Plasmodesmata Conductivity Regulation: A Mechanistic Model
Abstract
:1. Introduction
2. Substructural Architecture of Plasmodesmata
3. Plasmodesmata-Associated Proteins (PdAPs)
3.1. Non-Secretory Pd Proteins
3.1.1. Actin, Myosin and Tubulin
3.1.2. Synaptotagmins
3.1.3. Remorin
3.1.4. Calreticulin
3.1.5. Non-Cell-Autonomous Pathway Proteins (NCAPPs)
3.1.6. Reticulons
3.2. Secreted PdAPs
3.2.1. Callose-Degrading β-1,3-glucanases (BG)
3.2.2. Pd-Associated Callose Binding Proteins (PDCBs)
3.2.3. Plasmodesmata-Located Protein 1 (PDLP1)
3.2.4. Plasmodesmata-Located Protein 5 (PDLP5)
3.2.5. β-1,6-N-acetylglucosaminyl Transferase-Like Enzyme (GnTL)
3.2.6. Cell Wall Pectin and Pectin Methylesterase (PME) as Factors Controlling Pd Permeability
3.2.7. Callose Synthase (CalS)
3.2.8. Formins
3.2.9. Class 1 Reversibly Glycosylated Polypeptide (C1RGP)
4. Mechanisms of PdAP Participation in Intercellular Cytoplasmic Connectivity
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jeandroz, S.; Lamotte, O. Editorial: Plant Responses to Biotic and Abiotic Stresses: Lessons from Cell Signaling. Front. Plant Sci. 2017, 8, 1772. [Google Scholar] [CrossRef] [Green Version]
- Aubry, E.; Dinant, S.; Vilaine, F.; Bellini, C.; Le Hir, R. Lateral Transport of Organic and Inorganic Solutes. Plants 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-Y. Plasmodesmata: A signaling hub at the cellular boundary. Curr. Opin. Plant Biol. 2015, 27, 133–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevilem, I.; Yadav, S.R.; Helariutta, Y. Plasmodesmata: Channels for intercellular signaling during plant growth and development. In Plasmodesmata: Methods and Protocols; Heinlein, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1217, pp. 3–24. [Google Scholar] [CrossRef]
- Chung, K.P.; Zeng, Y. An Overview of Protein Secretion in Plant Cells. In Plant Protein Secretion: Methods and Protocols; Jiang, L., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1662, pp. 19–32. [Google Scholar] [CrossRef]
- Davis, D.J.; Kang, B.-H.; Heringer, A.S.; Wilkop, T.E.; Drakakaki, G. Unconventional Protein Secretion in Plants. In Unconventional Protein Secretion: Methods and Protocols; Pompa, A., De Marchis, F., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; Volume 1459, pp. 47–63. [Google Scholar] [CrossRef]
- Goring, D.R.; Di Sansebastiano, G.P. Protein and membrane trafficking routes in plants: Conventional or unconventional? J. Exp. Bot. 2017, 69, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellucci, M.; De Marchis, F.; Pompa, A. The endoplasmic reticulum is a hub to sort proteins toward unconventional traffic pathways and endosymbiotic organelles. J. Exp. Bot. 2018, 69, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.; Li, R.; Pan, J.; Ding, Z.; Lin, J. Endocytosis and its regulation in plants. Trends Plant Sci. 2015, 20, 388–397. [Google Scholar] [CrossRef]
- Rosquete, M.R.; Drakakaki, G. Plant TGN in the stress response: A compartmentalized overview. Curr. Opin. Plant Biol. 2018, 46, 122–129. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, J.; Lin, J. At the intersection of exocytosis and endocytosis in plants. New Phytol. 2019. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Jwa, N.-S.; Lebrun, M.-H.; Job, D.; Rakwal, R. Plant secretome: Unlocking secrets of the secreted proteins. Proteomics 2010, 10, 799–827. [Google Scholar] [CrossRef]
- Ding, Y.; Robinson, D.G.; Jiang, L. Unconventional protein secretion (UPS) pathways in plants. Curr. Opin. Cell Biol. 2014, 29, 107–115. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, J.; Wang, J.; Stierhof, Y.-D.; Robinson, D.G.; Jiang, L. Unconventional protein secretion. Trends Plant Sci. 2012, 17, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Marchis, F.D.; Bellucci, M.; Pompa, A. Unconventional pathways of secretory plant proteins from the endoplasmic reticulum to the vacuole bypassing the Golgi complex. Plant Signal. Behav. 2013, 8, e25129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickel, W.; Seedorf, M. Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu. Rev. Cell Dev. Biol. 2008, 24, 287–308. [Google Scholar] [CrossRef] [PubMed]
- Brunkard, J.O.; Zambryski, P.C. Plasmodesmata enable multicellularity: New insights into their evolution, biogenesis, and functions in development and immunity. Curr. Opin. Plant Biol. 2017, 35, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medrano, R.; Xoconostle-Cazares, B.; Kragler, F. The plasmodesmatal transport pathway for homeotic proteins, silencing signals and viruses. Curr. Opin. Plant Biol. 2004, 7, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X. Intercellular and systemic trafficking of RNAs in plants. Nat. Plants 2018, 4, 869–878. [Google Scholar] [CrossRef]
- Reagan, B.C.; Ganusova, E.E.; Fernandez, J.C.; McCray, T.N.; Burch-Smith, T.M. RNA on the move: The plasmodesmata perspective. Plant Sci. Int. J. Exp. Plant Biol. 2018, 275, 1–10. [Google Scholar] [CrossRef]
- Cheval, C.; Faulkner, C. Plasmodesmal regulation during plant-pathogen interactions. New Phytol. 2018, 217, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Ganusova, E.E.; Burch-Smith, T.M. Review: Plant-pathogen interactions through the plasmodesma prism. Plant Sci. Int. J. Exp. Plant Biol. 2019, 279, 70–80. [Google Scholar] [CrossRef]
- Otero, S.; Helariutta, Y.; Benitez-Alfonso, Y. Symplastic communication in organ formation and tissue patterning. Curr. Opin. Plant Biol. 2016, 29, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Julius, B.T.; Leach, K.A.; Tran, T.M.; Mertz, R.A.; Braun, D.M. Sugar Transporters in Plants: New Insights and Discoveries. Plant Cell Physiol. 2017, 58, 1442–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liesche, J. Sucrose transporters and plasmodesmal regulation in passive phloem loading. J. Integr. Plant Biol. 2017, 59, 311–321. [Google Scholar] [CrossRef]
- Liesche, J.; Gao, C.; Binczycki, P.; Andersen, S.R.; Rademaker, H.; Schulz, A.; Martens, H.J. Direct Comparison of Leaf Plasmodesma Structure and Function in Relation to Phloem-Loading Type. Plant Physiol. 2019, 179, 1768–1778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burch-Smith, T.M.; Stonebloom, S.; Xu, M.; Zambryski, P.C. Plasmodesmata during development: Re-examination of the importance of primary, secondary, and branched plasmodesmata structure versus function. Protoplasma 2011, 248, 61–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burch-Smith, T.M.; Zambryski, P.C. Plasmodesmata paradigm shift: Regulation from without versus within. Annu. Rev. Plant Biol. 2012, 63, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, C. Plasmodesmata and the symplast. Curr. Biol. 2018, 28, R1374–R1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patrick, J.W.; Botha, F.C.; Birch, R.G. Metabolic engineering of sugars and simple sugar derivatives in plants. Plant Biotechnol. J. 2013, 11, 142–156. [Google Scholar] [CrossRef]
- Sager, R.; Lee, J.-Y. Plasmodesmata in integrated cell signalling: Insights from development and environmental signals and stresses. J. Exp. Bot. 2014, 65, 6337–6358. [Google Scholar] [CrossRef] [Green Version]
- Sager, R.E.; Lee, J.-Y. Plasmodesmata at a glance. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Huang, D.; Chen, X. Dynamic regulation of plasmodesmatal permeability and its application to horticultural research. Hortic. Res. 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Tilsner, J.; Nicolas, W.; Rosado, A.; Bayer, E.M. Staying Tight: Plasmodesmal Membrane Contact Sites and the Control of Cell-to-Cell Connectivity in Plants. Annu. Rev. Plant Biol. 2016, 67, 337–364. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Erlanger, M.; Rosenthal, M.; Epel, B.L. A plasmodesmata-associated beta-1,3-glucanase in Arabidopsis. Plant J. Cell Mol. Biol. 2007, 49, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Zavaliev, R.; Ueki, S.; Epel, B.L.; Citovsky, V. Biology of callose (β-1,3-glucan) turnover at plasmodesmata. Protoplasma 2011, 248, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-W.; Kumar, R.; Iswanto, A.B.B.; Kim, J.-Y. Callose balancing at plasmodesmata. J. Exp. Bot. 2018, 69, 5325–5339. [Google Scholar] [CrossRef]
- Ueki, S.; Citovsky, V. Plasmodesmata-associated proteins: Can we see the whole elephant? Plant Signal. Behav. 2014, 9, e27899. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Calvino, L.; Faulkner, C.; Walshaw, J.; Saalbach, G.; Bayer, E.; Benitez-Alfonso, Y.; Maule, A. Arabidopsis plasmodesmal proteome. PLoS ONE 2011, 6, e18880. [Google Scholar] [CrossRef] [Green Version]
- Roberts, I.M.; Boevink, P.; Roberts, A.G.; Sauer, N.; Reichel, C.; Oparka, K.J. Dynamic changes in the frequency and architecture of plasmodesmata during the sink-source transition in tobacco leaves. Protoplasma 2001, 218, 31–44. [Google Scholar] [CrossRef]
- Tilsner, J.; Amari, K.; Torrance, L. Plasmodesmata viewed as specialised membrane adhesion sites. Protoplasma 2011, 248, 39–60. [Google Scholar] [CrossRef]
- Nicolas, W.J.; Grison, M.S.; Bayer, E.M. Shaping intercellular channels of plasmodesmata: The structure-to-function missing link. J. Exp. Bot. 2017, 69, 91–103. [Google Scholar] [CrossRef] [Green Version]
- Ross-Elliott, T.J.; Jensen, K.H.; Haaning, K.S.; Wager, B.M.; Knoblauch, J.; Howell, A.H.; Mullendore, D.L.; Monteith, A.G.; Paultre, D.; Yan, D.; et al. Phloem unloading in Arabidopsis roots is convective and regulated by the phloem-pole pericycle. eLIFE 2017, 6, e24125. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, W.J.; Grison, M.S.; Trépout, S.; Gaston, A.; Fouché, M.; Cordelières, F.P.; Oparka, K.; Tilsner, J.; Brocard, L.; Bayer, E.M. Architecture and permeability of post-cytokinesis plasmodesmata lacking cytoplasmic sleeves. Nat. Plants 2017, 3, 17082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oparka, K.J.; Roberts, A.G.; Boevink, P.; Santa Cruz, S.; Roberts, I.; Pradel, K.S.; Imlau, A.; Kotlizky, G.; Sauer, N.; Epel, B. Simple, but not branched, plasmodesmata allow the nonspecific trafficking of proteins in developing tobacco leaves. Cell 1999, 97, 743–754. [Google Scholar] [CrossRef] [Green Version]
- Amsbury, S.; Benitez-Alfonso, Y. Tightening the pores to unload the phloem. Nat. Plants 2019, 5, 561–562. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Huang, L.-J.; Feng, D.; Jiang, W.; Miu, W.; Li, N. Plasmodesmata-Related Structural and Functional Proteins: The Long Sought-After Secrets of a Cytoplasmic Channel in Plant Cell Walls. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackman, L.M.; Overall, R.L. Immunolocalisation of the cytoskeleton to plasmodesmata of Chara corallina. Plant J. 1998, 14, 733–741. [Google Scholar] [CrossRef]
- Radford, J.E.; White, R.G. Localization of a myosin-like protein to plasmodesmata. Plant J. Cell Mol. Biol. 1998, 14, 743–750. [Google Scholar] [CrossRef] [Green Version]
- White, R.G.; Badelt, K.; Overall, R.L.; Vesk, M. Actin associated with plasmodesmata. Protoplasma 1994, 180, 169–184. [Google Scholar] [CrossRef]
- Baluska, F.; Samaj, J.; Napier, R.; Volkmann, D. Maize calreticulin localizes preferentially to plasmodesmata in root apex. Plant J. Cell Mol. Biol. 1999, 19, 481–488. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-H.; Tian, G.-W.; Gafni, Y.; Citovsky, V. Effects of calreticulin on viral cell-to-cell movement. Plant Physiol. 2005, 138, 1866–1876. [Google Scholar] [CrossRef] [Green Version]
- Ye, C.-M.; Chen, S.; Payton, M.; Dickman, M.B.; Verchot, J. TGBp3 triggers the unfolded protein response and SKP1-dependent programmed cell death. Mol. Plant Pathol. 2013, 14, 241–255. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Yoo, B.-C.; Rojas, M.R.; Gomez-Ospina, N.; Staehelin, L.A.; Lucas, W.J. Selective trafficking of non-cell-autonomous proteins mediated by NtNCAPP1. Science 2003, 299, 392–396. [Google Scholar] [CrossRef]
- Sheshukova, E.V.; Komarova, T.V.; Pozdyshev, D.V.; Ershova, N.M.; Shindyapina, A.V.; Tashlitsky, V.N.; Sheval, E.V.; Dorokhov, Y.L. The intergenic interplay between aldose 1-epimerase-like protein and pectin methylesterase in abiotic and biotic stress control. Front. Plant Sci. 2017, 8, 1646. [Google Scholar] [CrossRef] [Green Version]
- Raffaele, S.; Bayer, E.; Lafarge, D.; Cluzet, S.; German Retana, S.; Boubekeur, T.; Leborgne-Castel, N.; Carde, J.-P.; Lherminier, J.; Noirot, E.; et al. Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs potato virus X movement. Plant Cell 2009, 21, 1541–1555. [Google Scholar] [CrossRef] [Green Version]
- Knox, K.; Wang, P.; Kriechbaumer, V.; Tilsner, J.; Frigerio, L.; Sparkes, I.; Hawes, C.; Oparka, K. Putting the Squeeze on Plasmodesmata: A Role for Reticulons in Primary Plasmodesmata Formation. Plant Physiol. 2015, 168, 1563–1572. [Google Scholar] [CrossRef] [Green Version]
- Kriechbaumer, V.; Botchway, S.W.; Slade, S.E.; Knox, K.; Frigerio, L.; Oparka, K.; Hawes, C. Reticulomics: Protein-Protein Interaction Studies with Two Plasmodesmata-Localized Reticulon Family Proteins Identify Binding Partners Enriched at Plasmodesmata, Endoplasmic Reticulum, and the Plasma Membrane1. Plant Physiol. 2015, 169, 1933–1945. [Google Scholar] [CrossRef]
- Levy, A.; Zheng, J.Y.; Lazarowitz, S.G. Synaptotagmin SYTA forms ER-plasma membrane junctions that are recruited to plasmodesmata for plant virus movement. Curr. Biol. 2015, 25, 2018–2025. [Google Scholar] [CrossRef] [Green Version]
- Lewis, J.D.; Lazarowitz, S.G. Arabidopsis synaptotagmin SYTA regulates endocytosis and virus movement protein cell-to-cell transport. Proc. Natl. Acad. Sci. USA 2010, 107, 2491–2496. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, A.; Shimada-Beltran, H.; Levy, A.; Zheng, J.Y.; Javia, P.A.; Lazarowitz, S.G. The Arabidopsis synaptotagmin SYTA regulates the cell-to-cell movement of diverse plant viruses. Front. Plant Sci. 2014, 5, 584. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Lazarowitz, S.G.; Citovsky, V. The Plasmodesmal Localization Signal of TMV MP Is Recognized by Plant Synaptotagmin SYTA. mBio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Doxey, A.C.; Yaish, M.W.F.; Moffatt, B.A.; Griffith, M.; McConkey, B.J. Functional divergence in the Arabidopsis beta-1,3-glucanase gene family inferred by phylogenetic reconstruction of expression states. Mol. Biol. Evol. 2007, 24, 1045–1055. [Google Scholar] [CrossRef] [Green Version]
- Zavaliev, R.; Levy, A.; Gera, A.; Epel, B.L. Subcellular dynamics and role of Arabidopsis β-1,3-glucanases in cell-to-cell movement of tobamoviruses. Mol. Plant-Microbe Interact. MPMI 2013, 26, 1016–1030. [Google Scholar] [CrossRef] [Green Version]
- Levy, A.; Guenoune-Gelbart, D.; Epel, B.L. β-1,3-Glucanases. Plant Signal. Behav. 2007, 2, 404–407. [Google Scholar] [CrossRef] [Green Version]
- Burch-Smith, T.M.; Cui, Y.; Zambryski, P.C. Reduced levels of class 1 reversibly glycosylated polypeptide increase intercellular transport via plasmodesmata. Plant Signal. Behav. 2012, 7, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Sagi, G.; Katz, A.; Guenoune-Gelbart, D.; Epel, B.L. Class 1 reversibly glycosylated polypeptides are plasmodesmal-associated proteins delivered to plasmodesmata via the golgi apparatus. Plant Cell 2005, 17, 1788–1800. [Google Scholar] [CrossRef] [Green Version]
- Zavaliev, R.; Sagi, G.; Gera, A.; Epel, B.L. The constitutive expression of Arabidopsis plasmodesmal-associated class 1 reversibly glycosylated polypeptide impairs plant development and virus spread. J. Exp. Bot. 2010, 61, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Cui, W.; Lee, J.-Y. Arabidopsis callose synthases CalS1/8 regulate plasmodesmal permeability during stress. Nat. Plants 2016, 2, 16034. [Google Scholar] [CrossRef]
- Diao, M.; Ren, S.; Wang, Q.; Qian, L.; Shen, J.; Liu, Y.; Huang, S. Arabidopsis formin 2 regulates cell-to-cell trafficking by capping and stabilizing actin filaments at plasmodesmata. eLIFE 2018, 7. [Google Scholar] [CrossRef]
- Oulehlovï, D.; Kollï Rovï, E.; Cifrovï, P.; Pejchar, P.; Žï Rskï, V.; Cvrčkovï, F. Arabidopsis Class I Formin FH1 Relocates between Membrane Compartments during Root Cell Ontogeny and Associates with Plasmodesmata. Plant Cell Physiol. 2019, 60, 1855–1870. [Google Scholar] [CrossRef]
- Zalepa-King, L.; Citovsky, V. A plasmodesmal glycosyltransferase-like protein. PLoS ONE 2013, 8, e58025. [Google Scholar] [CrossRef]
- Benitez-Alfonso, Y.; Faulkner, C.; Pendle, A.; Miyashima, S.; Helariutta, Y.; Maule, A. Symplastic intercellular connectivity regulates lateral root patterning. Dev. Cell 2013, 26, 136–147. [Google Scholar] [CrossRef] [Green Version]
- Simpson, C.; Thomas, C.; Findlay, K.; Bayer, E.; Maule, A.J. An Arabidopsis GPI-Anchor Plasmodesmal Neck Protein with Callose Binding Activity and Potential to Regulate Cell-to-Cell Trafficking. Plant Cell 2009, 21, 581–594. [Google Scholar] [CrossRef] [Green Version]
- Amari, K.; Boutant, E.; Hofmann, C.; Schmitt-Keichinger, C.; Fernandez-Calvino, L.; Didier, P.; Lerich, A.; Mutterer, J.; Thomas, C.L.; Heinlein, M.; et al. A Family of Plasmodesmal Proteins with Receptor-Like Properties for Plant Viral Movement Proteins. PLoS Pathog. 2010, 6, e1001119. [Google Scholar] [CrossRef] [Green Version]
- Bayer, E.; Thomas, C.; Maule, A. Symplastic domains in the Arabidopsis shoot apical meristem correlate with PDLP1 expression patterns. Plant Signal. Behav. 2008, 3, 853–855. [Google Scholar] [CrossRef] [Green Version]
- Lim, G.-H.; Shine, M.B.; de Lorenzo, L.; Yu, K.; Cui, W.; Navarre, D.; Hunt, A.G.; Lee, J.-Y.; Kachroo, A.; Kachroo, P. Plasmodesmata Localizing Proteins Regulate Transport and Signaling during Systemic Acquired Immunity in Plants. Cell Host Microbe 2016, 19, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.L.; Bayer, E.M.; Ritzenthaler, C.; Fernandez-Calvino, L.; Maule, A.J. Specific targeting of a plasmodesmal protein affecting cell-to-cell communication. PLoS Biol. 2008, 6, e7. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-Y.; Wang, X.; Cui, W.; Sager, R.; Modla, S.; Czymmek, K.; Zybaliov, B.; van Wijk, K.; Zhang, C.; Lu, H.; et al. A plasmodesmata-localized protein mediates crosstalk between cell-to-cell communication and innate immunity in Arabidopsis. Plant Cell 2011, 23, 3353–3373. [Google Scholar] [CrossRef] [Green Version]
- Vaattovaara, A.; Brandt, B.; Rajaraman, S.; Safronov, O.; Veidenberg, A.; Luklová, M.; Kangasjärvi, J.; Löytynoja, A.; Hothorn, M.; Salojärvi, J.; et al. Mechanistic insights into the evolution of DUF26-containing proteins in land plants. Commun. Biol. 2019, 2, 56. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, P.; Qu, S.; Zhao, J.; Singh, P.K.; Wang, W. Ectodomain of plasmodesmata-localized protein 5 in Arabidopsis: Expression, purification, crystallization and crystallographic analysis. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2017, 73, 532–535. [Google Scholar] [CrossRef]
- Ning-Jing, L.; Tao, Z.; Zhao-Hui, L.; Xin, C.; Hui-Shan, G.; Bai-Hang, J.; Yuan-Yuan, Z.; Guo-Zhu, L.; Qiang-Hui, Z.; Yong-Mei, Q.; et al. Phytosphinganine affects plasmodesmata permeability via facilitating pdlp5-stimulated callose accumulation in arabidopsis. Mol. Plant. 2019. [Google Scholar] [CrossRef]
- Knox, J.P.; Benitez-Alfonso, Y. Roles and regulation of plant cell walls surrounding plasmodesmata. Curr. Opin. Plant Biol. 2014, 22, 93–100. [Google Scholar] [CrossRef]
- Morvan, O.; Quentin, M.; Jauneau, A.; Mareck, A.; Morvan, C. Immunogold localization of pectin methylesterases in the cortical tissues of flax hypocotyl. Protoplasma 1998, 202, 175–184. [Google Scholar] [CrossRef]
- Zavaliev, R.; Dong, X.; Epel, B.L. Glycosylphosphatidylinositol (GPI) Modification Serves as a Primary Plasmodesmal Sorting Signal. Plant Physiol. 2016, 172, 1061–1073. [Google Scholar] [CrossRef] [Green Version]
- Mirabet, V.; Krupinski, P.; Hamant, O.; Meyerowitz, E.M.; Jönsson, H.; Boudaoud, A. The self-organization of plant microtubules inside the cell volume yields their cortical localization, stable alignment, and sensitivity to external cues. PLoS Comput. Biol. 2018, 14, e1006011. [Google Scholar] [CrossRef]
- Yuan, C.; Lazarowitz, S.G.; Citovsky, V. Identification of a Functional Plasmodesmal Localization Signal in a Plant Viral Cell-To-Cell-Movement Protein. mBio 2016, 7, e02052-15. [Google Scholar] [CrossRef] [Green Version]
- Yuan, C.; Lazarowitz, S.G.; Citovsky, V. Identification of Plasmodesmal Localization Sequences in Proteins in Planta. J. Vis. Exp. JoVE 2017. [Google Scholar] [CrossRef] [Green Version]
- Pitzalis, N.; Heinlein, M. The roles of membranes and associated cytoskeleton in plant virus replication and cell-to-cell movement. J. Exp. Bot. 2017, 69, 117–132. [Google Scholar] [CrossRef]
- Reymond, P.; Kunz, B.; Paul-Pletzer, K.; Grimm, R.; Eckerskorn, C.; Farmer, E.E. Cloning of a cDNA encoding a plasma membrane-associated, uronide binding phosphoprotein with physical properties similar to viral movement proteins. Plant Cell 1996, 8, 2265–2276. [Google Scholar] [CrossRef]
- Verchot, J. The ER quality control and ER associated degradation machineries are vital for viral pathogenesis. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Baluska, F.; Cvrcková, F.; Kendrick-Jones, J.; Volkmann, D. Sink plasmodesmata as gateways for phloem unloading. Myosin VIII and calreticulin as molecular determinants of sink strength? Plant Physiol. 2001, 126, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Laporte, C.; Vetter, G.; Loudes, A.-M.; Robinson, D.G.; Hillmer, S.; Stussi-Garaud, C.; Ritzenthaler, C. Involvement of the secretory pathway and the cytoskeleton in intracellular targeting and tubule assembly of Grapevine fanleaf virus movement protein in tobacco BY-2 cells. Plant Cell 2003, 15, 2058–2075. [Google Scholar] [CrossRef] [Green Version]
- Christensen, A.; Svensson, K.; Thelin, L.; Zhang, W.; Tintor, N.; Prins, D.; Funke, N.; Michalak, M.; Schulze-Lefert, P.; Saijo, Y.; et al. Higher plant calreticulins have acquired specialized functions in Arabidopsis. PLoS ONE 2010, 5, e11342. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, S.E.; Tsou, P.-L.; Robertson, D. Expression of the high capacity calcium-binding domain of calreticulin increases bioavailable calcium stores in plants. Transgenic Res. 2002, 11, 1–10. [Google Scholar] [CrossRef]
- Xoconostle-Cázares, B.; Xiang, Y.; Ruiz-Medrano, R.; Wang, H.L.; Monzer, J.; Yoo, B.C.; McFarland, K.C.; Franceschi, V.R.; Lucas, W.J. Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 1999, 283, 94–98. [Google Scholar] [CrossRef]
- Lucas, W.J.; Ham, B.-K.; Kim, J.-Y. Plasmodesmata-bridging the gap between neighboring plant cells. Trends Cell Biol. 2009, 19, 495–503. [Google Scholar] [CrossRef]
- Taoka, K.-I.; Ham, B.-K.; Xoconostle-Cázares, B.; Rojas, M.R.; Lucas, W.J. Reciprocal phosphorylation and glycosylation recognition motifs control NCAPP1 interaction with pumpkin phloem proteins and their cell-to-cell movement. Plant Cell 2007, 19, 1866–1884. [Google Scholar] [CrossRef] [Green Version]
- Sparkes, I.; Tolley, N.; Aller, I.; Svozil, J.; Osterrieder, A.; Botchway, S.; Mueller, C.; Frigerio, L.; Hawes, C. Five Arabidopsis reticulon isoforms share endoplasmic reticulum location, topology, and membrane-shaping properties. Plant Cell 2010, 22, 1333–1343. [Google Scholar] [CrossRef] [Green Version]
- Kriechbaumer, V.; Maneta-Peyret, L.; Fouillen, L.; Botchway, S.W.; Upson, J.; Hughes, L.; Richardson, J.; Kittelmann, M.; Moreau, P.; Hawes, C. The odd one out: Arabidopsis reticulon 20 does not bend ER membranes but has a role in lipid regulation. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Nziengui, H.; Bouhidel, K.; Pillon, D.; Der, C.; Marty, F.; Schoefs, B. Reticulon-like proteins in Arabidopsis thaliana: Structural organization and ER localization. FEBS Lett. 2007, 581, 3356–3362. [Google Scholar] [CrossRef] [Green Version]
- Wakana, Y.; Koyama, S.; Nakajima, K.; Hatsuzawa, K.; Nagahama, M.; Tani, K.; Hauri, H.-P.; Melançon, P.; Tagaya, M. Reticulon 3 is involved in membrane trafficking between the endoplasmic reticulum and Golgi. Biochem. Biophys. Res. Commun. 2005, 334, 1198–1205. [Google Scholar] [CrossRef]
- Moore, A.E.; Stone, B.A. Effect of infection with TMV and other viruses on the level of a β-1,3-glucan hydrolase in leaves of Nicotiana glutinosa. Virology 1972, 50, 791–798. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Komarova, T.V.; Petrunia, I.V.; Frolova, O.Y.; Pozdyshev, D.V.; Gleba, Y.Y. Airborne signals from a wounded leaf facilitate viral spreading and induce antibacterial resistance in neighboring plants. PLoS Pathog. 2012, 8, e1002640. [Google Scholar] [CrossRef] [Green Version]
- den Hollander, P.W.; Kieper, S.N.; Borst, J.W.; van Lent, J.W.M. The role of plasmodesma-located proteins in tubule-guided virus transport is limited to the plasmodesmata. Arch. Virol. 2016, 161, 2431–2440. [Google Scholar] [CrossRef] [Green Version]
- Grison, M.S.; Brocard, L.; Fouillen, L.; Nicolas, W.; Wewer, V.; Dörmann, P.; Nacir, H.; Benitez-Alfonso, Y.; Claverol, S.; Germain, V.; et al. Specific Membrane Lipid Composition Is Important for Plasmodesmata Function in Arabidopsis. Plant Cell 2015, 27, 1228–1250. [Google Scholar] [CrossRef] [Green Version]
- Caillaud, M.-C.; Wirthmueller, L.; Sklenar, J.; Findlay, K.; Piquerez, S.J.M.; Jones, A.M.E.; Robatzek, S.; Jones, J.D.G.; Faulkner, C. The plasmodesmal protein PDLP1 localises to haustoria-associated membranes during downy mildew infection and regulates callose deposition. PLoS Pathog. 2014, 10, e1004496. [Google Scholar] [CrossRef]
- Ali, U.; Li, H.; Wang, X.; Guo, L. Emerging Roles of Sphingolipid Signaling in Plant Response to Biotic and Abiotic Stresses. Mol. Plant 2018, 11, 1328–1343. [Google Scholar] [CrossRef] [Green Version]
- Iswanto, A.B.B.; Kim, J.-Y. Lipid Raft, Regulator of Plasmodesmal Callose Homeostasis. Plants 2017, 6, 15. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Yadav, S.R.; Paterlini, A.; Nicolas, W.J.; Petit, J.D.; Brocard, L.; Belevich, I.; Grison, M.S.; Vaten, A.; Karami, L.; et al. Sphingolipid biosynthesis modulates plasmodesmal ultrastructure and phloem unloading. Nat. Plants 2019, 5, 604–615. [Google Scholar] [CrossRef]
- Contreras, F.-X.; Ernst, A.M.; Haberkant, P.; Björkholm, P.; Lindahl, E.; Gönen, B.; Tischer, C.; Elofsson, A.; von Heijne, G.; Thiele, C.; et al. Molecular recognition of a single sphingolipid species by a protein’s transmembrane domain. Nature 2012, 481, 525–529. [Google Scholar] [CrossRef] [Green Version]
- Peiró, A.; Martínez-Gil, L.; Tamborero, S.; Pallás, V.; Sánchez-Navarro, J.A.; Mingarro, I. The Tobacco mosaic virus movement protein associates with but does not integrate into biological membranes. J. Virol. 2014, 88, 3016–3026. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, C.; Akman, O.E.; Bell, K.; Jeffree, C.; Oparka, K. Peeking into Pit Fields: A Multiple Twinning Model of Secondary Plasmodesmata Formation in Tobacco. Plant Cell 2008, 20, 1504–1518. [Google Scholar] [CrossRef] [Green Version]
- Dorokhov, Y.L.; Sheshukova, E.V.; Komarova, T.V. Methanol in Plant Life. Front. Plant Sci. 2018, 9, 1623. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.H.; Sheng, J.; Hind, G.; Handa, A.K.; Citovsky, V. Interaction between the tobacco mosaic virus movement protein and host cell pectin methylesterases is required for viral cell-to-cell movement. EMBO J. 2000, 19, 913–920. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Mäkinen, K.; Frolova, O.Y.; Merits, A.; Saarinen, J.; Kalkkinen, N.; Atabekov, J.G.; Saarma, M. A novel function for a ubiquitous plant enzyme pectin methylesterase: The host-cell receptor for the tobacco mosaic virus movement protein. FEBS Lett. 1999, 461, 223–228. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-H.; Citovsky, V. Systemic movement of a tobamovirus requires host cell pectin methylesterase. Plant J. Cell Mol. Biol. 2003, 35, 386–392. [Google Scholar] [CrossRef] [Green Version]
- Amsbury, S.; Kirk, P.; Benitez-Alfonso, Y. Emerging models on the regulation of intercellular transport by plasmodesmata-associated callose. J. Exp. Bot. 2017, 69, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Han, X.; Lu, T. Callose synthesis during reproductive development in monocotyledonous and dicotyledonous plants. Plant Signal. Behav. 2016, 11, e1062196. [Google Scholar] [CrossRef] [Green Version]
- Drakakaki, G.; van de Ven, W.; Pan, S.; Miao, Y.; Wang, J.; Keinath, N.F.; Weatherly, B.; Jiang, L.; Schumacher, K.; Hicks, G.; et al. Isolation and proteomic analysis of the SYP61 compartment reveal its role in exocytic trafficking in Arabidopsis. Cell Res. 2012, 22, 413–424. [Google Scholar] [CrossRef] [Green Version]
- Kulich, I.; Vojtíková, Z.; Sabol, P.; Ortmannová, J.; Neděla, V.; Tihlaříková, E.; Žárský, V. Exocyst Subunit EXO70H4 Has a Specific Role in Callose Synthase Secretion and Silica Accumulation. Plant Physiol. 2018, 176, 2040–2051. [Google Scholar] [CrossRef] [Green Version]
- Schneider, R.; Hanak, T.; Persson, S.; Voigt, C.A. Cellulose and callose synthesis and organization in focus, what’s new? Curr. Opin. Plant Biol. 2016, 34, 9–16. [Google Scholar] [CrossRef]
- Vatén, A.; Dettmer, J.; Wu, S.; Stierhof, Y.-D.; Miyashima, S.; Yadav, S.R.; Roberts, C.J.; Campilho, A.; Bulone, V.; Lichtenberger, R.; et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 2011, 21, 1144–1155. [Google Scholar] [CrossRef] [Green Version]
- Langeveld, S.M.J.; Vennik, M.; Kottenhagen, M.; van Wijk, R.; Buijk, A.; Kijne, J.W.; de Pater, S. Glucosylation Activity and Complex Formation of Two Classes of Reversibly Glycosylated Polypeptides. Plant Physiol. 2002, 129, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Delgado, I.J.; Wang, Z.; de Rocher, A.; Keegstra, K.; Raikhel, N.V. Cloning and characterization of AtRGP1. A reversibly autoglycosylated arabidopsis protein implicated in cell wall biosynthesis. Plant Physiol. 1998, 116, 1339–1350. [Google Scholar] [CrossRef] [Green Version]
- Dhugga, K.S.; Ulvskov, P.; Gallagher, S.R.; Ray, P.M. Plant polypeptides reversibly glycosylated by UDP-glucose. Possible components of Golgi beta-glucan synthase in pea cells. J. Biol. Chem. 1991, 266, 21977–21984. [Google Scholar]
- Dhugga, K.S.; Tiwari, S.C.; Ray, P.M. A reversibly glycosylated polypeptide (RGP1) possibly involved in plant cell wall synthesis: Purification, gene cloning, and trans-Golgi localization. Proc. Natl. Acad. Sci. USA 1997, 94, 7679–7684. [Google Scholar] [CrossRef] [Green Version]
- Rautengarten, C.; Ebert, B.; Herter, T.; Petzold, C.J.; Ishii, T.; Mukhopadhyay, A.; Usadel, B.; Scheller, H.V. The Interconversion of UDP-Arabinopyranose and UDP-Arabinofuranose Is Indispensable for Plant Development in Arabidopsis. Plant Cell 2011, 23, 1373–1390. [Google Scholar] [CrossRef] [Green Version]
- Drakakaki, G.; Zabotina, O.; Delgado, I.; Robert, S.; Keegstra, K.; Raikhel, N. Arabidopsis reversibly glycosylated polypeptides 1 and 2 are essential for pollen development. Plant Physiol. 2006, 142, 1480–1492. [Google Scholar] [CrossRef] [Green Version]
- Park, K.; Knoblauch, J.; Oparka, K.; Jensen, K.H. Controlling intercellular flow through mechanosensitive plasmodesmata nanopores. Nat. Commun. 2019, 10, 3564. [Google Scholar] [CrossRef]
- Maule, A.J.; Benitez-Alfonso, Y.; Faulkner, C. Plasmodesmata-membrane tunnels with attitude. Curr. Opin. Plant Biol. 2011, 14, 683–690. [Google Scholar] [CrossRef]
- Reagan, B.C.; Burch-Smith, T.M. Viruses Reveal the Secrets of Plasmodesmal Cell Biology. Mol. Plant-Microbe Interact. MPMI 2019. [Google Scholar] [CrossRef]

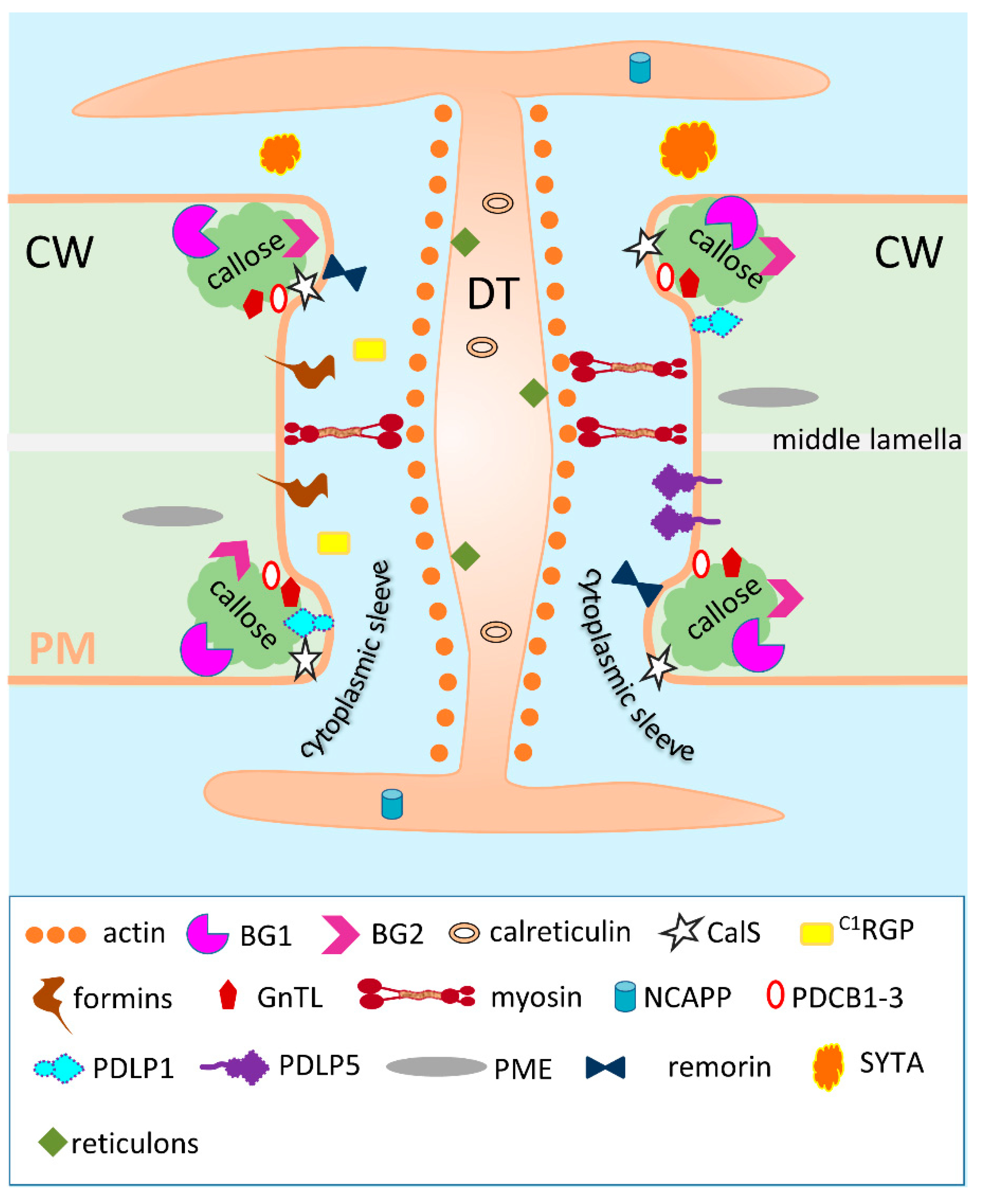
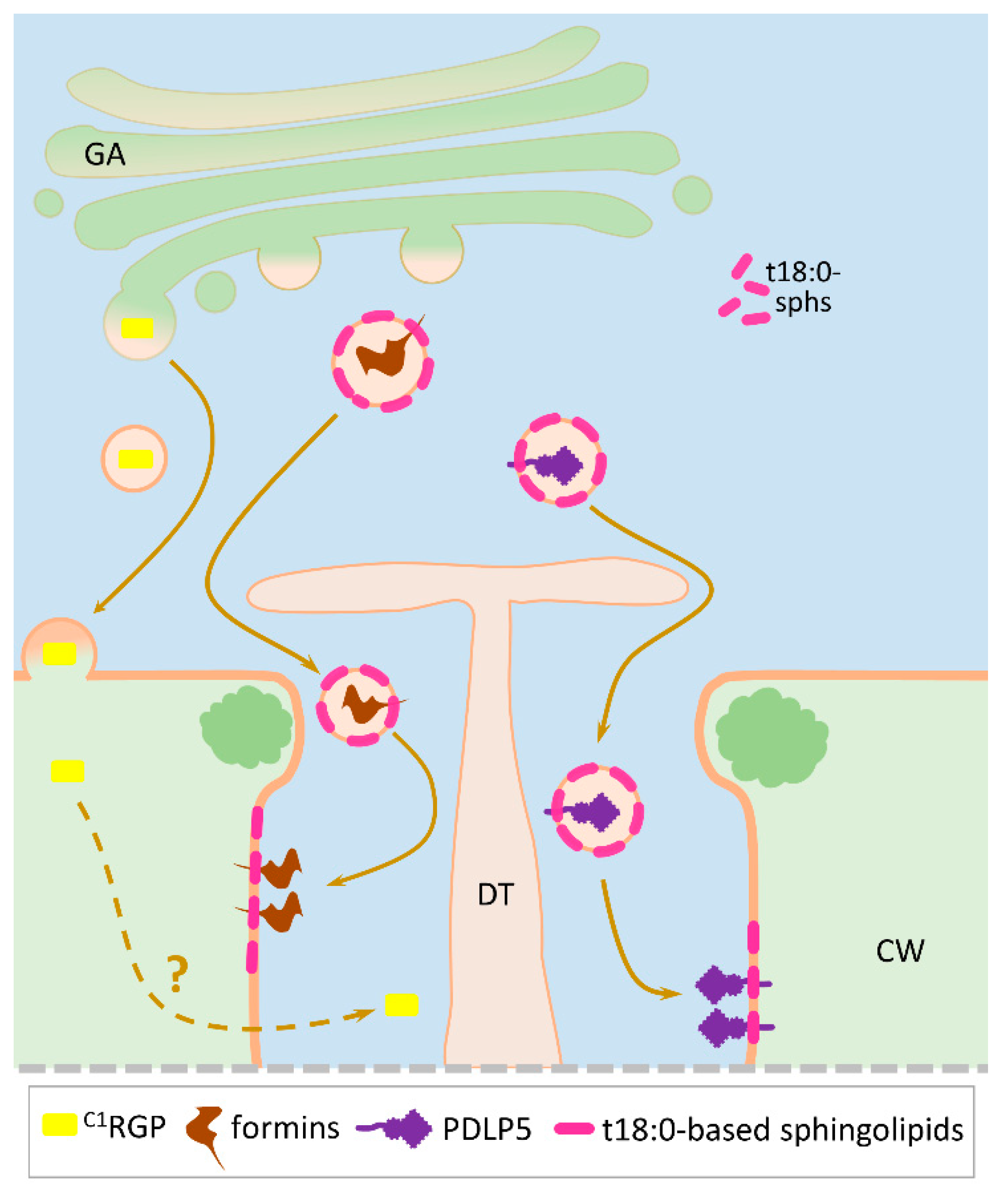

| Protein | Function/Description | Signal Sequence | Predicted N-Glycosylation Sites | Pd Localization | Protein Relocation After Stress Impact | References |
|---|---|---|---|---|---|---|
| Pd non-secretory proteins | ||||||
| Actin, myosin and tubulin | Actin-myosin filaments and tubulin are localized within the Pd cytoplasmic sleeve and negatively control Pd permeability | No | No | Cytoplasmic sleeves in the Pd cavity | No | [48,49,50] |
| A. thaliana calreticulin-1 (AtCRT1) (UniProt O04151) | Ca2+-sequestering protein chaperone and ER protein that negatively control Pd permeability | Yes | Asn59, Asn154, Asn399 | Associates with the desmotubule | Yes | [51,52,53] |
| Tobacco non-cell autonomous pathway protein (NCAPP) (UniProt Q947H5) | NCAPP has homology to aldose 1-epimerase; positively controls Pd permeability and PME/methanol production | Yes | Asn76 and Asn100 | Localizes in the ER | N/A | [54,55] |
| Remorin | Associates with PM raft-like structures and probably serves as a negative regulator of Pd permeability. Antagonist of Potato virus X triple gene block protein 1 | No | No | In the cytosolic surface of the Pd plasma membrane | N/A | [56] |
| A. thaliana reticulons, AtRTNLB3 (UniProt Q9SH59) and AtRTNLB6 (UniProt Q6DBN4) | ER-localized proteins with three TM domains that negatively control Pd permeability | No | No | Accumulates in the desmotubule | N/A | [57,58] |
| Arabidopsis synaptotagmin SYTA (AtSYTA) (UniProt - Q9SKR2) | ER-PM tethering and endocytic recycling | No | No | Interacts with TMV movement protein (MP) Pd localization signal (PLS) for cell-to-cell transport and participates in the formation of virus replication sites | Suggested that SYTA relocates to the Pd cavity after TMV infection | [59,60,61,62] |
| Pd secretory proteins | ||||||
| A. thaliana (1,3)-β-glucanase 1 (AtBG1) (UniProt - Q9M2M0) | Callose-degrading enzymes that positively control Pd permeability | Yes | Asn291 | Colocalizes with callose near Pd orifices | No | [36,63,64] |
| A. thaliana (1,3)-β-glucanase 2 (AtBG2) (UniProt P33157) | Yes | No | Colocalizes with callose near Pd orifices in the extracellular space | AtBG2 is not delivered to the extracellular space in TMV-infected cells, but associates with viral MP in Pd cytoplasmic sleeve | [36,64] | |
| A. thaliana (1,3)-β-glucanase 3 (AtBG3) (UniProt F4j270) | Yes | No | Constitutive Pd-associated enzyme but not stress-regulated | No | [36,64] | |
| *A. thaliana β-1,3-glucanase_putative Pd-associated protein (AtBG_ppap) (UniProt Q9FHX5) | Yes | No | Localizes to the Pd neck region | No | [35,36,65] | |
| A. thaliana Class 1 reversibly glycosylated polypeptide (AtC1RGP) (UniProt Q9SRT9) | AtC1RGP acts as a negative Pd regulator. Despite having no signal sequence, it is found in the GA and ultimately in the Pd | No | No | N/A | [66,67,68] | |
| A. thaliana Callose synthase (CalS) (UniProt Q9AUE0) | Callose-synthesizing enzyme encoded by glucan synthase-like (GSL) gene that negatively controls Pd permeability | No (needs exocyst-positive organelle (EXPO)-mediated secretion for Pd localization) | No | CalS localizes at callose depositions | No | [36,37,69] |
| A. thaliana formin-like protein 1 (UniProt Q9SE97) and 2 (UniProt O22824) (AtFH1 and AtFH2) | Negatively regulates Pd permeability by interacting with actin filaments | Yes | Multiple Asn sites | Localizes in the Pd cavity and interacts with actin | N/A | [70,71] |
| A. thaliana β-1,6-N-acetylglucosaminyl transferase-like enzyme (GnTL) (UniProt Q9SUZ8) | Interacts with calreticulin and probably serves as a negative regulator of Pd permeability | Yes | Asn287 and Asn316 | Colocalizes with callose-binding protein near Pd orifices | N/A | [72] |
| *A. thaliana Pd callose-binding proteins 1,2 and 3 (PDCB1-3) (At5g61130) | Pd callose-binding protein that negatively controls Pd permeability | Yes | Asn154 and Asn179 | Localizes to the Pd neck | No | [73,74] |
| A. thaliana Pd-located protein 1 (PDLP1) (UniProt Q8GXV7) | Membrane receptor-like protein with two extracellular DUF26 domains. PDLP1 overexpression causes restricted cell-to-cell trafficking. Acts as a negative Pd regulator by promoting callose deposition. Stimulates the transport of viruses that use tubule-guided movement by redundantly interacting with tubule-forming MPs within Pds | Yes | No | PDLP1 is targeted to Pd via the Brefeldin A–sensitive secretory pathway and resides at Pd with its C-terminus in the cytoplasmic space and its N-terminus in the apoplast. | No | [75,76,77,78] |
| A. thaliana Pd located protein 5 (PDLP5) (UniProt Q8GUJ2) | A member of the PDLP family that has 30% amino acid sequence identity to PDLP1. Contains sphingolipid binding motif in the TMD. Acts as a negative Pd regulator by promoting callose deposition. Delays systemic movement of TMV | Yes | Asn69 and Asn132 | PDLP5 localizes inside the central Pd region similar to TMV MP. However, PDLP5/MP overlap is not complete | Transmembrane secretory protein with ectopic localization | [79,80,81,82] |
| Tobacco pectin methylesterase (PME) (UniProt Q9LEBO) | Non-direct regulator of Pd permeability that participates in the de-methylesterification of cell wall HG through the formation of methanol | Yes | Asn43, Asn101 and Asn220 in proPME | Immunogold localization of PME is preferentially around Pd | No | [83,84] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dorokhov, Y.L.; Ershova, N.M.; Sheshukova, E.V.; Komarova, T.V. Plasmodesmata Conductivity Regulation: A Mechanistic Model. Plants 2019, 8, 595. https://doi.org/10.3390/plants8120595
Dorokhov YL, Ershova NM, Sheshukova EV, Komarova TV. Plasmodesmata Conductivity Regulation: A Mechanistic Model. Plants. 2019; 8(12):595. https://doi.org/10.3390/plants8120595
Chicago/Turabian StyleDorokhov, Yuri L., Natalia M. Ershova, Ekaterina V. Sheshukova, and Tatiana V. Komarova. 2019. "Plasmodesmata Conductivity Regulation: A Mechanistic Model" Plants 8, no. 12: 595. https://doi.org/10.3390/plants8120595
APA StyleDorokhov, Y. L., Ershova, N. M., Sheshukova, E. V., & Komarova, T. V. (2019). Plasmodesmata Conductivity Regulation: A Mechanistic Model. Plants, 8(12), 595. https://doi.org/10.3390/plants8120595




