Glassman, K.; Dolde, D.; Hu, T.X.; Jones, T.J.; Gruis, D.F.; Obukosia, S.; Wambugu, F.; Albertsen, M.C. Elevated vitamin e content improves all-trans β-carotene accumulation and stability in biofortified sorghum. Proc. Natl. Acad. Sci. USA 2016, 113, 11040–11045. [Google Scholar] [CrossRef] [Green Version]
- Lipkie, T.E.; De Moura, F.F.; Zhao, Z.-Y.; Albertsen, M.C.; Che, P.; Glassman, K.; Ferruzzi, M.G. Bioaccessibility of carotenoids from transgenic provitamin a biofortified sorghum. J. Agric. Food Chem. 2013, 61, 5764–5771. [Google Scholar] [CrossRef]
- Ye, X.; Al-Babili, S.; Kloti, A.; Zhang, J.; Lucca, P.; Beyer, P.; Potrykus, I. Engineering the provitamin a (β-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science 2000, 287, 303–305. [Google Scholar] [CrossRef] [Green Version]
- Paine, J.A.; Shipton, C.A.; Chaggar, S.; Howells, R.M.; Kennedy, M.J.; Vernon, G.; Wright, S.Y.; Hinchliffe, E.; Adams, J.L.; Silverstone, A.L.; et al. Improving the nutritional value of golden rice through increased pro-vitamin a content. Nat. Biotechnol. 2005, 23, 482–487. [Google Scholar] [CrossRef]
- Lu, S.; Van Eck, J.; Zhou, X.; Lopez, A.B.; O’Halloran, D.M.; Cosman, K.M.; Conlin, B.J.; Paolillo, D.J.; Garvin, D.F.; Vrebalov, J.; et al. The cauliflower or gene encodes a DNAj cysteine-rich domain-containing protein that mediates high levels of beta-carotene accumulation. Plant Cell 2006, 18, 3594–3605. [Google Scholar] [CrossRef] [Green Version]
- Simkin, A.J.; Laizet, Y.; Kuntz, M. Plastid lipid associated proteins of the fibrillin family: Structure, localisation, functions and gene expression. In Recent Research Developmetns in Biochemistry; Pandalai, S.G., Ed.; Research Signpost: Kerala, India, 2004; Volume 5, pp. 307–316. [Google Scholar]
- Yazdani, M.; Sun, Z.; Yuan, H.; Zeng, S.; Thannhauser, T.W.; Vrebalov, J.; Ma, Q.; Xu, Y.; Fei, Z.; Van Eck, J.; et al. Ectopic expression of orange promotes carotenoid accumulation and fruit development in tomato. Plant Biotechnol. J. 2019, 17, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yang, Y.; Xu, Q.; Owsiany, K.; Welsch, R.; Chitchumroonchokchai, C.; Lu, S.; Van Eck, J.; Deng, X.-X.; Failla, M.; et al. The or gene enhances carotenoid accumulation and stability during post-harvest storage of potato tubers. Mol. Plant 2012, 5, 339–352. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A.B.; Van Eck, J.; Conlin, B.J.; Paolillo, D.J.; O’Neill, J.; Li, L. Effect of the cauliflower or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J. Exp. Bot. 2008, 59, 213–223. [Google Scholar] [CrossRef]
- D’Ambrosio, C.; Giorio, G.; Marino, I.; Merendino, A.; Petrozza, A.; Salfi, L.; Stigliani, A.L.; Cellini, F. Virtually complete conversion of lycopene into β-carotene in fruits of tomato plants transformed with the tomato lycopene β-cyclase (tlcy-b) cDNA. Plant Sci. 2004, 166, 207–214. [Google Scholar] [CrossRef]
- Morris, W.L.; Ducreux, L.J.M.; Hedden, P.; Millam, S.; Taylor, M.A. Overexpression of a bacterial 1-deoxy-d-xylulose 5-phosphate synthase gene in potato tubers perturbs the isoprenoid metabolic network: Implications for the control of the tuber life cycle. J. Exp. Bot. 2006, 57, 3007–3018. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.A.; Parrott, W.A.; Hildebrand, D.F.; Berg, R.H.; Cooksey, A.; Pendarvis, K.; He, Y.; McCarthy, F.; Herman, E.M. Transgenic soya bean seeds accumulating β-carotene exhibit the collateral enhancements of oleate and protein content traits. Plant Biotechnol. J. 2015, 13, 590–600. [Google Scholar] [CrossRef] [Green Version]
- Paul, J.Y.; Khanna, H.; Kleidon, J.; Hoang, P.; Geijskes, J.; Daniells, J.; Zaplin, E.; Rosenberg, Y.; James, A.; Mlalazi, B.; et al. Golden bananas in the field: Elevated fruit pro-vitamin a from the expression of a single banana transgene. Plant Biotechnol. J. 2017, 15, 520–532. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.; Zhu, C.; Farre, G.; Ramessar, K.; Bassie, L.; Breitenbach, J.; Perez Conesa, D.; Ros, G.; Sandmann, G.; Capell, T.; et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 7762–7767. [Google Scholar] [CrossRef] [Green Version]
- Eastmond, P.; Koláčná, L.; Rawsthorne, S. Photosynthesis by developing embryos of oilseed rape (brassica napus l.). J. Exp. Bot. 1996, 47, 1763–1769. [Google Scholar] [CrossRef] [Green Version]
- Ruuska, S.A.; Schwender, J.; Ohlrogge, J.B. The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiol. 2004, 136, 2700–2709. [Google Scholar] [CrossRef] [Green Version]
- Cahoon, E.B.; Hall, S.E.; Ripp, K.G.; Ganzke, T.S.; Hitz, W.D.; Coughlan, S.J. Metabolic redesign of vitamin e biosynthesis in plants for tocotrienol production and increased antioxidant content. Nat. Biotechnol. 2003, 21, 1082–1087. [Google Scholar] [CrossRef]
- Gannon, B.; Kaliwile, C.; Arscott, S.A.; Schmaelzle, S.; Chileshe, J.; Kalungwana, N.; Mosonda, M.; Pixley, K.V.; Masi, C.; Tanumihardjo, S.A. Biofortified orange maize is as efficacious as a vitamin a supplement in zambian children even in the presence of high liver reserves of vitamin a: A community-based, randomized placebo-controlled trial. Am. J. Clin. Nutr. 2014, 100, 1541–1550. [Google Scholar] [CrossRef]
- Palmer, A.C.; Healy, K.; Barffour, M.A.; Siamusantu, W.; Chileshe, J.; Schulze, K.J.; West, K.P., Jr.; Labrique, A.B. Provitamin a carotenoid-biofortified maize consumption increases pupillary responsiveness among zambian children in a randomized controlled trial. J. Nutr. 2016, 146, 2551–2558. [Google Scholar] [CrossRef] [Green Version]
- DellaPenna, D. Biofortification of plant-based food: Enhancing folate levels by metabolic engineering. Proc. Natl. Acad. Sci. USA 2007, 104, 3675–3676. [Google Scholar] [CrossRef] [Green Version]
- Basset, G.; Quinlivan, E.P.; Gregory, J.F.; Hanson, A.D. Folate synthesis and metabolism in plants and prospects for biofortification. Crop. Sci. Soc. Am. 2005, 45, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Ravanel, S.; Douce, R.; Rebeille, F. Metabolism of folates in plants. Adv. Bot. Res. 2011, 59, 67–106. [Google Scholar]
- Bailey, L.B.; Gregory, J.F., III. Folate metabolism and requirements. J. Nutr. 1999, 129, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Poletta, F.A.; Rittler, M.; Saleme, C.; Campana, H.; Gili, J.A.; Pawluk, M.S.; Gimenez, L.G.; Cosentino, V.R.; Castilla, E.E.; Lopez-Camelo, J.S. Neural tube defects: Sex ratio changes after fortification with folic acid. PLoS ONE 2018, 13, e0193127. [Google Scholar] [CrossRef]
- Eskes, T.K. From anemia to spina bifida—The story of folic acid. A tribute to professor richard smithells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2000, 90, 119–123. [Google Scholar] [CrossRef]
- Lucock, M. Folic acid: Nutritional biochemistry, molecular biology, and role in disease processes. Mol. Genet. Metab. 2000, 71, 121–138. [Google Scholar] [CrossRef]
- Molloy, A.M.; Scott, J.M. Folates and prevention of disease. Public Health Nutr. 2001, 4, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Iyer, R.; Tomar, S.K. Folate: A functional food constituent. J. Food Sci. 2009, 74, R114–R122. [Google Scholar] [CrossRef]
- Díaz de la Garza, R.; Quinlivan, E.P.; Klaus, S.M.J.; Basset, G.J.C.; Gregory, J.F.; Hanson, A.D. Folate biofortification in tomatoes by engineering the pteridine branch of folate synthesis. Proc. Natl. Acad. Sci. USA 2004, 101, 13720–13725. [Google Scholar] [CrossRef] [Green Version]
- Díaz de la Garza, R.I.; Gregory, J.F.; Hanson, A.D. Folate biofortification of tomato fruit. Proc. Natl. Acad. Sci. USA 2007, 104, 4218–4222. [Google Scholar] [CrossRef] [Green Version]
- Hossain, T.; Rosenberg, I.; Selhub, J.; Kishore, G.; Beachy, R.; Schubert, K. Enhancement of folates in plants through metabolic engineering. Proc. Natl. Acad. Sci. USA 2004, 101, 5158–5163. [Google Scholar] [CrossRef] [Green Version]
- Basset, G.; Quinlivan, E.P.; Ziemak, M.J.; Diaz De La Garza, R.; Fischer, M.; Schiffmann, S.; Bacher, A.; Gregory, J.F.; Hanson, A.D. Folate synthesis in plants: The first step of the pterin branch is mediated by a unique bimodular gtp cyclohydrolase i. Proc. Natl. Acad. Sci. USA 2002, 99, 12489–12494. [Google Scholar] [CrossRef] [Green Version]
- Ramírez Rivera, N.G.; García-Salinas, C.; Aragão, F.J.L.; Díaz de la Garza, R.I. Metabolic engineering of folate and its precursors in mexican common bean (phaseolus vulgaris l.). Plant Biotechnol. J. 2016, 14, 2021–2032. [Google Scholar] [CrossRef]
- Dong, W.; Cheng, Z.J.; Lei, C.L.; Wang, X.L.; Wang, J.L.; Wang, J.; Wu, F.Q.; Zhang, X.; Guo, X.P.; Zhai, H.Q.; et al. Overexpression of folate biosynthesis genes in rice (oryza sativa l.) and evaluation of their impact on seed folate content. Plant Foods Hum. Nutr. 2014, 69, 379–385. [Google Scholar] [CrossRef]
- Kiekens, F.; Blancquaert, D.; Devisscher, L.; Van Daele, J.; Stove, V.V.; Delanghe, J.R.; Van Der Straeten, D.; Lambert, W.E.; Stove, C.P. Folates from metabolically engineered rice: A long-term study in rats. Mol. Nutr. Food Res. 2015, 59, 490–500. [Google Scholar] [CrossRef]
- De Steur, H.; Gellynck, X.; Blancquaert, D.; Lambert, W.; Van Der Straeten, D.; Qaim, M. Potential impact and cost-effectiveness of multi-biofortified rice in china. N. Biotechnol. 2012, 29, 432–442. [Google Scholar] [CrossRef]
- De Steur, H.; Blancquaert, D.; Lambert, W.; Van Der Straeten, D.; Gellynck, X. Conceptual framework for ex-ante evaluation at the micro/macro level of gm crops with health benefits. Trends Food Sci. Technol. 2014, 39, 116–134. [Google Scholar] [CrossRef]
- Nunes, A.C.S.; Kalkmann, D.C.; Aragão, F.J.L. Folate biofortification of lettuce by expression of a codon optimized chicken gtp cyclohydrolase i gene. Transgenic Res. 2009, 18, 661. [Google Scholar] [CrossRef]
- Blancquaert, D.; Storozhenko, S.; Van Daele, J.; Stove, C.; Visser, R.G.F.; Lambert, W.; Van Der Straeten, D. Enhancing pterin and para-aminobenzoate content is not sufficient to successfully biofortify potato tubers and arabidopsis thaliana plants with folate. J. Exp. Bot. 2013, 64, 3899–3909. [Google Scholar] [CrossRef] [Green Version]
- Storozhenko, S.; De Brouwer, V.; Volckaert, M.; Navarrete, O.; Blancquaert, D.; Zhang, G.-F.; Lambert, W.; Van Der Straeten, D. Folate fortification of rice by metabolic engineering. Nat. Biotechnol. 2007, 25, 1277–1279. [Google Scholar] [CrossRef]
- Roth, J.R.; Lawrence, J.G.; Bobik, T.A. Cobalamin (coenzyme b12): Synthesis and biological significance. Annu. Rev. Microbiol. 1996, 50, 137–181. [Google Scholar] [CrossRef] [Green Version]
- Watanaba, F. Vitamin b12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef]
- Woo, K.S.; Kwok, T.C.Y.; Celermajer, D.S. Vegan diet, subnormal vitamin b-12 status and cardiovascular health. Nutrients 2014, 6, 3259–3273. [Google Scholar] [CrossRef] [Green Version]
- Masalha, R.; Chudakov, B.; Morad, M.; Rudoy, I.; Volkov, I.; Wirguin, I. Cobalamin-responsive psychosis as the sole manifestation of vitamin b12 deficiency. Isr. Med Assoc. J. 2001, 9, 701–703. [Google Scholar]
- Biemans, E.; Hart, H.E.; Rutten, G.E.H.M.; Cuellar Renteria, V.G.; Kooijman-Buiting, A.M.J.; Beulens, J.W.J. Cobalamin status and its relation with depression, cognition and neuropathy in patients with type 2 diabetes mellitus using metformin. Acta Diabetol. 2015, 52, 383–393. [Google Scholar] [CrossRef]
- Pruthi, R.K.; Tefferi, A. Pernicious anemia revisited. Mayo Clin. Proc. 1994, 69, 144–150. [Google Scholar] [CrossRef]
- Warren, M.J.; Raux, E.; Schubert, H.L.; Escalante-Semerena, J.C. The biosynthesis of adenosylcobalamin (vitamin b12). Nat. Prod. Rep. 2002, 19, 390–412. [Google Scholar] [CrossRef]
- McGoldrick, H.M.; Roessner, C.A.; Raux, E.; Lawrence, A.D.; McLean, K.J.; Munro, A.W.; Santabarbara, S.; Rigby, S.E.J.; Heathcote, P.; Scott, A.I.; et al. Identification and characterization of a novel vitamin b12 (cobalamin) biosynthetic enzyme (cobz) from rhodobacter capsulatus, containing flavin, heme, and fe-s cofactors. J. Biol. Chem. 2005, 280, 1086–1094. [Google Scholar] [CrossRef] [Green Version]
- Warren, M.J. Finding the final pieces of the vitamin b12 biosynthetic jigsaw. Proc. Natl. Acad. Sci. USA 2006, 103, 4799–4800. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, A.D.; Nemoto-Smith, E.; Deery, E.; Baker, J.A.; Schroeder, S.; Brown, D.G.; Tullet, J.M.A.; Howard, M.J.; Brown, I.R.; Smith, A.G.; et al. Construction of fluorescent analogs to follow the uptake and distribution of cobalamin (vitamin b12) in bacteria, worms, and plants. Cell Chem. Biol. 2018, 25, 941–951. [Google Scholar] [CrossRef]
- Fang, H.; Kang, J.; Zhang, D. Microbial production of vitamin b(12): A review and future perspectives. Microb. Cell Factories 2017, 16, 15. [Google Scholar] [CrossRef] [Green Version]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin c content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef] [Green Version]
- Duarte, T.L.; Lunec, J. Reviewpart of the series: From dietary antioxidants to regulators in cellular signalling and gene expressionreview: When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin c. Free Radic. Res. 2005, 39, 671–686. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin c. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef] [Green Version]
- Harrison, F.E.; May, J.M. Vitamin c function in the brain: Vital role of the ascorbate transporter svct2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Young, J.I.; Züchner, S.; Wang, G. Regulation of the epigenome by vitamin c. Annu. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef] [Green Version]
- Camarena, V.; Wang, G. The epigenetic role of vitamin c in health and disease. Cell. Mol. Life Sci. 2016, 73, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta Rev. Cancer 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Leong, S.Y.; Oey, I. Effects of processing on anthocyanins, carotenoids and vitamin c in summer fruits and vegetables. Food Chem. 2012, 133, 1577–1587. [Google Scholar] [CrossRef]
- Da Silva Dias, J.C. Nutritional and health benefits of carrots and their seed extracts. Food Nutr. Sci. 2014, 5, 2147–2156. [Google Scholar] [CrossRef] [Green Version]
- Müller-Moulé, P.; Conklin, P.L.; Niyogi, K.K. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2002, 128, 970–977. [Google Scholar] [CrossRef] [Green Version]
- Müller-Moulé, P.; Golan, T.; Niyogi, K.K. Ascorbate-deficient mutants of arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol. 2004, 134, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Esteban, R.; Moran, J.F.; Becerril, J.M.; García-Plazaola, J.I. Versatility of carotenoids: An integrated view on diversity, evolution, functional roles and environmental interactions. Environ. Exp. Bot. 2015, 119, 63–75. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin c in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Lorence, A.; Chevone, B.I.; Mendes, P.; Nessler, C.L. Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004, 134, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Wolucka, B.A.; Van Montagu, M. Gdp-mannose 3’,5’-epimerase forms gdp-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin c in plants. J. Biol. Chem. 2003, 278, 47483–47490. [Google Scholar] [CrossRef] [Green Version]
- Wolucka, B.A.; Van Montagu, M. The vtc2 cycle and the de novo biosynthesis pathways for vitamin c in plants: An opinion. Phytochemistry 2007, 68, 2602–2613. [Google Scholar] [CrossRef]
- Agius, F.; González-Lamothe, R.; Caballero, J.L.; Muñoz-Blanco, J.; Botella, M.A.; Valpuesta, V. Engineering increased vitamin c levels in plants by overexpression of a d-galacturonic acid reductase. Nat. Biotechnol. 2003, 21, 177–181. [Google Scholar] [CrossRef]
- Loewus, F.A. Biosynthesis and metabolism of ascorbic acid in plants and of analogs of ascorbic acid in fungi. Phytochemistry 1999, 52, 193–210. [Google Scholar] [CrossRef]
- Conklin, P.L.; Norris, S.R.; Wheeler, G.L.; Williams, E.H.; Smirnoff, N.; Last, R.L. Genetic evidence for the role of gdp-mannose in plant ascorbic acid (vitamin c) biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 4198–4203. [Google Scholar] [CrossRef] [Green Version]
- Linster, C.L.; Gomez, T.A.; Christensen, K.C.; Adler, L.N.; Young, B.D.; Brenner, C.; Clarke, S.G. Arabidopsis vtc2 encodes a gdp-l-galactose phosphorylase, the last unknown enzyme in the smirnoff-wheeler pathway to ascorbic acid in plants. J. Biol. Chem. 2007, 282, 18879–18885. [Google Scholar] [CrossRef] [Green Version]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar] [CrossRef]
- Ishikawa, T.; Dowdle, J.; Smirnoff, N. Progress in manipulating ascorbic acid biosynthesis and accumulation in plants. Physiol. Plant. 2006, 126, 343–355. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhang, Y.; Cai, X.; Gong, P.; Zhang, J.; Wang, T.; Li, H.; Ye, Z. Overexpression of slgmes leads to ascorbate accumulation with enhanced oxidative stress, cold, and salt tolerance in tomato. Plant Cell Rep. 2011, 30, 389–398. [Google Scholar] [CrossRef]
- Bulley, S.; Wright, M.; Rommens, C.; Yan, H.; Rassam, M.; Lin-Wang, K.; Andre, C.; Brewster, D.; Karunairetnam, S.; Allan, A.C.; et al. Enhancing ascorbate in fruits and tubers through over-expression of the l-galactose pathway gene gdp-l-galactose phosphorylase. Plant Biotechnol. J. 2012, 10, 390–397. [Google Scholar] [CrossRef]
- Bulley, S.M.; Rassam, M.; Hoser, D.; Otto, W.; Schünemann, N.; Wright, M.; MacRae, E.; Gleave, A.; Laing, W. Gene expression studies in kiwifruit and gene over-expression in arabidopsis indicates that gdp-l-galactose guanyltransferase is a major control point of vitamin c biosynthesis. J. Exp. Bot. 2009, 60, 765–778. [Google Scholar] [CrossRef] [Green Version]
- Locato, V.; Cimini, S.; De Gara, L. Strategies to increase vitamin c in plants: From plant defense perspective to food biofortification. Front. Plant Sci. 2013, 4, 152. [Google Scholar] [CrossRef] [Green Version]
- Pignocchi, C.; Fletcher, J.M.; Wilkinson, J.E.; Barnes, J.D.; Foyer, C.H. The function of ascorbate oxidase in tobacco. Plant Physiol. 2003, 132, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, A.; Bhuiyan, M.N.H.; Waditee, R.; Tanaka, Y.; Esaka, M.; Oba, K.; Jagendorf, A.T.; Takabe, T. Suppressed expression of the apoplastic ascorbate oxidase gene increases salt tolerance in tobacco and arabidopsis plants. J. Exp. Bot. 2005, 56, 1785–1796. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Young, T.E.; Ling, J.; Chang, S.-C.; Gallie, D.R. Increasing vitamin c content of plants through enhanced ascorbate recycling. Proc. Natl. Acad. Sci. USA 2003, 100, 3525–3530. [Google Scholar] [CrossRef] [Green Version]
- Hemavathi, A.; Upadhyaya, C.P.; Akula, N.; Young, K.E.; Chun, S.C.; Kim, D.H.; Park, S.W. Enhanced ascorbic acid accumulation in transgenic potato confers tolerance to various abiotic stresses. Biotechnol. Lett. 2010, 32, 321–330. [Google Scholar] [CrossRef]
- Jain, A.K.; Nessler, C.L. Metabolic engineering of an alternative pathway for ascorbic acid biosynthesis in plants. Mol. Breed. 2000, 6, 73–78. [Google Scholar] [CrossRef]
- Radzio, J.A.; Lorence, A.; Chevone, B.I.; Nessler, C.L. L-gulono-1,4-lactone oxidase expression rescues vitamin c-deficient arabidopsis (vtc) mutants. Plant Mol. Biol. 2003, 53, 837–844. [Google Scholar] [CrossRef]
- Suekawa, M.; Fujikawa, Y.; Inoue, A.; Kondo, T.; Uchida, E.; Koizumi, T.; Esaka, M. High levels of expression of multiple enzymes in the smirnoff-wheeler pathway are important for high accumulation of ascorbic acid in acerola fruits. Biosci. Biotechnol. Biochem. 2019, 83, 1713–1716. [Google Scholar] [CrossRef]
- Schwanz, P.; Kimball, B.A.; Idso, S.B.; Hendrix, D.L.; Polle, A. Antioxidants in sun and shade leaves of sour orange trees (citrus aurantium) after long-term acclimation to elevated CO2. J. Exp. Bot. 1996, 47, 1941–1950. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.-X.; Feng, K.; Wang, G.-L.; Wu, X.-J.; Duan, A.-Q.; Yin, L.; Shen, D.; Xu, Z.-S.; Xiong, A.-S. Effect of elevated CO2 on ascorbate accumulation and the expression levels of genes involved in ascorbate metabolism in celery. J. Plant Growth Regul. 2019, 1–15. [Google Scholar] [CrossRef]
- Robinson, J.M.; Sicher, R.C. Antioxidant levels decrease in primary leaves of barley during growth at ambient and elevated carbon dioxide levels. Int. J. Plant Sci. 2004, 165, 965–972. [Google Scholar] [CrossRef]
- Cahoon, E.B.; Coughlan, S.J.; Cahoon, R.E.; Butler, K.H. Compositions and Methods for Altering Tocotrienal Content. French Patent WO2003082899A8, 30 June 2005. [Google Scholar]
- Cober, E.R.; Morrison, M.J. Soybean yield and seed composition changes in response to increasing atmospheric CO2 concentration in short-season canada. Plants 2019, 8, 250. [Google Scholar] [CrossRef] [Green Version]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.B.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 threatens human nutrition. Nature 2014, 510, 139. [Google Scholar] [CrossRef]
- Zhu, C.; Kobayashi, K.; Loladze, I.; Zhu, J.; Jiang, Q.; Xu, X.; Liu, G.; Seneweera, S.; Ebi, K.L.; Drewnowski, A.; et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018, 4, eaaq1012. [Google Scholar] [CrossRef] [Green Version]
- Aglawe, S.B.; Barbadikar, K.M.; Mangrauthia, S.K.; Madhav, M.S. New breeding technique “genome editing” for crop improvement: Applications, potentials and challenges. 3 Biotech 2018, 8, 336. [Google Scholar] [CrossRef]
- Georges, F.; Ray, H. Genome editing of crops: A renewed opportunity for food security. GM Crop. Food 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wilson, F.; Harrison, K.; Armitage, A.D.; Simkin, A.J.; Harrison, R.J. Crispr/cas9-mediated mutagenesis of phytoene desaturase in diploid and octoploid strawberry. BMC Plant Methods 2019, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- Exposito-Rodriguez, M.; Laissue, P.P.; Lopez-Calcagno, P.E.; Mullineaux, P.M.; Raines, C.A.; Simkin, A.J. Development of pgemini, a plant gateway destination vector allowing the simultaneous integration of two cdna via a single lr-clonase reaction. Plants 2017, 6, 55. [Google Scholar] [CrossRef] [Green Version]
- Engler, C.; Gruetzner, R.; Kandzia, R.; Marillonnet, S. Golden gate shuffling: A one-pot DNA shuffling method based on type iis restriction enzymes. PLoS ONE 2009, 4, e5553. [Google Scholar] [CrossRef] [Green Version]
- Engler, C.; Kandzia, R.; Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS ONE 2008, 3, e3647. [Google Scholar] [CrossRef] [Green Version]
- Engler, C.; Youles, M.; Gruetzner, R.; Ehnert, T.M.; Werner, S.; Jones, J.D.; Patron, N.J.; Marillonnet, S. A golden gate modular cloning toolbox for plants. ACS Synth Biol. 2014, 3, 839–843. [Google Scholar] [CrossRef]
- Marillonnet, S.; Werner, S. Assembly of multigene constructs using golden gate cloning. In Glyco-Engineering: Methods and Protocols; Castilho, A., Ed.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Alotaibi, S.S.; Sparks, C.A.; Parry, M.A.J.; Simkin, A.J.; Raines, C.A. Identification of leaf promoters for use in transgenic wheat. Plants 2018, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Stasolla, C.; Brule-Babel, A.; Ayele, B.T. Isolation and characterization of rubisco small subunit gene promoter from common wheat (triticum aestivum l.). Plant Signal. Behav. 2015, 10, e989033. [Google Scholar] [CrossRef] [Green Version]
- Alotaibi, S.S.; Alyassi, H.; Alshehawi, A.; Gaber, A.; Hassan, M.M.; Aljuaid, B.S.; Simkin, A.J.; Raines, C.A. Functional analysis of sbpase gene promoter in transgenic wheat under different growth conditions. Biotechnology 2019, 1, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Simkin, A.J.; Qian, T.; Caillet, V.; Michoux, F.; Ben Amor, M.; Lin, C.; Tanksley, S.; McCarthy, J. Oleosin gene family of coffea canephora: Quantitative expression analysis of five oleosin genes in developing and germinating coffee grain. J. Plant Physiol. 2006, 163, 691–708. [Google Scholar] [CrossRef]
- Simkin, A.J.; McCarthy, J.; Petiard, V.; Tanksley, S.; Lin, C. Oleosin Genes and Promoters from Coffee. French Patent WO2,007,005,928, 11 January 2007. [Google Scholar]
- Kuntz, M.; Chen, H.C.; Simkin, A.J.; Römer, S.; Shipton, C.A.; Drake, R.; Schuch, W.; Bramley, P.M. Upregulation of two ripening-related genes from a non-climacteric plant (pepper) in a transgenic climacteric plant (tomato). Plant J. 1998, 13, 351–361. [Google Scholar] [CrossRef]
- Regis, E. Golden Rice: The Imperiled Birth of a Gmo Superfood; Johns Hopkins University Press: Baltimore, MD, USA, 2019. [Google Scholar]
- Davison, J. Gm plants: Science, politics and ec regulations. Plant Sci. 2010, 178, 94–98. [Google Scholar] [CrossRef]
- Davison, J.; Ammann, K. New gmo regulations for old: Determining a new future for eu crop biotechnology. GM Crop. Food 2017, 8, 13–34. [Google Scholar] [CrossRef] [Green Version]
- Smart, R.D.; Blum, M.; Wesseler, J. Trends in approval times for genetically engineered crops in the United States and the European Union. J. Agric. Econ. 2017, 68, 182–198. [Google Scholar] [CrossRef] [Green Version]




| Manipulation | Plant | Transgene(s) Expressed | Biomass and Yield | Ref | ||
|---|---|---|---|---|---|---|
| CBC and Photorespiration | Arabidopsis Col-0 | SBPase | - | - | 42% increase in dry weight. 53% increase in seed yield under LL (39% increase in seed yield under HL) | [41] |
| - | Ald | - | 32% increase in dry weight. 35% increase in seed yield under LL (36% increase in seed yield under HL) | |||
| SBPase | Ald | - | 41% increase in dry weight. 49% increase in seed yield under LL (20% increase in seed yield under HL) | |||
| - | - | GDCH | 50% increase in dry weight. 0% increase in seed yield under LL (0% increase in seed yield under HL) | |||
| SBPase | Ald | GDCH | 71% increase in dry weight. 42% increase in seed yield under LL (62% increase in seed yield under HL) | |||
| CBC and CO2 transport | Tobacco cv Samsun | SBPase | - | - | 30–34% increase in dry weight under HL (52% under LL) | [42] |
| - | - | ictB | 71% increase in dry weight (HL) | |||
| SBPase | - | ictB | 92% increase in dry weight under HL (76% under LL) | |||
| SBPase | Ald | - | 62% increase in dry weight under HL (54% under LL) | |||
| SBPase | Ald | ictB | 103% increase in dry weight under HL (79% under LL) | |||
| CBC and CO2 transport | Rice | FS Bif | - | - | no increase in biomass observed | [88] |
| - | - | ictB | no increase in biomass observed | |||
| FS Bif | - | ictB | increase in biomass demonstrating the synergistic effect | |||
| Plant | Transgene(s) Expressed | Metabolite Analysis | Ref | ||
|---|---|---|---|---|---|
| Tomato fruit | crtB | - | - | phytoene content increase (1.6–3.1-fold). Lycopene (1.8–2.1-fold) and β-carotene (1.6–2.7-fold) were increased | [125] |
| - | crtL | - | β -carotene content increased about threefold, up to 45% of the total carotenoid content | [124] | |
| - | - | SlLyc | Increase in total carotenoids (2.3-fold). β-carotene increased (11.8-fold) and Lycopene decrease (10-fold) | [145] | |
| - | - | AtOr | Increases in Lycopene (1.6-fold), α-carotene 2.6-fold) and β-carotene (2.7-fold) | [142] | |
| - | - | CaFib | Increases in Lycopene (2.2-fold) and β-carotene (1.6-fold) | [126] | |
| Cassava tubers | crtB | - | - | ~15-fold increases in carotenoids (as all-trans-β-carotene) (40–60 µg/g DW compared to CN 0.5–1 µg/g DW) | [132] |
| crtB | AtDxs | - | 20- to 30-fold increases in carotenoids (as all-trans-β-carotene) (25 µg/g DW) compared to CN 0.5–1 µg/g DW) | ||
| - | - | BoOr | ~2-fold increases in carotenoids (as all-trans-β-carotene) (3–4 µg/g DW) compared to CN 0.5–1 µg/g DW) | ||
| Potato tubers | - | DXS | - | 2-fold increase in total carotenoids and 6- to 7- fold increase in phytoene | [146] |
| crtB | - | - | Carotenoid levels reached 35 μg/g. β-carotene levels in the transgenic tubers reached ~11 μg/g DW | [133] | |
| crtB | AtDxs | - | 37–109 µg/g DW total carotenoids (CN 8 µg/g) | [132] | |
| crtB | crtL | crtY | 20-fold increase (to 114 µg/g DW) with β-carotene 3600-fold higher (47 µg /g DW) | [131] | |
| - | - | BoOr | The total carotenoid contents were 6-old higher than CN. Increasing from ~4 µg/g DW to ~22 µg/g DW | [140] | |
| Canola seed | crtB | - | - | 50-fold increase in carotenoids with α- and β-carotene. Lutein, the predominant carotenoid in CN seeds remained at similar levels in transgenic seeds | [130] |
| Soybean | crtB | - | - | Accumulate 845µg/g DW of β carotene. An increase of 1500-fold compared to CN | [147] |
| Wheat | ZmPsy | ctrI | - | Increase β-carotene from 0.81µg /g DW to 2.3–4.9 µg /g DW in the best lines | [129] |
| Cavendish Banana | MtPsy | - | - | Increase in β-carotene content from 3.1 µg/g DW in fully ripe fruit to up to 8.3 µg/g DW. | [148] |
| ZmPsy | - | - | Increase in β-carotene content from 3.1 µg/g DW in fully ripe fruit to up to 9.0 µg/g DW. | ||
| ZmPsy | ctrI | - | Increase in β-carotene content from 3.1 µg/g DW in fully ripe fruit to up to 13.2 µg/g DW. | ||
| Maize | ZmPsy | ctrI | - | Increase β-carotene from 0.35 µg /g DW to 15–59 µg /g DW in the best lines. Up to 100-fold increase in total carotenoids (see Section 3.6) | [149] |
| crtB | ctrI | - | Increase β-carotene from 0.39 µg /g DW to 9.8 µg /g DW in the best line | [127] | |
| Rice | NpPsy | crtI | - | β-carotene, + small amounts of lutein and zeaxanthin | [138] |
| NpPsy | crtI | NpLyc | 1.6 µg/g carotenoid in the endosperm | ||
| NpPsy | crtI | - | 0.8–1.2 µg/g (up to 68% β-carotene) | [139] | |
| SlPsy | crtI | - | 0.9–1.2 µg/g (up to 68% β-carotene) | ||
| CaPsy | crtI | - | 1.1–4.7 µg/g (up to 80% β-carotene) | ||
| ZmPsy | crtI | - | Up to 14.5 µg/g (up to 89% β-carotene) | ||
| OsPsy | crtI | - | Up to 18.4 µg/g (up to 86% β-carotene) | ||
| Sorghum | AtDxs | ZmPsy | ctrI, PMI | β-carotene levels ranged from 2.5 to 9.1 μg/g DW in the mature seeds compared to CN 0.5 μg/g DW (+10-fold) | [136] |
| HGGTAtDxs | ZmPsy, | ctrI, PMI | all-trans β-carotene levels ranged from 7.3 to 12.3 μg/g DW in the mature seeds compared to CN 0.5 μg/g DW (~19-fold increase) +1.8-fold increase in α-tocopherol | ||
| Plant | Transgene(s) Expressed | Metabolite Analysis | Ref | |||
|---|---|---|---|---|---|---|
| Pterins | PABA | Folate | ||||
| Arabidopsis | GCHI | - | 1250-fold increase | NR | 2- to 4-fold increase. | [166] |
| Mexican Bean | GCHI | - | 150-fold increase | Increase | Up to 3-fold increase in desiccated beans | [168] |
| Lettuce | GCHI | - | NR | NR | 2- to 8.5-fold increase | [173] |
| Potato | GCHI | Approx. 18-fold increase | Decrease | Up to 2-fold increase | [174] | |
| GCHI | ADCS | Approx. 33-fold increase | >6-fold increase | Up to 3-fold increase | ||
| Tomato Fruit | GCHI | - | 3- to 140-fold increase | Severely depleted | average 2-fold increase in ripe fruit | [164,165] |
| - | ADCS | No increase observed | Up to 20-fold increase | No increase observed in ripe fruit | ||
| GCHI | ADCS | Up to 30-fold increase | Up to 20-fold increase | Up to 25-fold increase in ripe fruit | ||
| Rice | GCHI | - | 25-fold increase | NR | No increase observed | [175] |
| - | ADCS | NR | 49 times higher than controls | 6 times lower than in controls | ||
| GCHI | ADCS | 4-fold increase | 25 times high than control | 15–100 times higher than CN | ||
| Corn | E. coli folE encoding GCHI | NR | NR | ~2-fold increase (see Section 3.6) | [149] | |
| Plant | Enzyme | Regulation | Metabolite Analysis | Ref |
|---|---|---|---|---|
| Tomato | GDP-Man-3’,5’-epimerase | Up | 1.2- to 1.6-fold increase in fruit | [209] |
| Arabidopsis | GDP-L-Gal phosphorylase | Up | Up to 4-fold increase in best lines | [211] |
| Tomato | GDP-L-Gal phosphorylase | Up | 3- to 6-fold increase in fruit ascorbate | [210] |
| Strawberry | GDP-L-Gal phosphorylase | Up | 2-fold increase in tuber ascorbate | [210] |
| Potato | GDP-L-Gal phosphorylase | Up | Up to 3-fold increase in fruit ascorbate | [210] |
| Tobacco | ascorbate oxidase | Down | 1.9-fold increase in ascorbate and increase in the Ascorbate to DHA ratio | [213] |
| Tobacco | ascorbate oxidase | Down | No increase in the ascorbate pool but increase in ratio of Ascorbate to DHA | [214] |
| Tobacco | dehydroascorbate reductase | Up | 2.2- to 3.9-fold increase in ascorbate and increase in the Ascorbate to DHA ratio | [215] |
| Maize | dehydroascorbate reductase | Up | 1.9-fold increase in ascorbate and increase in the Ascorbate to DHA ratio | [215] |
| Arabidopsis | D-glucuronate reductase | Up | 2- to 3-fold increase in ascorbate | [203] |
| Potato | D-galacturonate reductase | Up | Up to 2-fold increase in tuber ascorbate | [216] |
| Tobacco | L-gulonolactone oxidase | Up | 7-fold increase in ascorbate | [217] |
| Lettuce | L-gulonolactone oxidase | Up | 4- to 7-fold increase in ascorbate | [217] |
| Arabidopsis | L-gulonolactone oxidase | Up | ~2.0-fold increase in ascorbate | [218] |
| Arabidopsis | myo-inositol oxygenase | Up | 2- to 3-fold increase in the ascorbate content of leaves compared with controls | [200] |
| Corn | dehydroascorbate reductase | Up | Up to 7.5-fold increase in ascorbate (see Section 3.6) | [149] |
| Tobacco | malate dehydrogenase | Down | 5.7-fold increase in ascorbate | [102] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simkin, A.J. Genetic Engineering for Global Food Security: Photosynthesis and Biofortification. Plants 2019, 8, 586. https://doi.org/10.3390/plants8120586
Simkin AJ. Genetic Engineering for Global Food Security: Photosynthesis and Biofortification. Plants. 2019; 8(12):586. https://doi.org/10.3390/plants8120586
Chicago/Turabian StyleSimkin, Andrew John. 2019. "Genetic Engineering for Global Food Security: Photosynthesis and Biofortification" Plants 8, no. 12: 586. https://doi.org/10.3390/plants8120586
APA StyleSimkin, A. J. (2019). Genetic Engineering for Global Food Security: Photosynthesis and Biofortification. Plants, 8(12), 586. https://doi.org/10.3390/plants8120586





